Robotic-Arm-Based Force Control in Neurosurgical Practice
Abstract
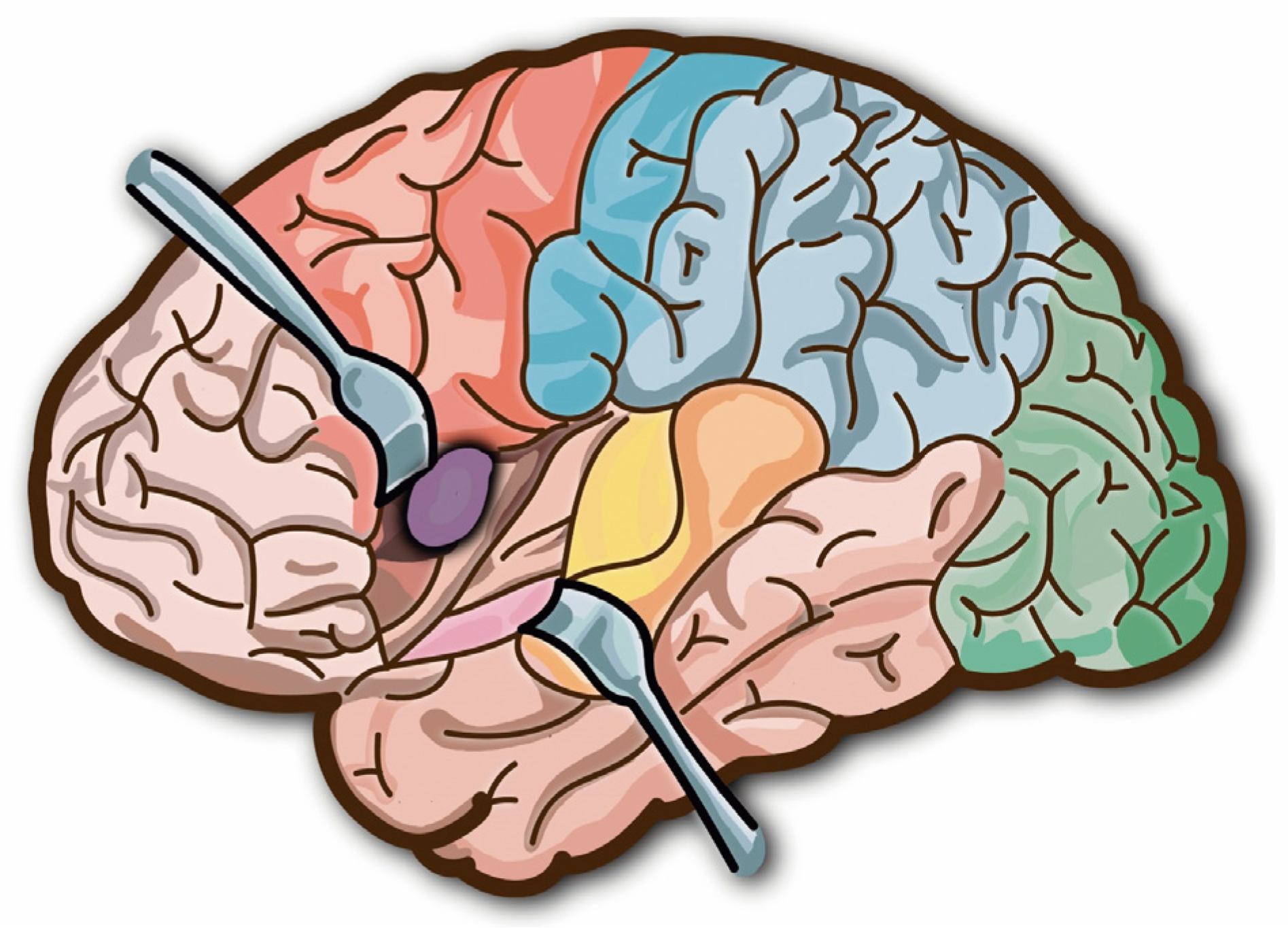
:1. Introduction
2. Problem Settlement and Control Proposal
3. Control Proposal
3.1. Cost Functional Proposal
3.2. Control Law Proposal
4. System Setup and Results
5. Results
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dewan, M.C.; Rattani, A.; Fieggen, G.; Arraez, M.A.; Servadei, F.; Boop, F.A.; Johnson, W.D.; Warf, B.C.; Park, K.B. Global Neurosurgery: The Current Capacity and Deficit in the Provision of Essential Neurosurgical Care. Executive Summary of the Global Neurosurgery Initiative at the Program in Global Surgery and Social Change. J. Neurosurg. 2019, 130, 1055–1064. [Google Scholar] [CrossRef]
- Bennett, M.H.; Albin, M.S.; Bunegin, L.; Dujovny, M.; Hellstrom, H.; Jannetta, P.J. Evoked Potential Changes during Brain Retraction in Dogs. Stroke 1977, 8, 487–492. [Google Scholar] [CrossRef]
- Andrews, R.J.; Bringas, J.R. A Review of Brain Retraction and Recommendations for Minimizing Intraoperative Brain Injury. Neurosurgery 1993, 33, 1052–1064. [Google Scholar] [CrossRef]
- Spetzler, R.F.; Sanai, N. The Quiet Revolution: Retractorless Surgery for Complex Vascular and Skull Base Lesions: Clinical Article. J. Neurosurg. 2012, 116, 291–300. [Google Scholar] [CrossRef]
- Rice, B.J.; Peerless, S.J.; Drake, C.G. Surgical Treatment of Unruptured Aneurysms of the Posterior Circulation. J. Neurosurg. 1990, 73, 165–173. [Google Scholar] [CrossRef]
- Nazzaro, J.M.; Shults, W.T.; Neuwelt, E.A. Neuro-Ophthalmological Function of Patients with Pineal Region Tumors Approached Transtentorially in the Semisitting Position. J. Neurosurg. 1992, 76, 746–751. [Google Scholar] [CrossRef]
- Laha, R.K.; Dujovny, M.; Rao, S.; Barrionuevo, P.J.; Bunegin, L.; Hellstrom, H.R.; Albin, M.S.; Taylor, F.H. Cerebellar Retraction: Significance and Sequelae. Surg. Neurol. 1979, 12, 209–215. [Google Scholar]
- Bell, B.A.; Symon, L.; Branston, N.M. CBF and Time Thresholds for the Formation of Ischemic Cerebral Edema, and Effect of Reperfusion in Baboons. J. Neurosurg. 1985, 62, 31–41. [Google Scholar] [CrossRef]
- Fukamachi, A.; Koizumi, H.; Nukui, H. Postoperative Intracerebral Hemorrhages: A Survey of Computed Tomographic Findings after 1074 Intracranial Operations. Surg. Neurol. 1985, 23, 575–580. [Google Scholar] [CrossRef]
- Hu, X.; Chen, A.; Luo, Y.; Zhang, C. Steerable catheters for minimally invasive surgery: A review and future directions. Comput. Assist. Surg. 2018, 23, 21–41. [Google Scholar] [CrossRef]
- Kalfas, I.H.; Little, J.R. Postoperative Hemorrhage: A Survey of 4992 Intracranial Procedures. Neurosurgery 1988, 23, 343–347. [Google Scholar] [CrossRef]
- Rosenørn, J. The Risk of Ischaemic Brain Damage during the Use of Self-Retaining Brain Retractors. Acta Neurol. Scand. 1989, 79, 1–30. [Google Scholar] [CrossRef]
- Dai, Z. Improvement of General Design Theory and Methodology with Its Application to Design of a Retractor for Ventral Hernia Repair Surgery. Master’s Thesis, University of Saskatchewan, Saskatoon, SK, Canada, March 2019. [Google Scholar]
- Yokoh, A.; Sugita, K.; Kobayashi, S. Clinical Study of Brain Retraction in Different Approaches and Diseases. Acta Neurochir. 1987, 87, 134–139. [Google Scholar] [CrossRef]
- Dujovny, M.; Wackenhut, N.; Kossovsky, N.; Leff, L.; Gómez, C.; Nelson, D. Biomechanics of Vascular Occlusion in Neurosurgery. Acta Neurol. Lat. 1980, 26, 123–127. [Google Scholar]
- DeLorenzo, C.; Papademetris, X.; Staib, L.H.; Vives, K.P.; Spencer, D.D.; Duncan, J.S. Volumetric Intraoperative Brain Deformation Compensation: Model Development and Phantom Validation. IEEE Trans. Med. Imaging 2012, 31, 1607–1619. [Google Scholar] [CrossRef]
- Hartkens, T.; Hill, D.L.G.; Castellano-Smith, A.D.; Hawkes, D.J.; Maurer, C.R.; Martin, A.J.; Hall, W.A.; Liu, H.; Truwit, C.L. Measurement and Analysis of Brain Deformation during Neurosurgery. IEEE Trans. Med. Imaging 2003, 22, 82–92. [Google Scholar] [CrossRef]
- Warfield, S.K.; Talos, F.; Tei, A.; Bharatha, A.; Nabavi, A.; Ferrant, M.; McL. Black, P.; Jolesz, F.A.; Kikinis, R. Real-Time Registration of Volumetric Brain MRI by Biomechanical Simulation of Deformation during Image Guided Neurosurgery. Comput. Vis. Sci. 2002, 5, 3–11. [Google Scholar] [CrossRef]
- Arani, A.; Min, H.-K.; Fattahi, N.; Wetjen, N.M.; Trzasko, J.D.; Manduca, A.; Jack, C.R.; Lee, K.H.; Ehman, R.L.; Huston, J. Acute Pressure Changes in the Brain Are Correlated with MR Elastography Stiffness Measurements: Initial Feasibility in an in Vivo Large Animal Model: MRE Stiffness Correlates With Changes in ICP. Magn. Reson. Med. 2018, 79, 1043–1051. [Google Scholar] [CrossRef]
- Budday, S.; Ovaert, T.C.; Holzapfel, G.A.; Steinmann, P.; Kuhl, E. Fifty Shades of Brain: A Review on the Mechanical Testing and Modeling of Brain Tissue. Arch. Comput. Methods Eng. 2020, 27, 1187–1230. [Google Scholar] [CrossRef]
- Chambers, I.R.; Martin, D.; Clark, A.; Nicklin, A.; Mendelow, A.D.; Mitchell, P. The Measurement of Brain Tissue Stiffness In-Vivo. In Acta Neurochirurgica Supplements; Steiger, H.-J., Ed.; Acta Neurochirurgica Supplementum; Springer: Vienna, Austria, 2008; Volume 102, pp. 287–289. ISBN 978-3-211-85577-5. [Google Scholar]
- Fallah, A.; Subramaniam, T.; Phillips, H.W.; Michalet, X.; Vinters, H.V.; Yong, W.H.; Wu, J.Y.; Salamon, N.; Ellingson, B.M.; Wang, A.C.; et al. Novel Tonometer Device Distinguishes Brain Stiffness in Epilepsy Surgery. Sci. Rep. 2020, 10, 20978. [Google Scholar] [CrossRef]
- Kyriacou, S.K.; Mohamed, A.; Miller, K.; Neff, S. Brain Mechanics For Neurosurgery: Modeling Issues. Biomech. Model. Mechanobiol. 2002, 1, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Cepolina, F.; Razzoli, R.P. An introductory review of robotically assisted surgical systems. Int. J. Med. Robot. 2022, 18, e2409. [Google Scholar] [CrossRef] [PubMed]
- Hoeckelmann, M.; Rudas, I.J.; Fiorini, P.; Kirchner, F.; Haidegger, T. Current Capabilities and Development Potential in Surgical Robotics. Int. J. Adv. Robot. Syst. 2015, 12, 61. [Google Scholar] [CrossRef]
- Attanasio, A.; Scaglioni, B.; De Momi, E.; Fiorini, P.; Valdastri, P. Autonomy in Surgical Robotics. Annu. Rev. Control Robot. Auton. Syst. 2021, 4, 651–679. [Google Scholar] [CrossRef]
- Davies, B.; Starkie, S.; Harris, S.J.; Agterhuis, E.; Paul, V.; Auer, L.M. Neurobot: A Special-Purpose Robot for Neurosurgery. In Proceedings of the 2000 ICRA. Millennium Conference. IEEE International Conference on Robotics and Automation. Symposia Proceedings (Cat. No.00CH37065), San Francisco, CA, USA, 24–28 April 2000; Volume 4, pp. 4103–4108. [Google Scholar]
- Švaco, M.; Koren, P.; Jerbić, B.; Vidaković, J.; Šekoranja, B.; Šuligoj, F. Validation of Three KUKA Agilus Robots for Application in Neurosurgery. In Advances in Service and Industrial Robotics; Ferraresi, C., Quaglia, G., Eds.; Mechanisms and Machine Science; Springer International Publishing: Cham, Switzerland, 2018; Volume 49, pp. 996–1006. ISBN 978-3-319-61275-1. [Google Scholar]
- Okamoto, J.; Iida, M.; Nambu, K.; Fujie, M.G.; Umezu, M. Development of Multi-Dof Brain Retract Manipulator with Safety Method. In Proceedings of the 2003 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS 2003) (Cat. No.03CH37453), Las Vegas, NV, USA, 27–31 October 2003; Volume 3, pp. 2594–2599. [Google Scholar]
- Watanabe, M.; Yoneyama, T.; Nakada, M.; Watanabe, T. Development of Disposable Pressure Sensible Retractor System for Preventing the Overloading. In Proceedings of the 2019 IEEE/SICE International Symposium on System Integration (SII), Paris, France, 14–16 January 2019; pp. 129–134. [Google Scholar]
- Malone, H.R.; Syed, O.N.; Downes, M.S.; D’Ambrosio, A.L.; Quest, D.O.; Kaiser, M.G. Simulation in Neurosurgery: A Review of Computer-Based Simulation Environments and Their Surgical Applications. Neurosurgery 2010, 67, 1105–1116. [Google Scholar] [CrossRef] [PubMed]
- Sase, K.; Fukuhara, A.; Tsujita, T.; Konno, A. GPU-Accelerated Surgery Simulation for Opening a Brain Fissure. Robomech J. 2015, 2, 17. [Google Scholar] [CrossRef]
- Coats, B.; Margulies, S.S. Material Properties of Porcine Parietal Cortex. J. Biomech. 2006, 39, 2521–2525. [Google Scholar] [CrossRef]
- Ying, H.S.; Liu, P.X.; Hou, W.G. A deformation model of pulsating brain tissue for neurosurgery simulation. Comput. Methods Programs Biomed. 2022, 218, 106729. [Google Scholar] [CrossRef]
- Gan, L.S.; Zareinia, K.; Lama, S.; Maddahi, Y.; Yang, F.W.; Sutherland, G.R. Quantification of Forces During a Neurosurgical Procedure: A Pilot Study. World Neurosurg. 2015, 84, 537–548. [Google Scholar] [CrossRef]
- Miller, K.; Wittek, A.; Joldes, G.; Horton, A.; Dutta-Roy, T.; Berger, J.; Morriss, L. Modelling Brain Deformations for Computer-Integrated Neurosurgery. Int. J. Numer. Meth. Biomed. Eng. 2010, 26, 117–138. [Google Scholar] [CrossRef]







| Parameter Name | Definition | Value with Units |
|---|---|---|
| Maximum force amplitude | 0.05 N | |
| Brain retractor contact surface area | 1 cm2 | |
| Maximum pressure in brain tissue | 500 Pa | |
| square position setpoint error ponderation coefficient | 10−4 m−2 | |
| square force term ponderation coefficient | 1 N−2 | |
| square speed term ponderation coefficient | 10−3 s2 m−2 | |
| xd | Displacement set point | 0.02 m |
| Linitial | displaced brain tissue length | m |
| Parameter Name | Definition | Units |
|---|---|---|
| brain tissue stretch | [-] | |
| x | brain tissue displacement | m |
| v | Robot arm speed or brain tissue speed | m.s−1 |
| F | Applied force by brain retractors | N |
| Normalised force | [-] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inziarte-Hidalgo, I.; Uriarte, I.; Fernandez-Gamiz, U.; Sorrosal, G.; Zulueta, E. Robotic-Arm-Based Force Control in Neurosurgical Practice. Mathematics 2023, 11, 828. https://doi.org/10.3390/math11040828
Inziarte-Hidalgo I, Uriarte I, Fernandez-Gamiz U, Sorrosal G, Zulueta E. Robotic-Arm-Based Force Control in Neurosurgical Practice. Mathematics. 2023; 11(4):828. https://doi.org/10.3390/math11040828
Chicago/Turabian StyleInziarte-Hidalgo, Ibai, Irantzu Uriarte, Unai Fernandez-Gamiz, Gorka Sorrosal, and Ekaitz Zulueta. 2023. "Robotic-Arm-Based Force Control in Neurosurgical Practice" Mathematics 11, no. 4: 828. https://doi.org/10.3390/math11040828







