Active Fault-Tolerant Control Applied to a Pressure Swing Adsorption Process for the Production of Bio-Hydrogen
Abstract
:1. Introduction
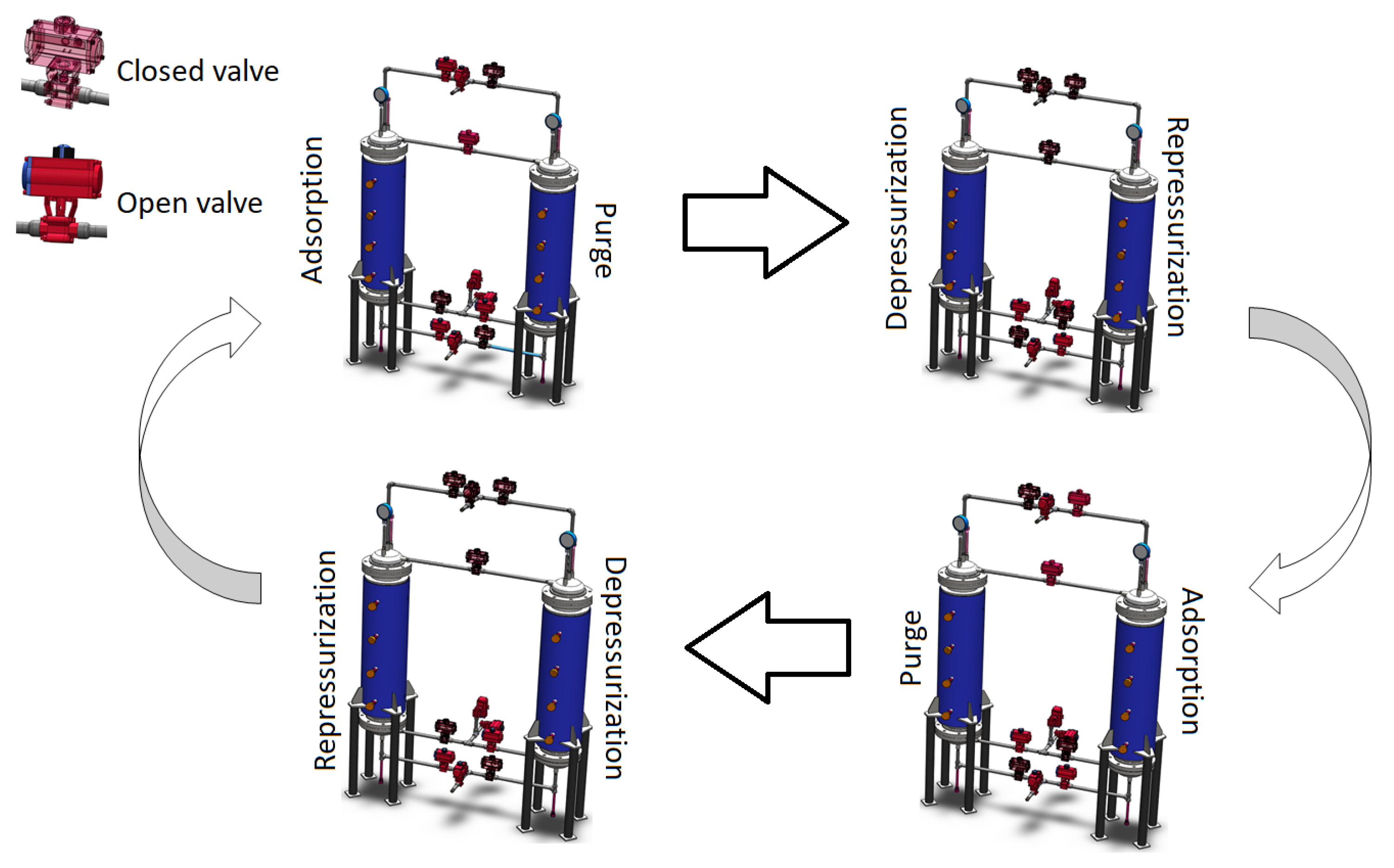
2. Characteristics and Configuration of the PSA Process for the Production of Bio-Hydrogen
Non-Linear Model of the PSA Process for the Production of Bio-Hydrogen
- There are no reactions between the elements of the mixture ( and ).
- The steam phase is convective and axial dispersion is constant.
- The Langmuir model is used in terms of partial pressure.
- The mass transfer coefficient is considered constant.
- Mass balance equation:
- Energy balance equation:
- Ergun equation for momentum balance:
- Equation of kinetics:
- Adsorption isotherms:
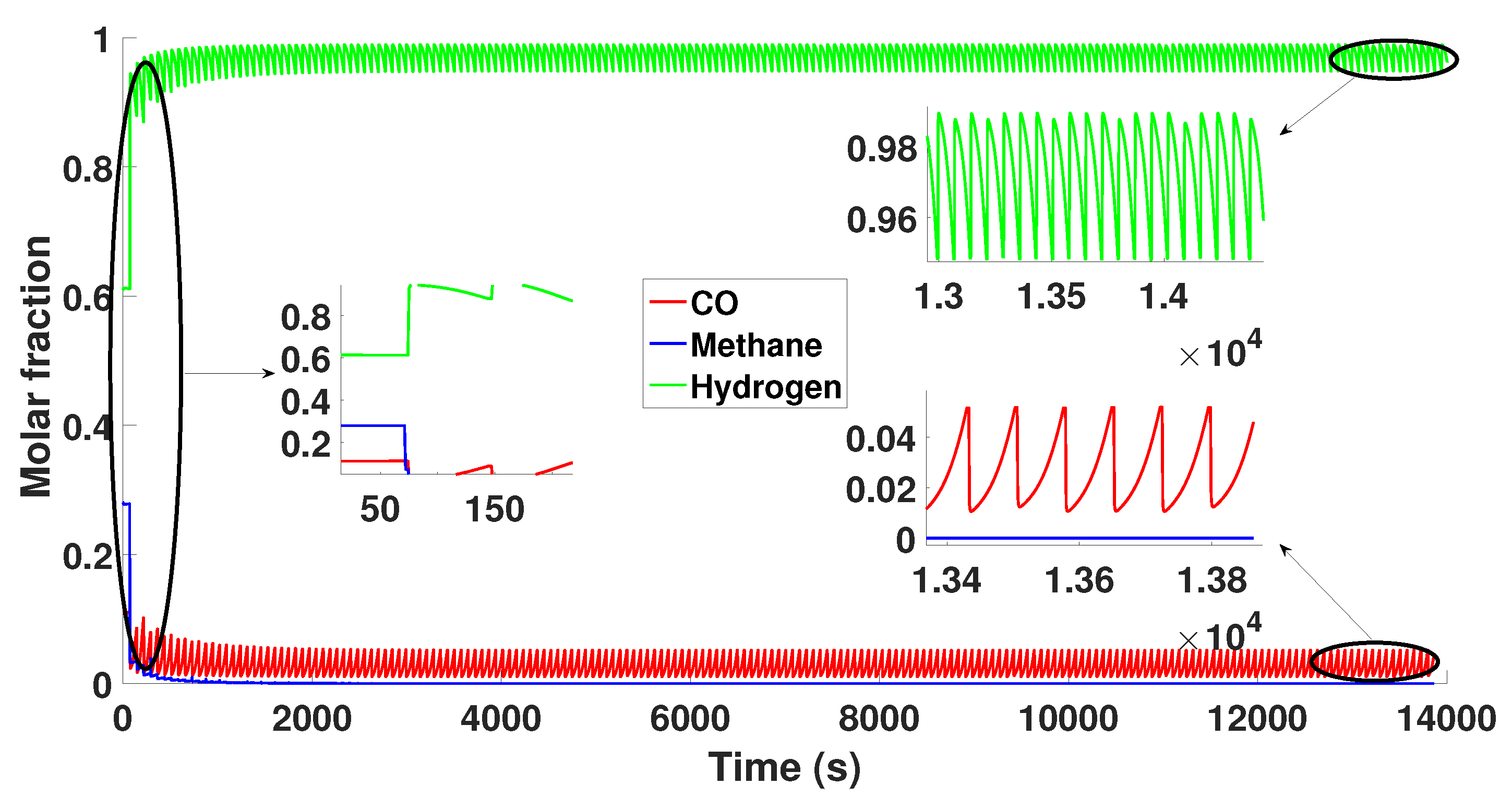
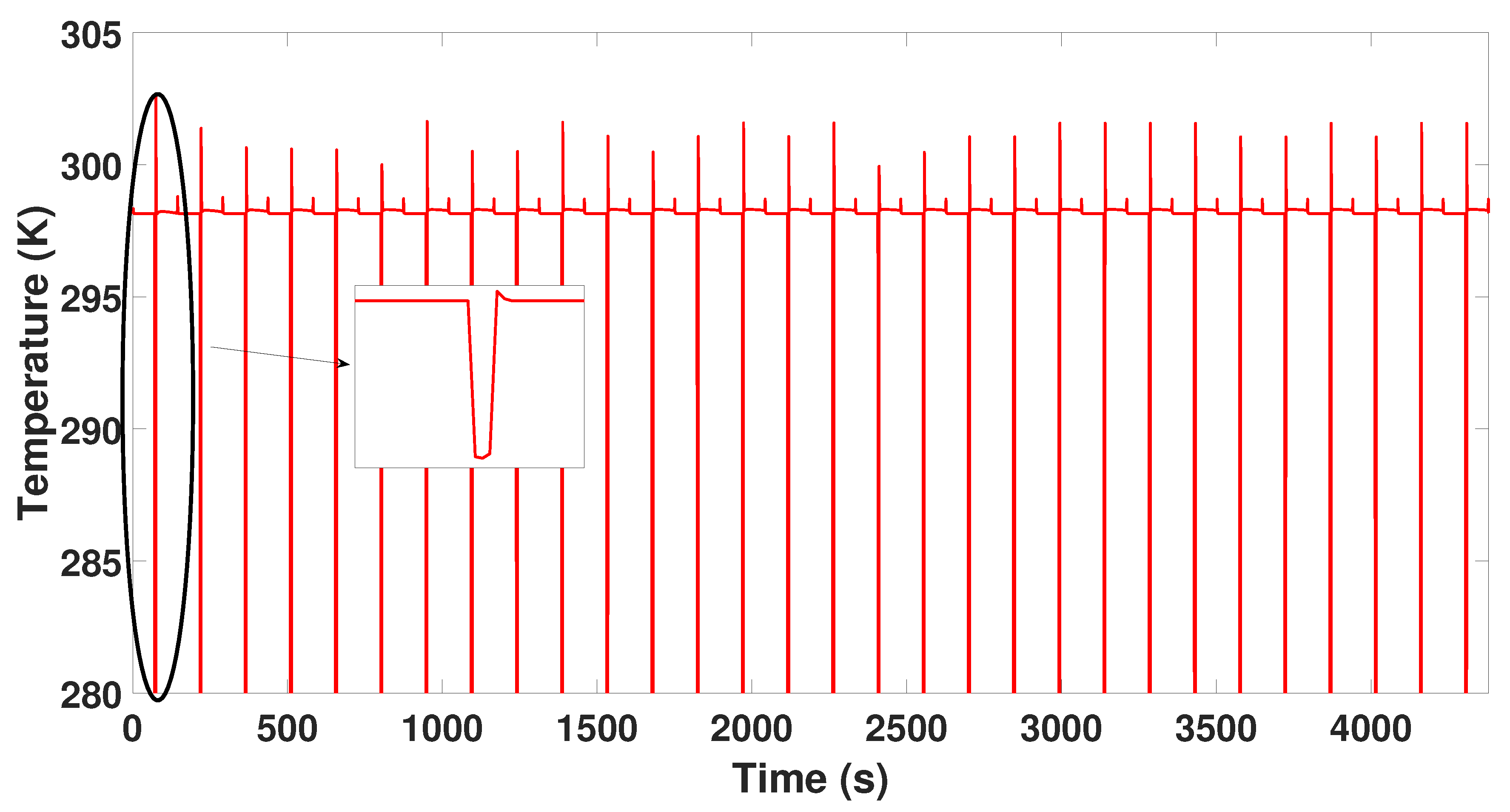
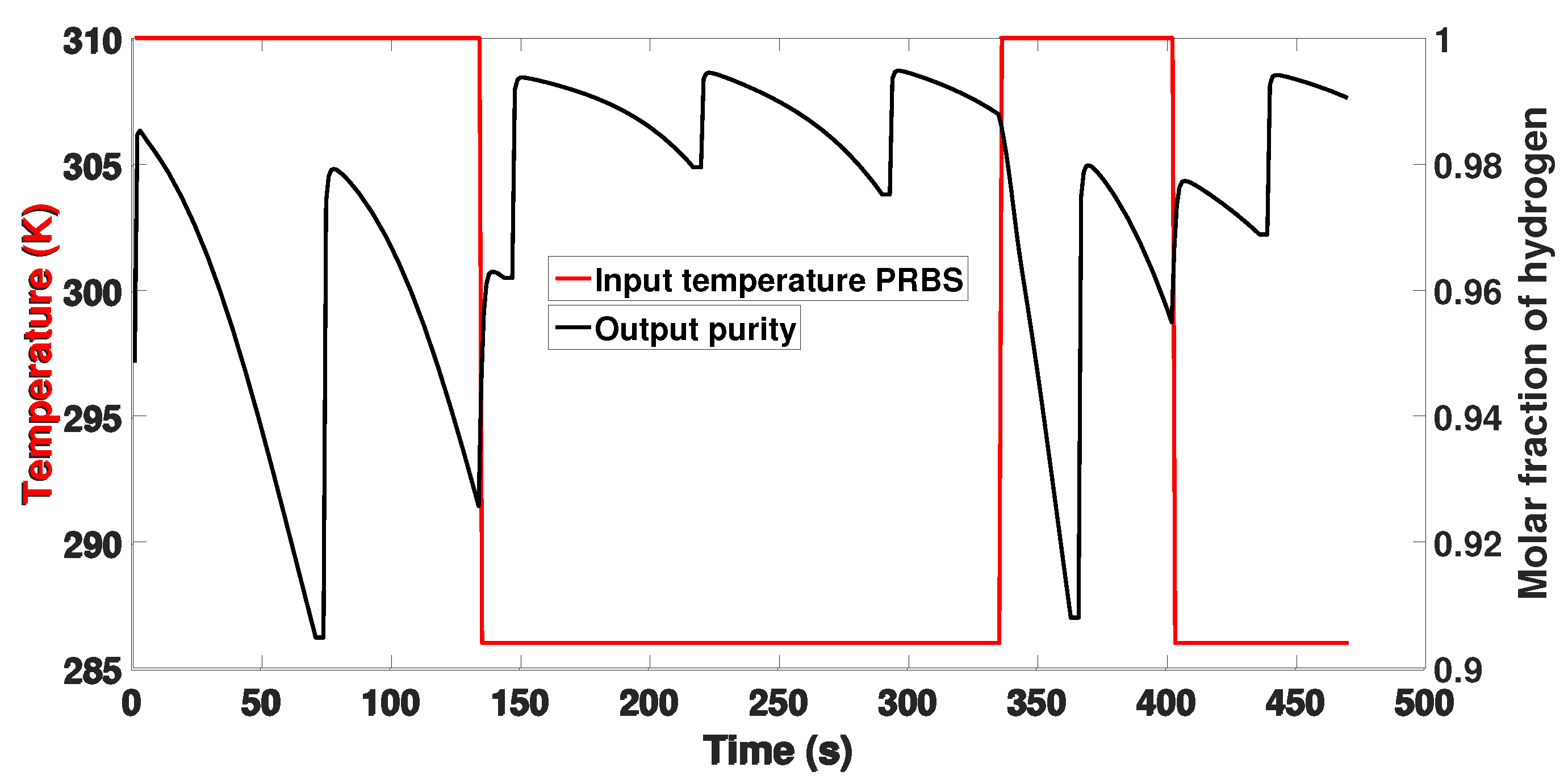
3. Simulation of the PSA Process for the Production of Bio-Hydrogen
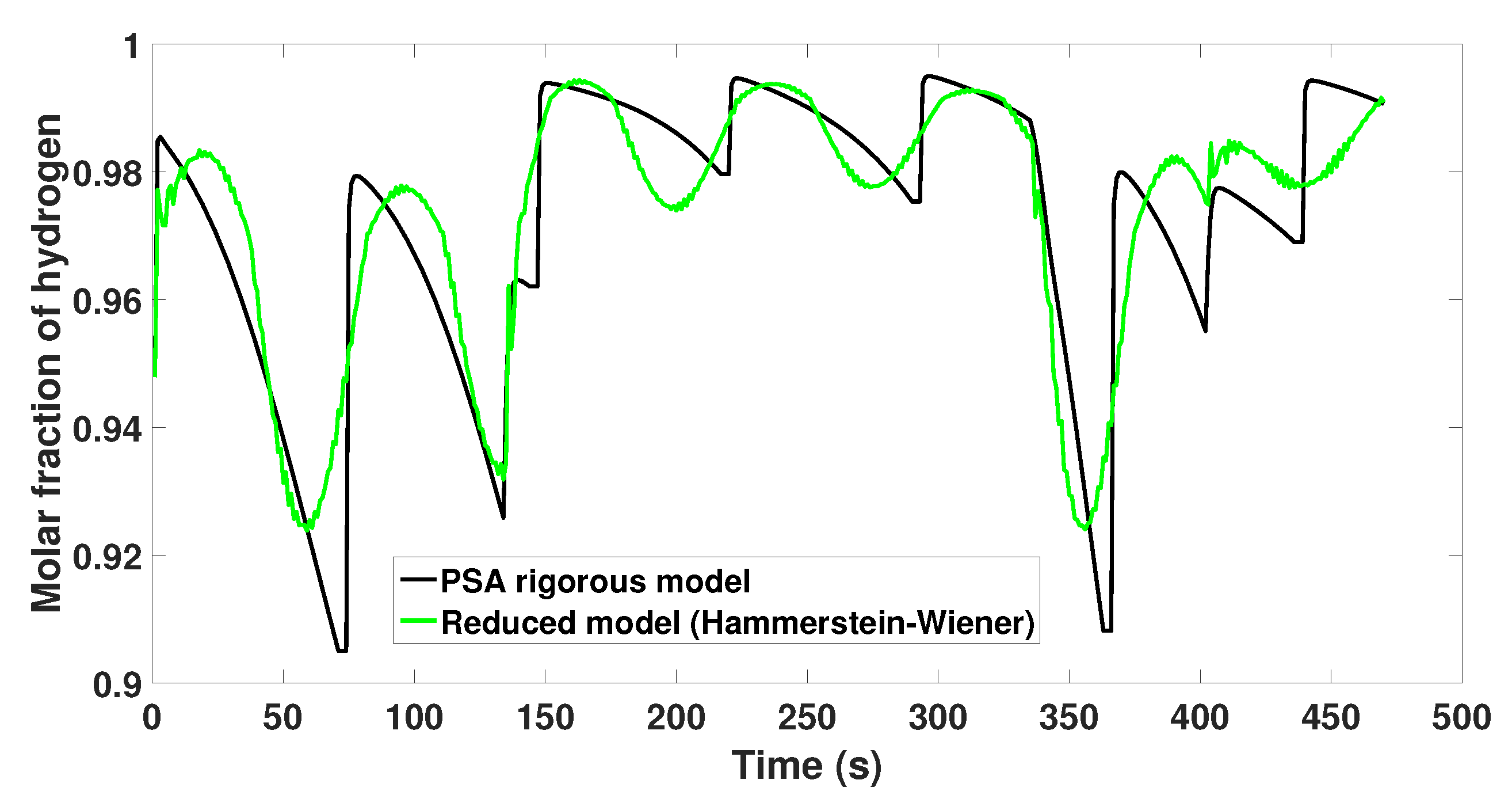
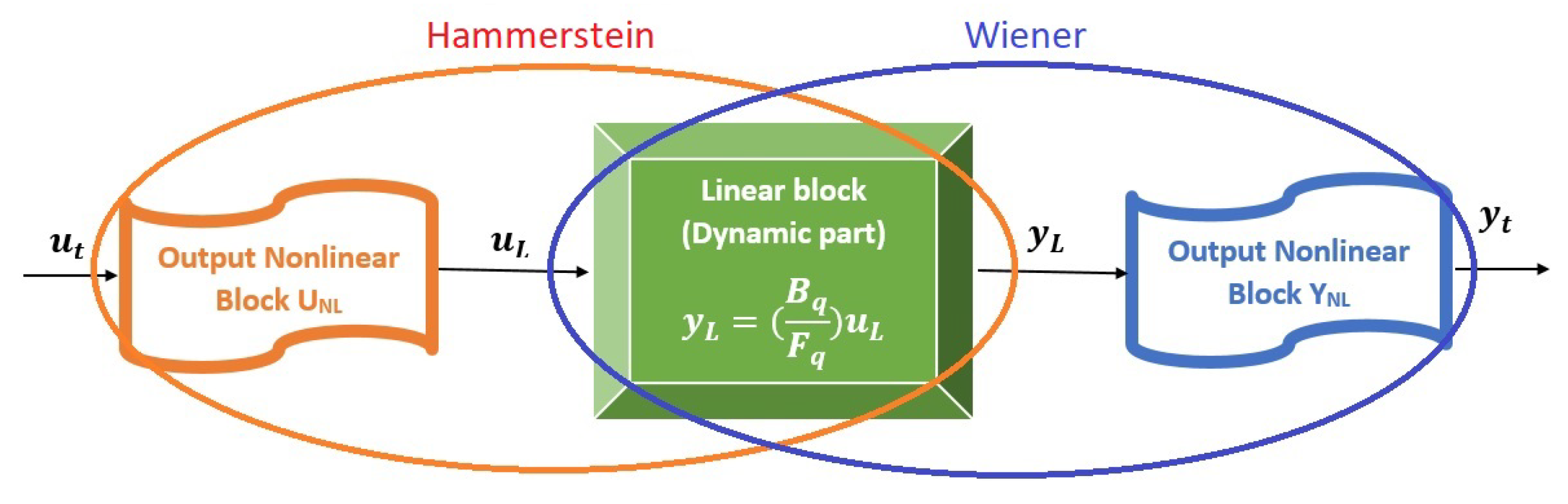
4. Identification of a Reduced Model
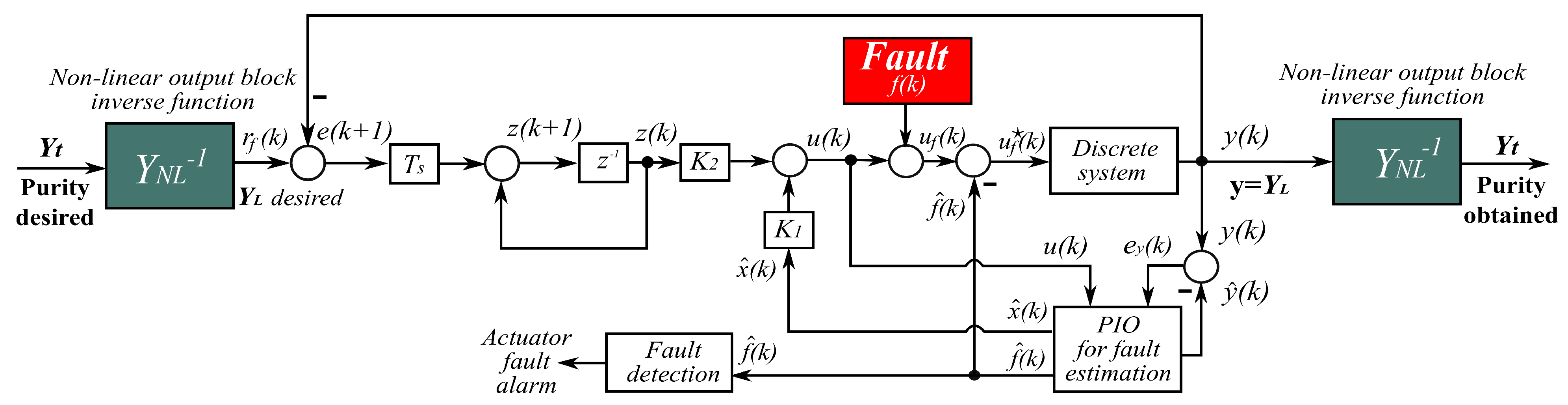
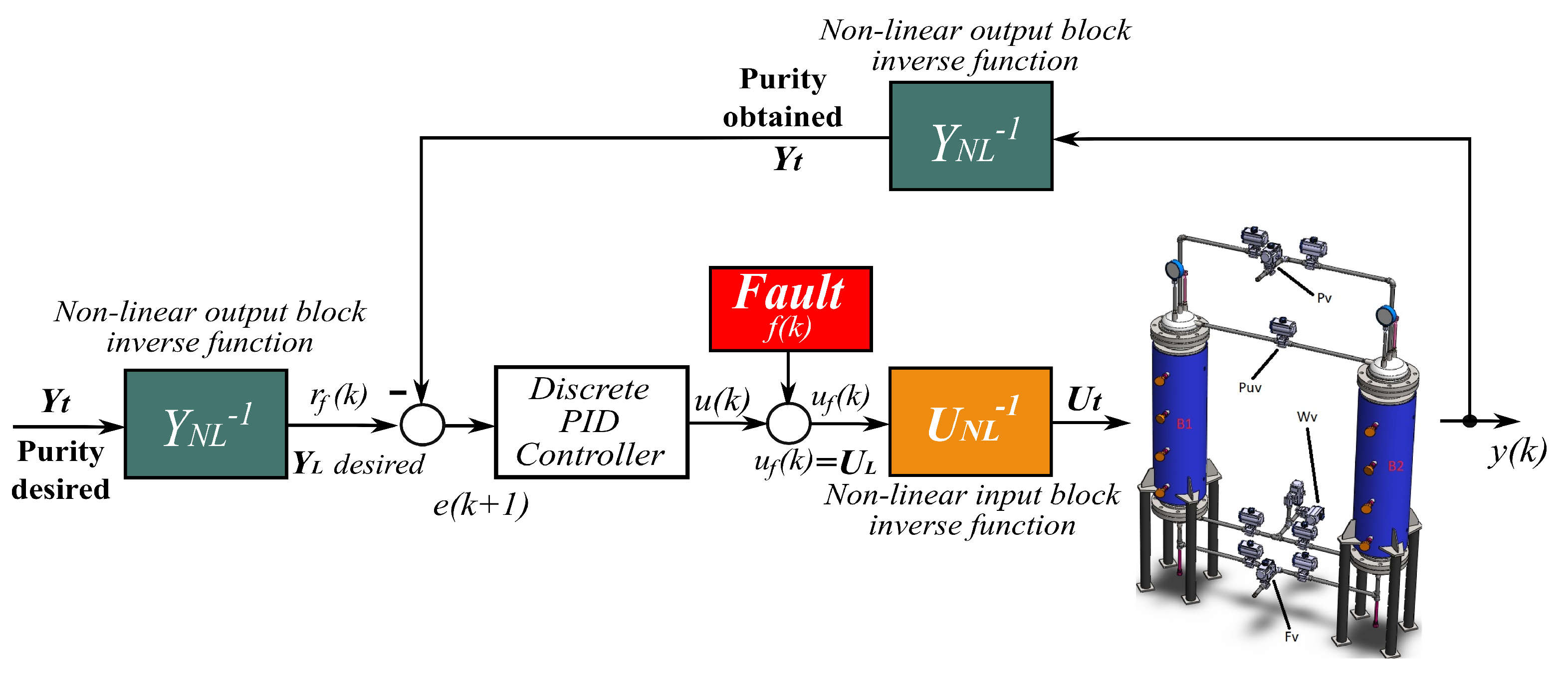
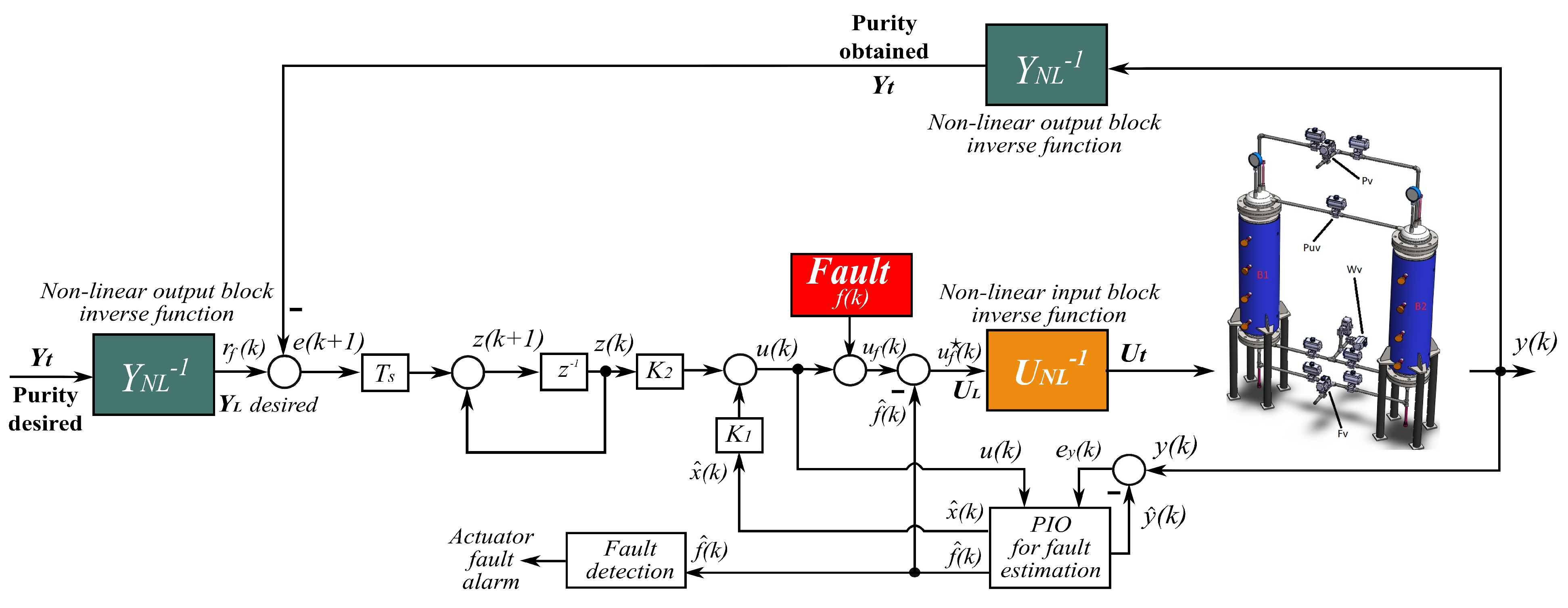
5. Active Fault-Tolerant Controller Design
5.1. Nominal Discrete Controller
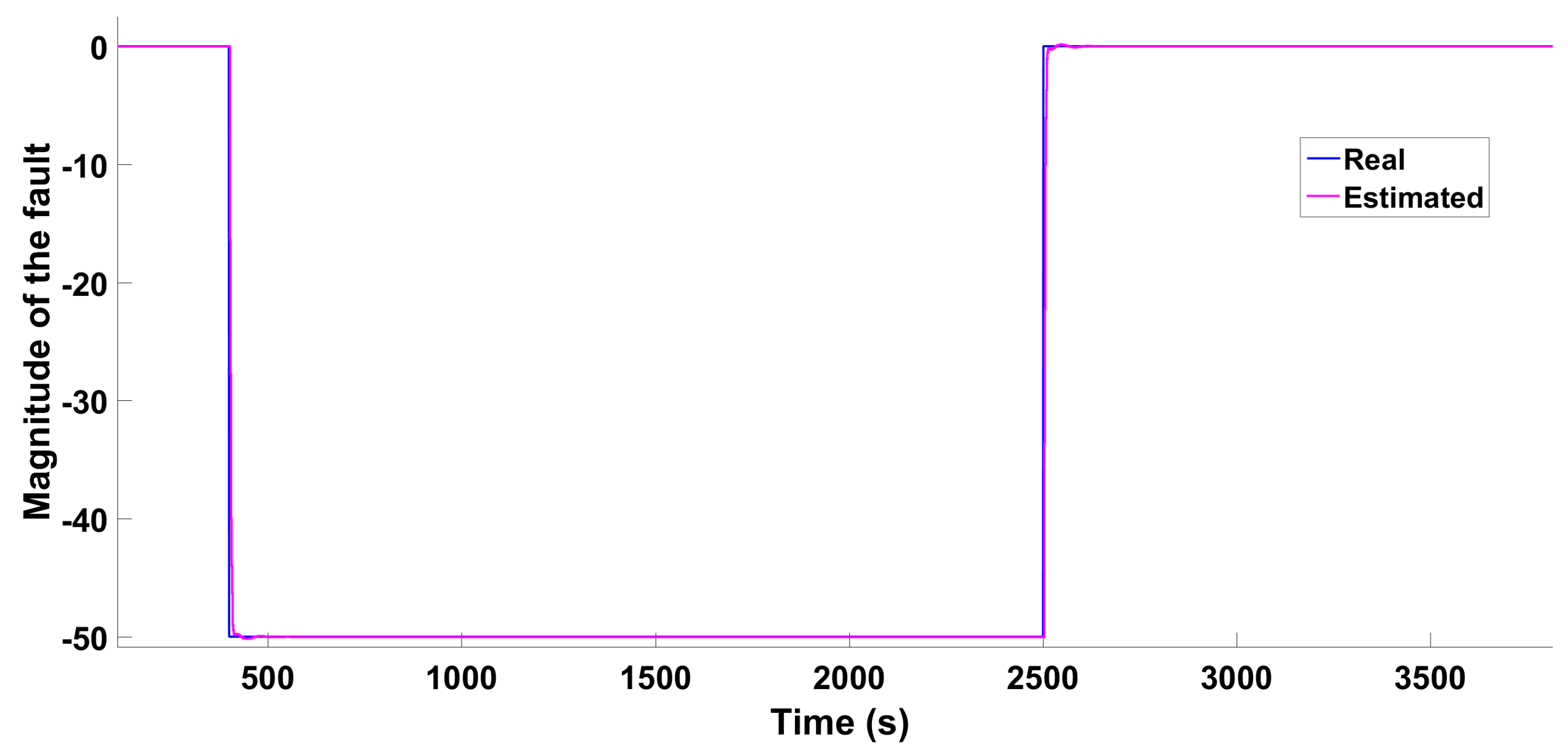
5.2. Fault Detection and Diagnosis System
5.3. Fault Accommodation Control Law
6. Results and Discussion
6.1. Scenario 1: Comparison between FTC and Discrete PID Controller on the Hammerstein–Wiener Model
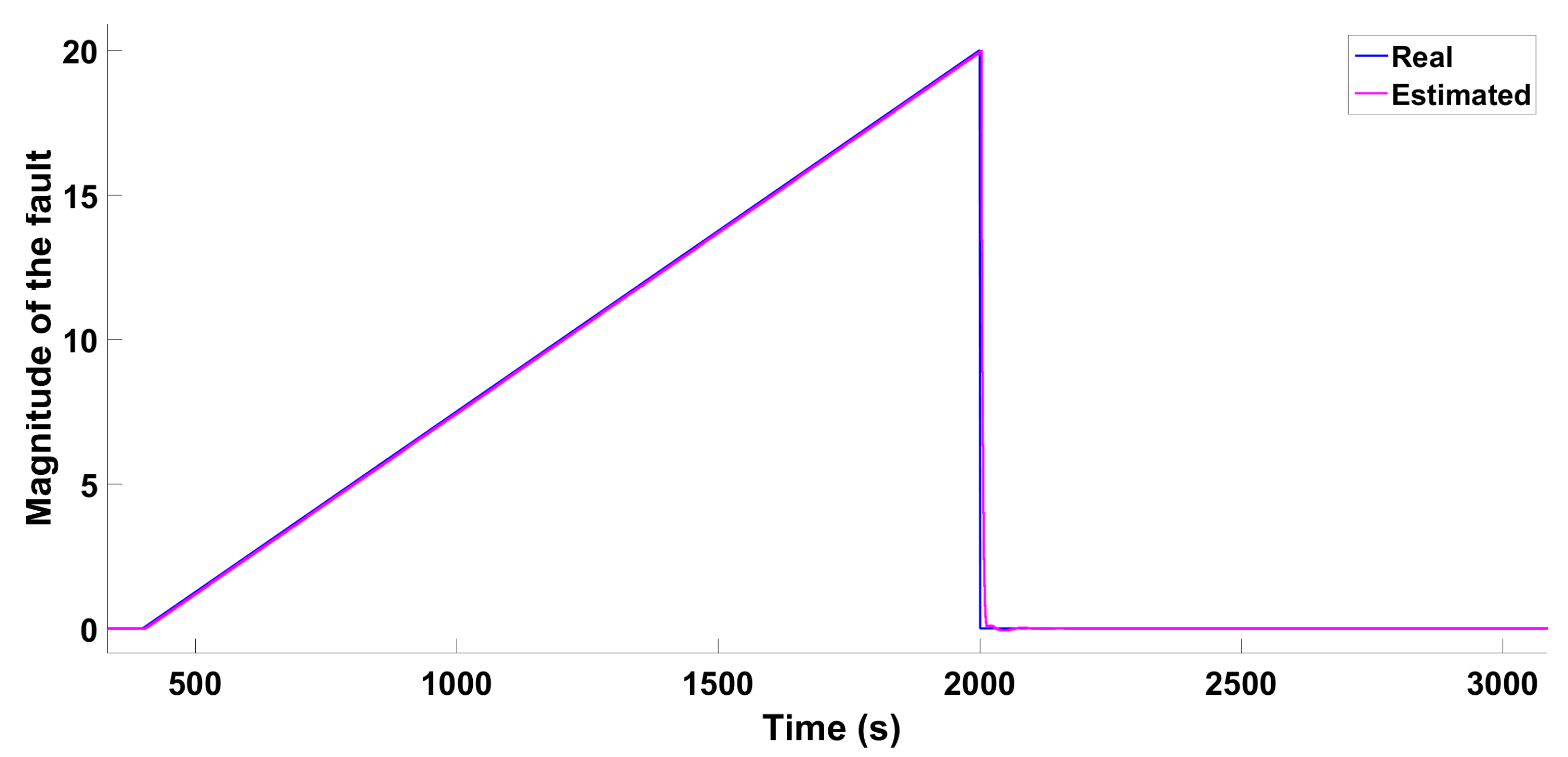
6.1.1. Scenario 1—Ramp-Type Fault
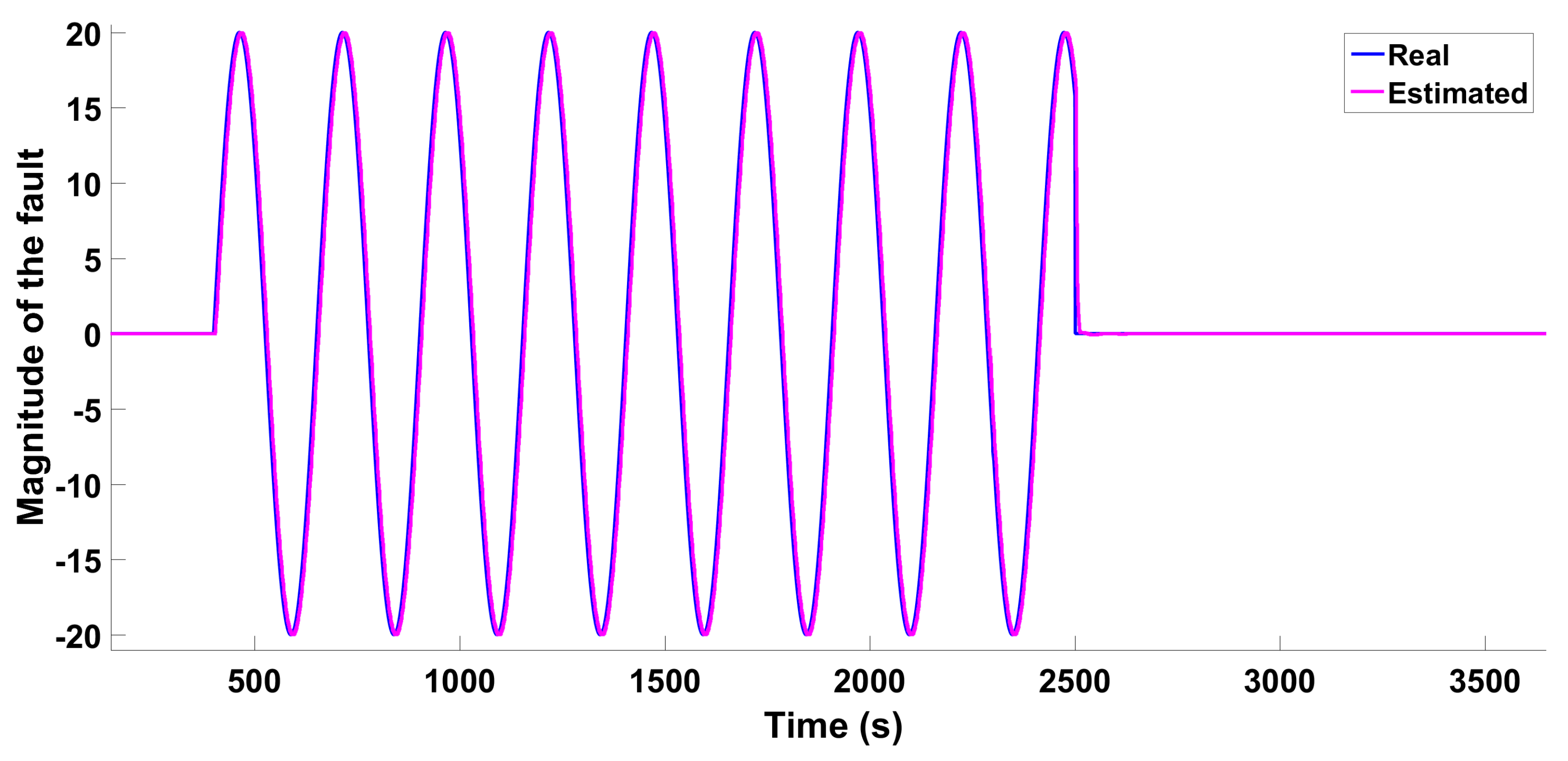
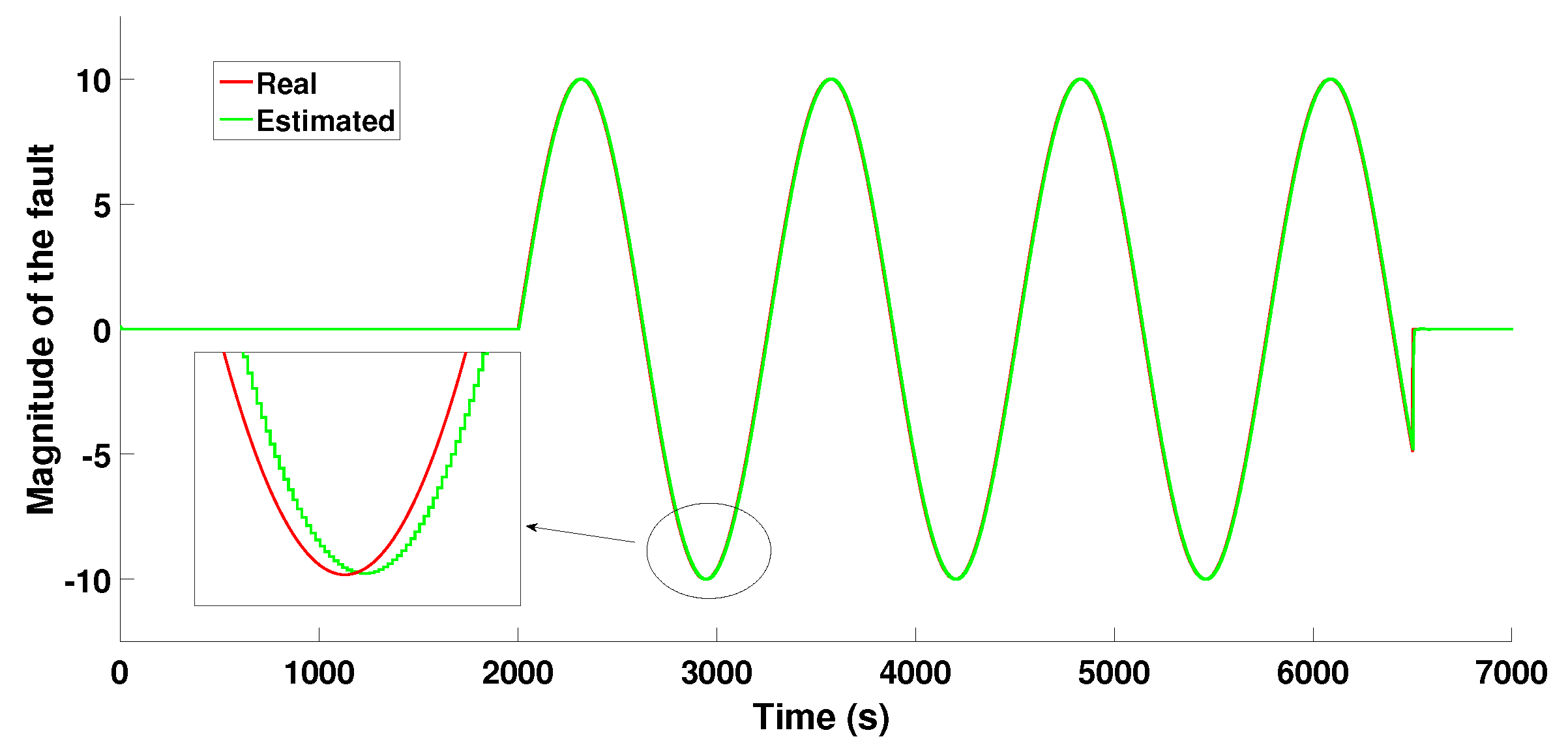
6.1.2. Scenario 1—Sine Wave-Type Fault
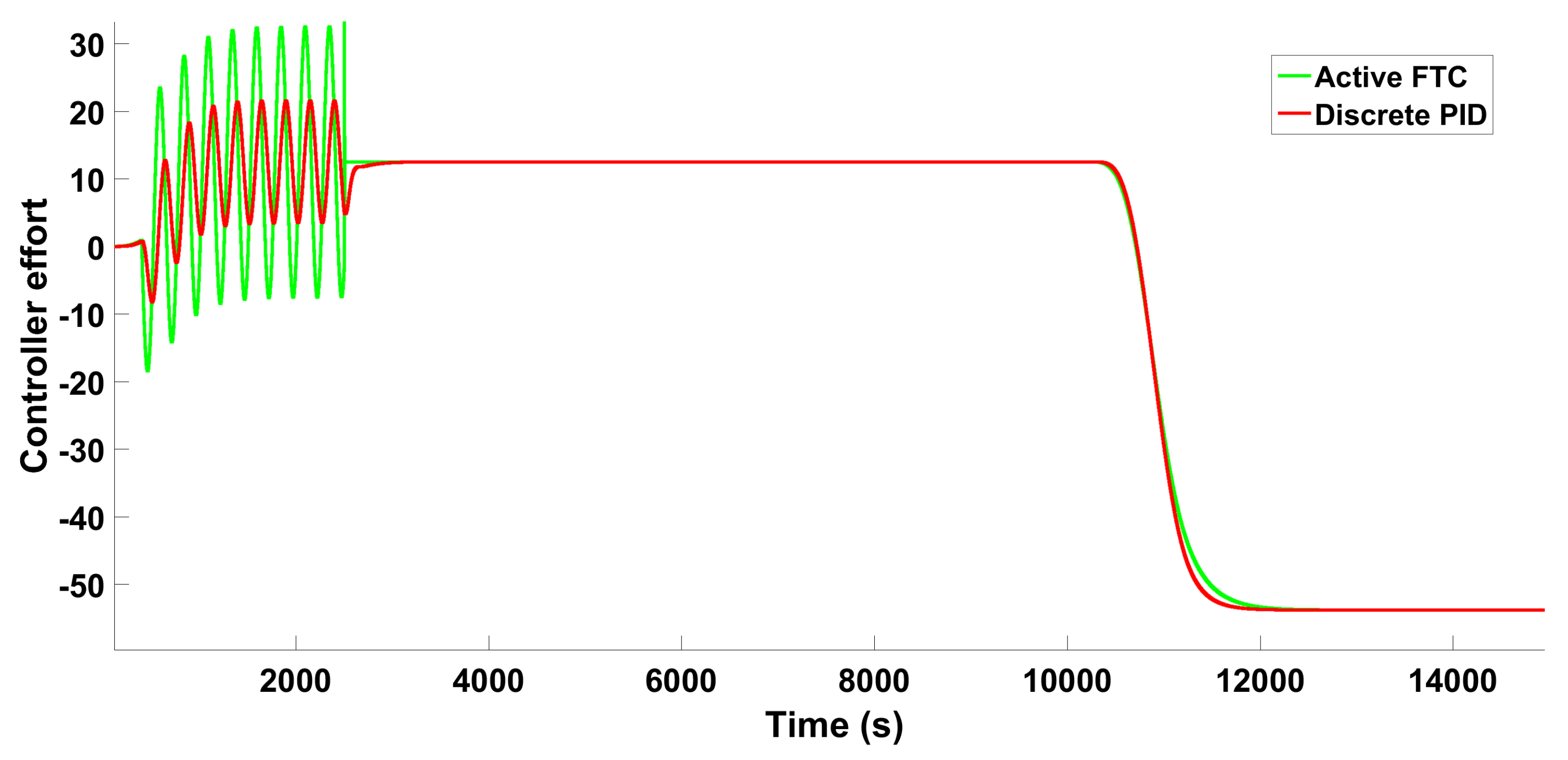
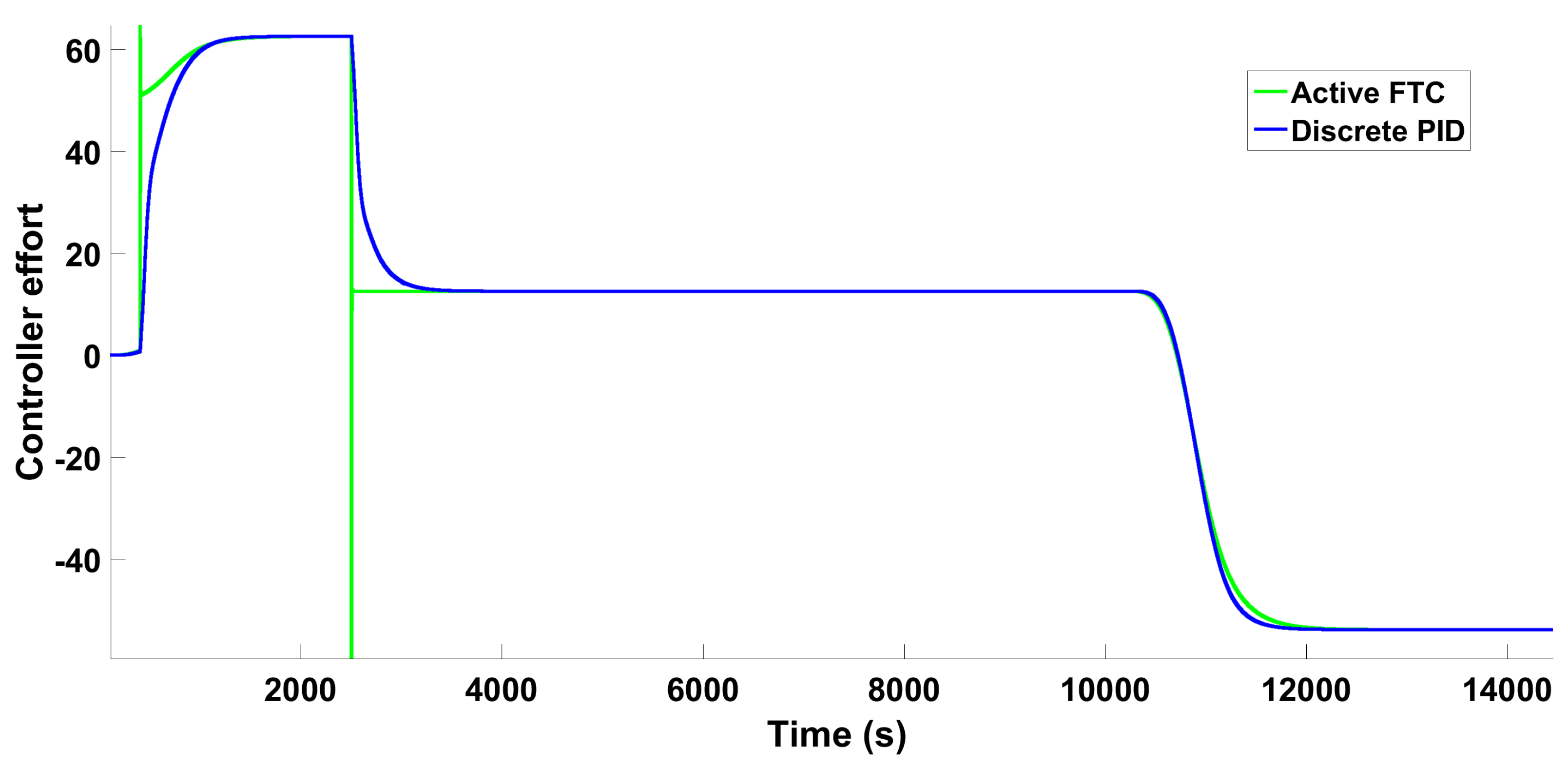
6.1.3. Scenario 1—Step-Type Fault
6.2. Scenario 2: Comparison between FTC and Discrete PID Controller on the PSA Process
6.2.1. Scenario 2—Nominal Controllers for the Following Trajectory
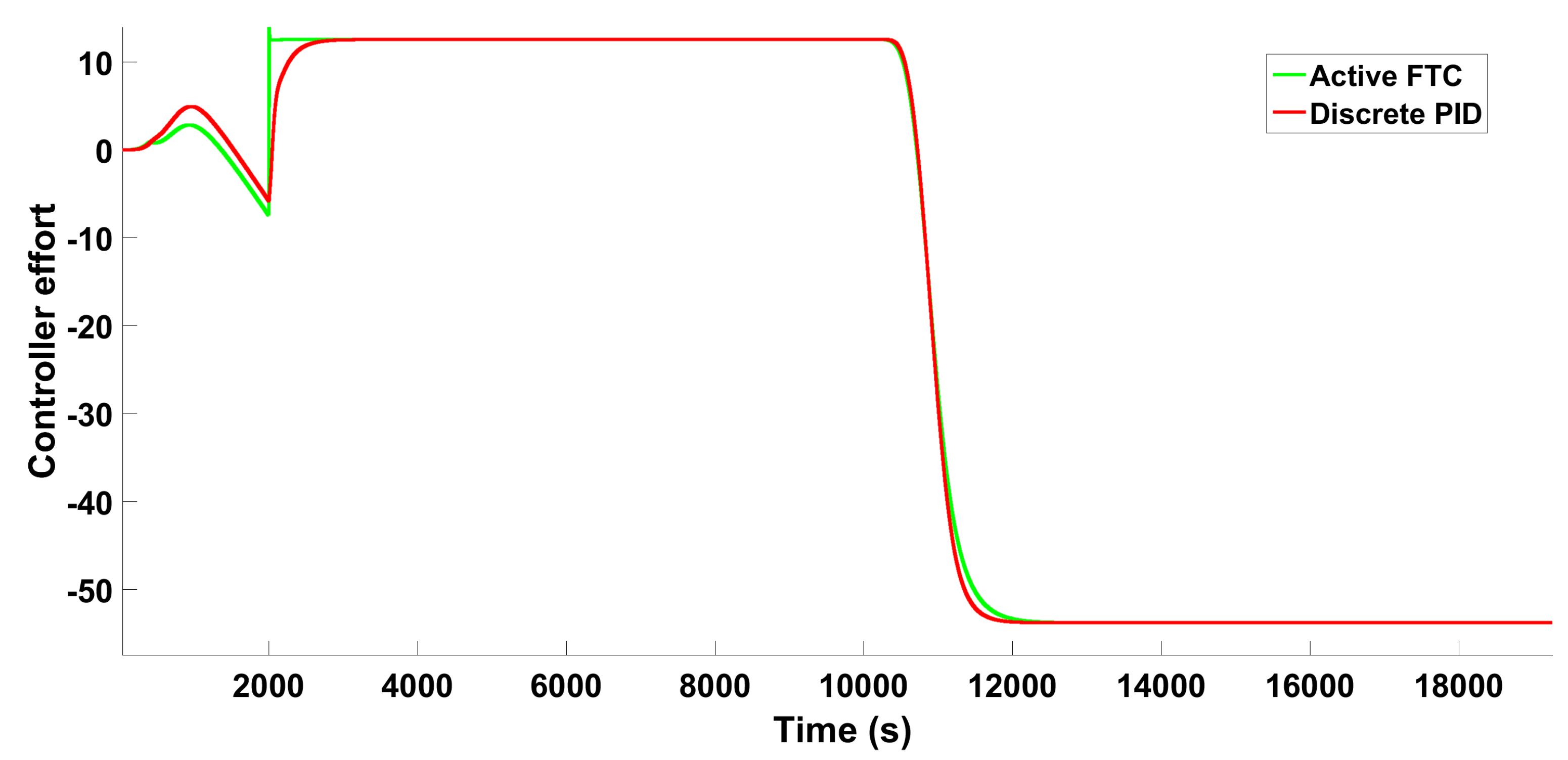
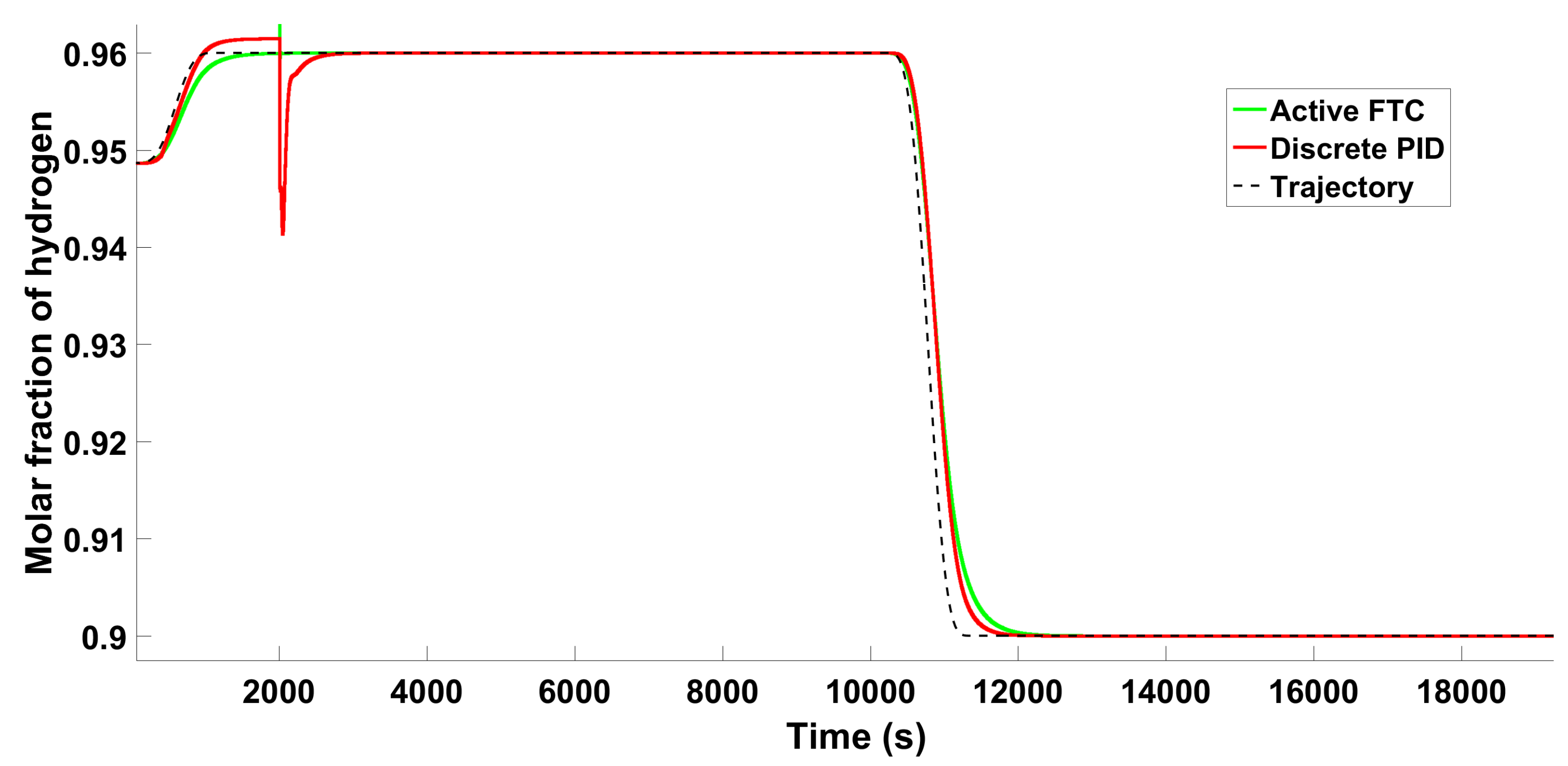
6.2.2. Scenario 2—Individual Fault
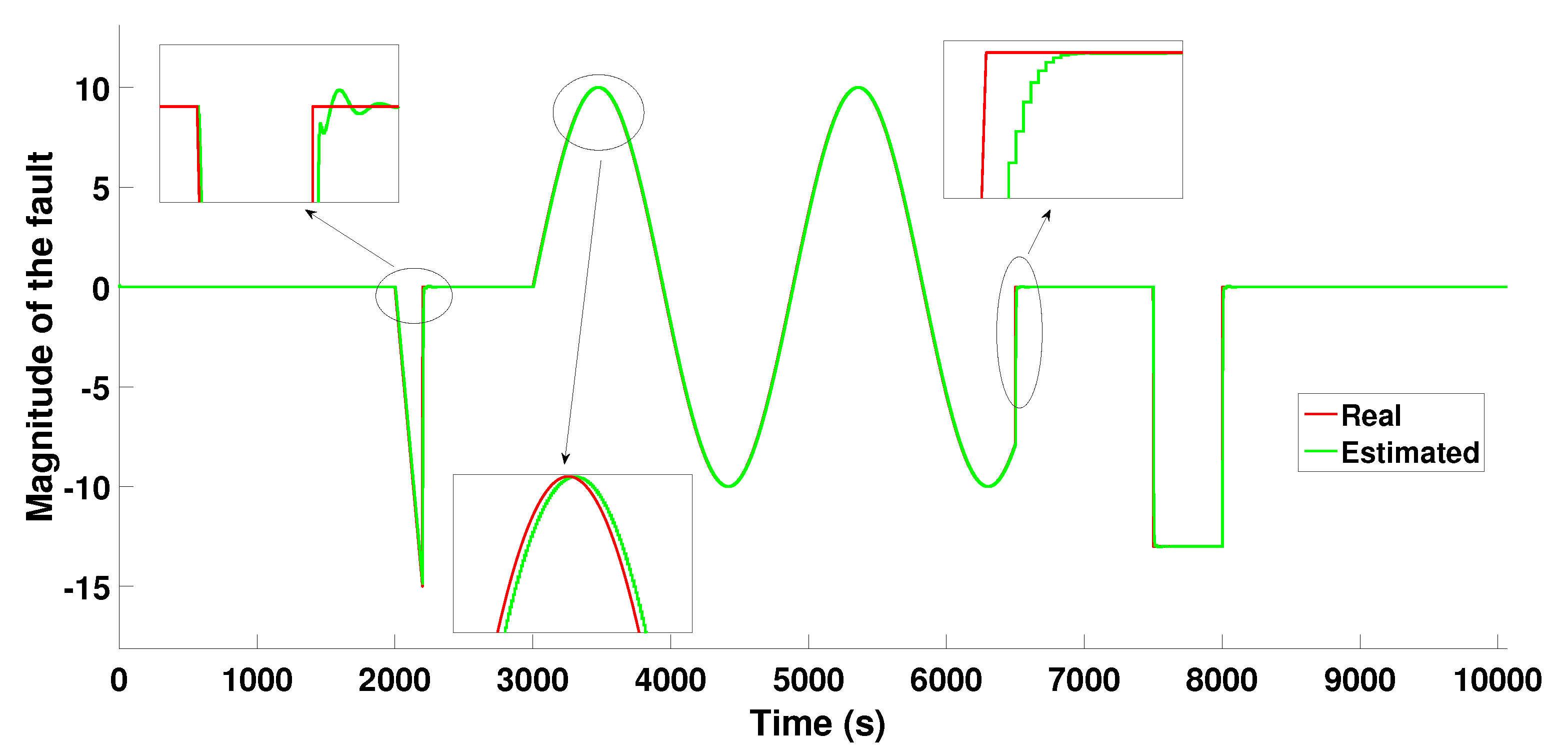
6.2.3. Scenario 2−Multiple Faults
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| Greek symbols | |
| Bed porosity | |
| Particle porosity | |
| Viscosity, N s m−2 | |
| Bed packing density, kg m−3 | |
| Particle density, kmol m−3 | |
| Parameter (glueckauf) | |
| Letters | |
| Specific particle surface, m2 m−3 | |
| Concentration, kmol m−3 | |
| heat capacity (gas), MJ kg−1 K−1 | |
| Heat capacity (adsorbent), MJ kmol−1 K−1 | |
| Bed diameter, | |
| Particle diameter, | |
| Axial dispersion, m2s | |
| Isotherm parameters for component i | |
| heat adsorption gradient, J s m−2 K−1 | |
| k | Molecular weight, Pa |
| M | Molecular weight, kg kmol−1 |
| Mass transfer coefficient solidt, s−1 | |
| P | Pressure, |
| Q | Isosteric heat of adsorption, |
| t | Time, |
| Steam temperature, | |
| Adsorbent temperature, | |
| T | Temperature, |
| Surface gas velocity, m s−1 | |
| Adsorbed amount, kmol kg−1 | |
| Adsorbed equilibrium amount, kmol kg−1 | |
| Molar fraction, i | |
| z | axial distance, |
| Subscripts | |
| F | flow |
| i | water (w) or ethanol (e) |
| g | gas phase |
| s | solid phase |
| p | particle |
| b | bulk or packed bed |
References
- Guan, Z.; Wang, Y.; Yu, X.; Shen, Y.; He, D.; Tang, Z.; Li, W.; Zhang, D. Simulation and analysis of dual-reflux pressure swing adsorption using silica gel for blue coal gas initial separation. Int. J. Hydrogen Energy 2021, 46, 683–696. [Google Scholar] [CrossRef]
- Chicano, J.; Dion, C.T.; Pasaogullari, U.; Valla, J.A. Simulation of 12-bed vacuum pressure-swing adsorption for hydrogen separation from methanol-steam reforming off-gas. Int. J. Hydrogen Energy 2021, 46, 28626–28640. [Google Scholar] [CrossRef]
- Zhang, N.; Xiao, J.; Bénard, P.; Chahine, R. Single- and double-bed pressure swing adsorption processes for H2/CO syngas separation. Int. J. Hydrogen Energy 2019, 44, 26405–26418. [Google Scholar] [CrossRef]
- Delgado Dobladez, J.A.; Águeda Maté, V.I.; Torrellas, S.Á.; Larriba, M.; Brea, P. Efficient recovery of syngas from dry methane reforming product by a dual pressure swing adsorption process. Int. J. Hydrogen Energy 2021, 46, 17522–17533. [Google Scholar] [CrossRef]
- Fakhroleslam, M.; Bozorgmehry Boozarjomehry, R.; Fatemi, S. Design of a dynamical hybrid observer for pressure swing adsorption processes. Int. J. Hydrogen Energy 2017, 42, 21027–21039. [Google Scholar] [CrossRef]
- Du, Z.; Liu, C.; Zhai, J.; Guo, X.; Xiong, Y.; Su, W.; He, G. A Review of Hydrogen Purification Technologies for Fuel Cell Vehicles. Catalysts 2021, 11, 393. [Google Scholar] [CrossRef]
- Zhang, R.; Shen, Y.; Tang, Z.; Li, W.; Zhang, D.A.; Raganati, F.; Zhang, R.; Shen, Y.; Tang, Z.; Li, W.; et al. A Review of Numerical Research on the Pressure Swing Adsorption Process. Processes 2022, 10, 812. [Google Scholar] [CrossRef]
- Luberti, M.; Ahn, H. Review of Polybed pressure swing adsorption for hydrogen purification. Int. J. Hydrogen Energy 2022, 47, 10911–10933. [Google Scholar] [CrossRef]
- Zhang, X.; Bao, C.; Zhou, F.; Lai, N.C. Modeling study on a two-stage hydrogen purification process of pressure swing adsorption and carbon monoxide selective methanation for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2023. [Google Scholar] [CrossRef]
- Di Marcoberardino, G.; Vitali, D.; Spinelli, F.; Binotti, M.; Manzolini, G. Green Hydrogen Production from Raw Biogas: A Techno-Economic Investigation of Conventional Processes Using Pressure Swing Adsorption Unit. Processes 2018, 6, 19. [Google Scholar] [CrossRef] [Green Version]
- Garcia, R.; Gómez-Díaz, D.; Kalman, V.; Voigt, J.; Jordan, C.; Harasek, M. Hydrogen Purification by Pressure Swing Adsorption: High-Pressure PSA Performance in Recovery from Seasonal Storage. Sustainability 2022, 14, 14037. [Google Scholar] [CrossRef]
- Zhang, N.; Bénard, P.; Chahine, R.; Yang, T.; Xiao, J. Optimization of pressure swing adsorption for hydrogen purification based on Box-Behnken design method. Int. J. Hydrogen Energy 2021, 46, 5403–5417. [Google Scholar] [CrossRef]
- Vo, N.D.; Oh, D.H.; Kang, J.H.; Oh, M.; Lee, C.H. Dynamic-model-based artificial neural network for H2 recovery and CO2 capture from hydrogen tail gas. Appl. Energy 2020, 273, 115263. [Google Scholar] [CrossRef]
- López Núñez, A.R.; Rumbo Morales, J.Y.; Salas Villalobos, A.U.; De La Cruz-Soto, J.; Ortiz Torres, G.; Rodríguez Cerda, J.C.; Calixto-Rodriguez, M.; Brizuela Mendoza, J.A.; Aguilar Molina, Y.; Zatarain Durán, O.A.; et al. Optimization and Recovery of a Pressure Swing Adsorption Process for the Purification and Production of Bioethanol. Fermentation 2022, 8, 293. [Google Scholar] [CrossRef]
- Morales, J.Y.; Lopez, G.L.; Alvarado, V.M.; Cantero, C.A.; Rivera, H.R. Optimal Predictive Control for a Pressure Oscillation Adsorption Process for Producing Bioethanol. Computación y Sistemas 2019, 23, 1593–1617. [Google Scholar] [CrossRef]
- Morales, J.Y.; Vidal, A.F.; Torres, G.O.; Villalobo, A.U.; de J. Sorcia Vázquez, F.; Mendoza, J.A.; De-la Torre, M.; Martínez, J.S. Adsorption and Separation of the H2O/H2SO4 and H2O/C2H5OH Mixtures: A Simulated and Experimental Study. Processes 2020, 8, 290. [Google Scholar] [CrossRef] [Green Version]
- Rumbo-Morales, J.Y.; Lopez-Lopez, G.; Alvarado, V.M.; Valdez-Martinez, J.S.; Sorcia-Vázquez, F.D.; Brizuela-Mendoza, J.A. Simulación y control de un proceso de adsorción por oscilación de presión para deshidratar etanol. Rev. Mex. Ing. Quím. 2018, 17, 1051–1081. [Google Scholar] [CrossRef]
- Rumbo Morales, J.Y.; Ortiz-Torres, G.; García, R.O.D.; Cantero, C.A.T.; Rodriguez, M.C.; Sarmiento-Bustos, E.; Oceguera-Contreras, E.; Hernández, A.A.F.; Cerda, J.C.R.; Molina, Y.A.; et al. Review of the Pressure Swing Adsorption Process for the Production of Biofuels and Medical Oxygen: Separation and Purification Technology. Adsorpt. Sci. Technol. 2022, 2022, 3030519. [Google Scholar] [CrossRef]
- Park, Y.; Kang, J.H.; Moon, D.K.; Jo, Y.S.; Lee, C.H. Parallel and series multi-bed pressure swing adsorption processes for H2 recovery from a lean hydrogen mixture. Chem. Eng. J. 2021, 408, 127299. [Google Scholar] [CrossRef]
- Shah, G.; Ahmad, E.; Pant, K.K.; Vijay, V.K. Comprehending the contemporary state of art in biogas enrichment and CO2 capture technologies via swing adsorption. Int. J. Hydrogen Energy 2021, 46, 6588–6612. [Google Scholar] [CrossRef]
- Martins, M.A.; Rodrigues, A.E.; Loureiro, J.M.; Ribeiro, A.M.; Nogueira, I.B. Artificial Intelligence-oriented economic non-linear model predictive control applied to a pressure swing adsorption unit: Syngas purification as a case study. Sep. Purif. Technol. 2021, 276, 119333. [Google Scholar] [CrossRef]
- Ye, F.; Ma, S.; Tong, L.; Xiao, J.; Bénard, P.; Chahine, R. Artificial neural network based optimization for hydrogen purification performance of pressure swing adsorption. Int. J. Hydrogen Energy 2019, 44, 5334–5344. [Google Scholar] [CrossRef]
- Xiao, J.; Li, C.; Fang, L.; Böwer, P.; Wark, M.; Bénard, P.; Chahine, R. Machine learning–based optimization for hydrogen purification performance of layered bed pressure swing adsorption. Int. J. Energy Res. 2020, 44, 4475–4492. [Google Scholar] [CrossRef]
- Yu, X.; Shen, Y.; Guan, Z.; Zhang, D.; Tang, Z.; Li, W. Multi-objective optimization of ANN-based PSA model for hydrogen purification from steam-methane reforming gas. Int. J. Hydrogen Energy 2021, 46, 11740–11755. [Google Scholar] [CrossRef]
- Renteria-Vargas, E.M.; Zuniga Aguilar, C.J.; Rumbo Morales, J.Y.; De-La-Torre, M.; Cervantes, J.A.; Lomeli Huerta, J.R.; Torres, G.O.; Vazquez, F.D.J.S.; Sanchez, R.O. Identification by Recurrent Neural Networks applied to a Pressure Swing Adsorption Process for Ethanol Purification. In Proceedings of the 2022 Signal Processing: Algorithms, Architectures, Arrangements, and Applications (SPA), Poznan, Poland, 21–22 September 2022; pp. 128–134. [Google Scholar] [CrossRef]
- Cantero, C.A.T.; Lopez, G.L.; Alvarado, V.M.; Escobar Jimenez, R.F.; Morales, J.Y.; Coronado, E.M. Control Structures Evaluation for a Salt Extractive Distillation Pilot Plant: Application to Bio-Ethanol Dehydration. Energies 2017, 10, 1276. [Google Scholar] [CrossRef] [Green Version]
- Torres Cantero, C.A.; Pérez Zúñiga, R.; Martínez García, M.; Ramos Cabral, S.; Calixto-Rodriguez, M.; Valdez Martínez, J.S.; Mena Enriquez, M.G.; Pérez Estrada, A.J.; Ortiz Torres, G.; Sorcia Vázquez, F.D.J.; et al. Design and Control Applied to an Extractive Distillation Column with Salt for the Production of Bioethanol. Processes 2022, 10, 1792. [Google Scholar] [CrossRef]
- Renteria-Vargas, E.M.; Zuniga Aguilar, C.J.; Rumbo Morales, J.Y.; Vazquez, F.D.J.S.; De-La-Torre, M.; Cervantes, J.A.; Bustos, E.S.; Calixto Rodriguez, M. Neural Network-Based Identification of a PSA Process for Production and Purification of Bioethanol. IEEE Access 2022, 10, 27771–27782. [Google Scholar] [CrossRef]
- Martínez García, M.; Rumbo Morales, J.Y.; Torres, G.O.; Rodríguez Paredes, S.A.; Vázquez Reyes, S.; Sorcia Vázquez, F.D.J.; Pérez Vidal, A.F.; Valdez Martínez, J.S.; Pérez Zúñiga, R.; Renteria Vargas, E.M. Simulation and State Feedback Control of a Pressure Swing Adsorption Process to Produce Hydrogen. Mathematics 2022, 10, 1762. [Google Scholar] [CrossRef]
- Rumbo Morales, J.Y.; López López, G.; Alvarado Martínez, V.M.; Sorcia Vázquez, F.D.J.; Brizuela Mendoza, J.A.; Martínez García, M. Parametric study and control of a pressure swing adsorption process to separate the water-ethanol mixture under disturbances. Sep. Purif. Technol. 2020, 236, 116214. [Google Scholar] [CrossRef]
- Urich, M.D.; Vemula, R.R.; Kothare, M.V. Implementation of an embedded model predictive controller for a novel medical oxygen concentrator. Comput. Chem. Eng. 2022, 160, 107706. [Google Scholar] [CrossRef]
- Rumbo Morales, J.Y.; Brizuela Mendoza, J.A.; Ortiz Torres, G.; Sorcia Vázquez, F.D.J.; Rojas, A.C.; Pérez Vidal, A.F. Fault-Tolerant Control implemented to Hammerstein–Wiener model: Application to Bio-ethanol dehydration. Fuel 2022, 308, 121836. [Google Scholar] [CrossRef]
- Oliveira, P.H.M.; Martins, M.A.F.; Rodrigues, A.E.; Loureiro, J.M.; Ribeiro, A.M.; Nogueira, I.B.R. A Robust Model Predictive Controller applied to a Pressure Swing Adsorption Process: An Analysis Based on a Linear Model Mismatch. IFAC-PapersOnLine 2021, 54, 219–224. [Google Scholar] [CrossRef]
- Lee, J.J.; Kim, M.K.; Lee, D.G.; Ahn, H.; Kim, M.J.; Lee, C.H. Heat-exchange pressure swing adsorption process for hydrogen separation. AIChE J. 2008, 54, 2054–2064. [Google Scholar] [CrossRef]
- Xiao, J.; Fang, L.; Bénard, P.; Chahine, R. Parametric study of pressure swing adsorption cycle for hydrogen purification using Cu-BTC. Int. J. Hydrogen Energy 2018, 43, 13962–13974. [Google Scholar] [CrossRef]
- Xiao, J.; Peng, Y.; Bénard, P.; Chahine, R. Thermal effects on breakthrough curves of pressure swing adsorption for hydrogen purification. Int. J. Hydrogen Energy 2016, 41, 8236–8245. [Google Scholar] [CrossRef]
- Duan, G.; Yu, H.H. LMIs in Control Systems: Analysis, Design and Applications; CRC Press: Boca Raton, FL, USA, 2013; p. 449. [Google Scholar]
- Caverly, R.J.; Forbes, J.R. LMI properties and applications in systems, stability, and control theory. arXiv 2019, arXiv:1903.08599. [Google Scholar]







| Equation | Description of Contributions |
|---|---|
| Material balance accounts for | |
| Mass balance for gas phase | Convection |
| Dispersion | |
| Accumulation | |
| Energy balance accounts for | |
| Gas phase energy balance | Thermal conduction (Solid) |
| Heat of adsorption | |
| Heat transfer (Gas–solid) | |
| Momentum balance accounts for | |
| Pressure drop | Karman–Kozeny |
| Burke–Plummer | |
| Ergun equation | |
| Kinetic equilibrium | |
| Kinetic models For solid | Linear Driving Force (LDF) |
| Diffusion Pore | |
| Mass Transfer Coefficient (Constant) | |
| Thermodynamic equilibrium | |
| Langmuir | Isotherm assumed for layer (Extended Langmuir 3) |
| I. Adsorption | |
| t = 0 | |
| z = 0 | |
| z = L | |
| II. Depressurization | |
| t = 0 | |
| z = 0 | |
| z = L | |
| III. Purge | |
| t = 0 | |
| z = 0 | |
| z = L | |
| IV. Repressurization | |
| t = 0 | |
| z = 0 | |
| z = L |
| Feed | Value |
|---|---|
| Molar fraction of carbon monoxide | 0.11 |
| Molar fraction of hydrogen | 0.61 |
| Molar fraction of methane | 0.28 |
| Production Temperature | 298.15 K |
| Production pressure | 980,000 Pa |
| Purge pressure | 101,300 Pa |
| Bed length l | 1 m |
| Bed Diamter D | 0.037 m |
| Inter-particle | 0.433 |
| Intra-particle | 0.347 |
| Bulk solid density of adsorbent | 850 kg m−3 |
| Constant mass transfer coefficients | 0.15 s−1 |
| Constant mass transfer coefficients | 0.7 s−1 |
| Constant mass transfer coefficients | 0.195 s−1 |
| Adsorbent particle radius | 0.0015 m |
| Isotherm parameter () | 0.03385 |
| Isotherm parameter () | 0.01694 |
| Isotherm parameter () | 0.02386 |
| Isotherm parameter () | 9.072 |
| Isotherm parameter () | 2.1 |
| Isotherm parameter () | 5.621 |
| Isotherm parameter () | 2.311 |
| Isotherm parameter () | 6.248 |
| Isotherm parameter () | 0.03478 |
| Isotherm parameter () | 1751.0 |
| Isotherm parameter () | 1229 |
| Isotherm parameter () | 1159 |
| Computational parameters | |
| Number of nodes | 10 |
| Discretization method to be used | UDS1 first order (Derivatióntiny of Upwind Differencing Scheme 1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz Torres, G.; Rumbo Morales, J.Y.; Ramos Martinez, M.; Valdez-Martínez, J.S.; Calixto-Rodriguez, M.; Sarmiento-Bustos, E.; Torres Cantero, C.A.; Buenabad-Arias, H.M. Active Fault-Tolerant Control Applied to a Pressure Swing Adsorption Process for the Production of Bio-Hydrogen. Mathematics 2023, 11, 1129. https://doi.org/10.3390/math11051129
Ortiz Torres G, Rumbo Morales JY, Ramos Martinez M, Valdez-Martínez JS, Calixto-Rodriguez M, Sarmiento-Bustos E, Torres Cantero CA, Buenabad-Arias HM. Active Fault-Tolerant Control Applied to a Pressure Swing Adsorption Process for the Production of Bio-Hydrogen. Mathematics. 2023; 11(5):1129. https://doi.org/10.3390/math11051129
Chicago/Turabian StyleOrtiz Torres, Gerardo, Jesse Yoe Rumbo Morales, Moises Ramos Martinez, Jorge Salvador Valdez-Martínez, Manuela Calixto-Rodriguez, Estela Sarmiento-Bustos, Carlos Alberto Torres Cantero, and Hector Miguel Buenabad-Arias. 2023. "Active Fault-Tolerant Control Applied to a Pressure Swing Adsorption Process for the Production of Bio-Hydrogen" Mathematics 11, no. 5: 1129. https://doi.org/10.3390/math11051129







