Deep Learning in COVID-19 Diagnosis, Prognosis and Treatment Selection
Abstract
1. Introduction
2. Deep-Learning Methods
2.1. Basic Neural Network Methods
2.2. Convolutional Neural Networks
2.3. Graph Neural Networks
2.4. Deep-Network Visualization
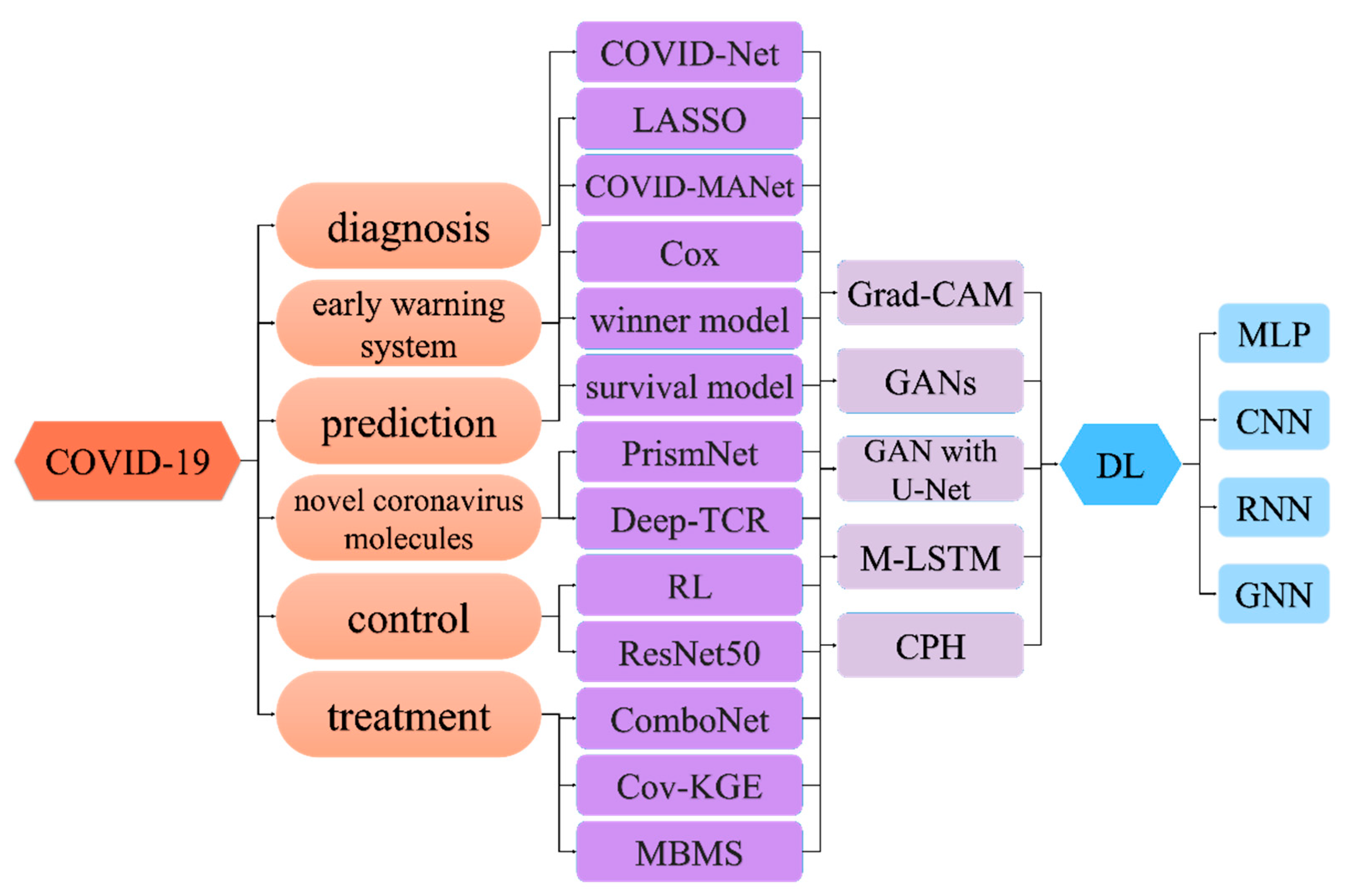
3. Deep Learning in COVID-19
3.1. Deep Learning for Diagnosis of COVID-19
3.2. Deep Learning for COVID-19 Early Warning System
3.3. Deep Learning for COVID-19 Prediction
3.4. Deep Learning for Novel Coronavirus Molecules
3.5. Deep Learning for COVID-19 Control
3.6. Deep Learning for COVID-19 Treatment
4. Challenges and Limitations: The Road to Clinical Implementation
4.1. AI Interpretability
4.2. Data Limitations
5. Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hosseini, E.S.; Kashani, N.R.; Nikzad, H.; Azadbakht, J.; Bafrani, H.H.; Kashani, H.H. The novel coronavirus disease-2019 (COVID-19): Mechanism of action, detection and recent therapeutic strategies. Virology 2020, 551, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 cell entry depends on ace2 and tmprss2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef] [PubMed]
- Taz, T.A.; Ahmed, K.; Paul, B.K.; Kawsar, M.; Aktar, N.; Mahmud, S.M.H.; Moni, M.A. Network-based identification genetic effect of SARS-CoV-2 infections to idiopathic pulmonary fibrosis (ipf) patients. Brief. Bioinform. 2020, 22, 1254–1266. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, S.M.H.; Al-Mustanjid, M.; Akter, F.; Rahman, M.S.; Ahmed, K.; Rahman, M.H.; Chen, W.; Moni, M.A. Bioinformatics and system biology approach to identify the influences of SARS-CoV-2 infections to idiopathic pulmonary fibrosis and chronic obstructive pulmonary disease patients. Brief. Bioinform. 2021, 22, bbab115. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Saleem, F.; Al-Ghamdi, A.S.A.; Alassafi, M.O.; AlGhamdi, S.A. Machine learning, deep learning, and mathematical models to analyze forecasting and epidemiology of COVID-19: A systematic literature review. Int. J. Environ. Res. Public Health 2022, 19, 5099. [Google Scholar] [CrossRef]
- Kucharski, A.J.; Russell, T.W.; Diamond, C.; Liu, Y.; Edmunds, J.; Funk, S.; Eggo, R.M. Early dynamics of transmission and control of COVID-19: A mathematical modelling study. Lancet Infect. Dis. 2020, 20, 553–558. [Google Scholar] [CrossRef]
- Zou, X.; Li, S.; Fang, M.; Hu, M.; Bian, Y.; Ling, J.; Yu, S.; Jing, L.; Li, D.; Huang, J. Acute physiology and chronic health evaluation ii score as a predictor of hospital mortality in patients of coronavirus disease 2019. Crit. Care Med. 2020, 48, e657–e665. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, P.; Ju, X.; Rao, J.; Huang, W.; Ren, L.; Zhang, S.; Xiong, T.; Xu, K.; Zhou, X.; et al. In vivo structural characterization of the SARS-CoV-2 rna genome identifies host proteins vulnerable to repurposed drugs. Cell 2021, 184, 1865–1883.e1820. [Google Scholar] [CrossRef] [PubMed]
- Mernea, M.; Martin, E.C.; Petrescu, A.J.; Avram, S. Deep learning in the quest for compound nomination for fighting COVID-19. Curr. Med. Chem. 2021, 28, 5699–5732. [Google Scholar] [CrossRef]
- Bagabir, S.A.; Ibrahim, N.K.; Bagabir, H.A.; Ateeq, R.H. COVID-19 and artificial intelligence: Genome sequencing, drug development and vaccine discovery. J. Infect. Public Health 2022, 15, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, P.; Mishra, N.K.; Fatimah, B.; Singh, P.; Gupta, A.; Joshi, S.D. COVID-19 image classification using deep learning: Advances, challenges and opportunities. Comput. Biol. Med. 2022, 144, 105350. [Google Scholar] [CrossRef] [PubMed]
- OPoirion, B.; Jing, Z.; Chaudhary, K.; Huang, S.; Garmire, L.X. Deepprog: An ensemble of deep-learning and machine-learning models for prognosis prediction using multi-omics data. Genome Med. 2021, 13, 112. [Google Scholar] [CrossRef]
- Stephanie, S.; Shum, T.; Cleveland, H.; Challa, S.R.; Herring, A.; Jacobson, F.L.; Hatabu, H.; Byrne, S.C.; Shashi, K.; Araki, T.; et al. Determinants of chest x-ray sensitivity for COVID-19: A multi-institutional study in the united states. Radiol. Cardiothorac Imaging 2020, 2, e200337. [Google Scholar] [CrossRef]
- Wang, L.; Lin, Z.Q.; Wong, A. Covid-net: A tailored deep convolutional neural network design for detection of COVID-19 cases from chest x-ray images. Sci. Rep. 2020, 10, 19549. [Google Scholar] [CrossRef]
- Rajawat, N.; Hada, B.S.; Meghawat, M.; Lalwani, S.; Kumar, R. C-covidnet: A cnn model for COVID-19 detection using image processing. Arab. J. Sci. Eng. 2022, 47, 10811–10822. [Google Scholar] [CrossRef]
- Wu, S.; Roberts, K.; Datta, S.; Du, J.; Ji, Z.; Si, Y.; Soni, S.; Wang, Q.; Wei, Q.; Xiang, Y.; et al. Deep learning in clinical natural language processing: A methodical review. J. Am. Med. Inform. Assoc. 2020, 27, 457–470. [Google Scholar] [CrossRef]
- Rifaioglu, A.S.; Atas, H.; Martin, M.J.; Cetin-Atalay, R.; Atalay, V.; Doğan, T. Recent applications of deep learning and machine intelligence on in silico drug discovery: Methods, tools and databases. Brief. Bioinform. 2019, 20, 1878–1912. [Google Scholar] [CrossRef] [PubMed]
- Wainberg, M.; Merico, D.; Delong, A.; Frey, B.J. Deep learning in biomedicine. Nat. Biotechnol. 2018, 36, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Bai, Q.; Liu, S.; Tian, Y.; Xu, T.; Banegas-Luna, A.J.; Pérez-Sánchez, H.; Huang, J.; Liu, H.; Yao, X. Application advances of deep learning methods for de novo drug design and molecular dynamics simulation. WIREs Comput. Mol. Sci. 2022, 12, e1581. [Google Scholar] [CrossRef]
- Qifeng, B.; Jian, M.; Shuo, L.; Tingyang, X.; Jesús, B.-L.A.; Horacio, P.-S.; Yanan, T.; Junzhou, H.; Huanxiang, L.; Xiaojun, Y. Waddaica: A webserver for aiding protein drug design by artificial intelligence and classical algorithm. Comput. Struct. Biotechnol. J. 2021, 19, 3573–3579. [Google Scholar]
- Bai, Q.; Tan, S.; Xu, T.; Liu, H.; Huang, J.; Yao, X. Molaical: A soft tool for 3d drug design of protein targets by artificial intelligence and classical algorithm. Brief. Bioinform. 2020, 22, bbaa161. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.M.; Beroza, G.C. Deep-learning seismology. Science 2022, 377, eabm4470. [Google Scholar] [CrossRef]
- Nabipour, M.; Nayyeri, P.; Jabani, H.; Mosavi, A.; Salwana, E.S.S. Deep learning for stock market prediction. Entropy 2020, 22, 840. [Google Scholar] [CrossRef]
- Dixon, M.; Klabjan, D.; Bang, J.H. Implementing Deep Neural Networks for Financial Market Prediction on the Intel Xeon Phi; Association for Computing Machinery: New York, NY, USA, 2015. [Google Scholar]
- Valle-Cruz, D.; Criado, J.I.; Almazan, R.S.; Gómez, E.R. Assessing the public policy-cycle framework in the age of artificial intelligence: From agenda-setting to policy evaluation. Gov. Inf. Q. 2020, 37, 101509. [Google Scholar] [CrossRef]
- Farabet, C.; Couprie, C.; Najman, L.; Lecun, Y. Learning hierarchical features for scene labeling. IEEE Trans. Pattern Anal. Mach. Intell. 2013, 35, 1915–1929. [Google Scholar] [CrossRef]
- Hannan, B. Connectionism and the mind: An introduction to parallel processing in networks. Philos. Books 1992, 33, 92–94. [Google Scholar]
- Dias, R.; Torkamani, A. Artificial intelligence in clinical and genomic diagnostics. Genome Med. 2019, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, K. Neocognitron: A self organizing neural network model for a mechanism of pattern recognition unaffected by shift in position. Biol. Cybern. 1980, 36, 193–202. [Google Scholar] [CrossRef]
- Hubel, D.H.; Wiesel, T.N. Receptive fields, binocular interaction and functional architecture in the cat′s visual cortex. J. Physiol. 1962, 160, 106–154. [Google Scholar] [CrossRef] [PubMed]
- Cun, Y.L. Learning Process in an Asymmetric Threshold Network; Springer: Berlin/Heidelberg, Germany, 1986; pp. 233–240. [Google Scholar]
- Alom, M.Z.; Taha, T.M.; Yakopcic, C.; Westberg, S.; Sidike, P.; Nasrin, M.S.; Essen, B.C.V.; Awwal, A.A.S.; Asari, V.K. The history began from alexnet: A comprehensive survey on deep learning approaches. arXiv 2018, arXiv:abs/1803.01164. [Google Scholar]
- Li, Z.; Liu, F.; Yang, W.; Peng, S.; Zhou, J. A survey of convolutional neural networks: Analysis, applications, and prospects. IEEE Trans. Neural Netw. Learn. Syst. 2022, 33, 6999–7019. [Google Scholar] [CrossRef]
- Tsubaki, M.; Tomii, K.; Sese, J. Compound-protein interaction prediction with end-to-end learning of neural networks for graphs and sequences. Bioinformatics 2019, 35, 309–318. [Google Scholar] [CrossRef]
- Mullin, R. And now: The drug plant of the future. Chem. Eng. News 2017, 95, 22–24. [Google Scholar]
- Elbasani, E.; Njimbouom, S.N.; Oh, T.J.; Kim, E.H.; Lee, H.; Kim, J.D. Gcrnn: Graph convolutional recurrent neural network for compound-protein interaction prediction. BMC Bioinform. 2022, 22 (Suppl. S5), 616. [Google Scholar] [CrossRef]
- Shi, W.; Tong, L.; Zhu, Y.; Wang, M.D. COVID-19 automatic diagnosis with radiographic imaging: Explainable attention transfer deep neural networks. IEEE J. Biomed. Health Inform. 2021, 25, 2376–2387. [Google Scholar] [CrossRef]
- Brunese, L.; Mercaldo, F.; Reginelli, A.; Santone, A. Explainable deep learning for pulmonary disease and coronavirus COVID-19 detection from x-rays. Comput. Methods Programs Biomed. 2020, 196, 105608. [Google Scholar] [CrossRef]
- Munir, K.; Elahi, H.; Ayub, A.; Frezza, F.; Rizzi, A. Cancer diagnosis using deep learning: A bibliographic review. Cancers 2019, 11, 1235. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Shelhamer, E.; Darrell, T. Fully convolutional networks for semantic segmentation. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Boston, MA, USA, 7–12 June 2015; pp. 3431–3440. [Google Scholar]
- Ronneberger, O.; Fischer, P.; Brox, T. U-Net: Convolutional Networks for Biomedical Image Segmentation; Springer International Publishing: Cham, Switzerland, 2015; pp. 234–241. [Google Scholar]
- Alom, M.Z.; Yakopcic, C.; Taha, T.M.; Asari, V.K. Nuclei segmentation with recurrent residual convolutional neural networks based u-net (r2u-net). In Proceedings of the NAECON 2018-IEEE National Aerospace and Electronics Conference, Dayton, OH, USA, 23–26 July 2018; pp. 228–233. [Google Scholar]
- Salehi, S.S.M.; Erdogmus, D.; Gholipour, A. Auto-context convolutional neural network (auto-net) for brain extraction in magnetic resonance imaging. IEEE Trans. Med. Imaging 2017, 36, 2319–2330. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Cheng, J.; Fu, H.; Zhou, K.; Hao, H.; Zhao, Y.; Zhang, T.; Gao, S.; Liu, J. Ce-net: Context encoder network for 2d medical image segmentation. IEEE Trans. Med. Imaging 2019, 38, 2281–2292. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Siddiquee, M.M.R.; Tajbakhsh, N.; Liang, J. Unet++: A nested u-net architecture for medical image segmentation. In Deep Learn Med Image Anal Multimodal Learn Clin Decis Support; Springer: Berlin/Heidelberg, Germany, 2018; Volume 11045, pp. 3–11. [Google Scholar]
- Chouhan, V.; Singh, S.K.; Khamparia, A.; Gupta, D.; Tiwari, P.; Moreira, C.; Damaševičius, R.; de Albuquerque, V.H.C. A novel transfer learning based approach for pneumonia detection in chest x-ray images. Appl. Sci. 2020, 10, 559. [Google Scholar] [CrossRef]
- Afshar, P.; Heidarian, S.; Naderkhani, F.; Oikonomou, A.; Plataniotis, K.N.; Mohammadi, A. Covid-caps: A capsule network-based framework for identification of COVID-19 cases from x-ray images. Pattern Recognit. Lett. 2020, 138, 638–643. [Google Scholar] [CrossRef]
- Bayoudh, K.; Hamdaoui, F.; Mtibaa, A. Hybrid-covid: A novel hybrid 2d/3d cnn based on cross-domain adaptation approach for COVID-19 screening from chest x-ray images. Phys. Eng. Sci. Med. 2020, 43, 1415–1431. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, L.; Chen, D.; Pan, W.; Shi, C.; Niu, Y.; Yao, X.; Xu, X.; Cheng, Y. Dense gan and multi-layer attention based lesion segmentation method for COVID-19 ct images. Biomed. Signal Process. Control 2021, 69, 102901. [Google Scholar] [CrossRef]
- Dong, Y.M.; Sun, J.; Li, Y.X.; Chen, Q.; Liu, Q.Q.; Sun, Z.; Pang, R.; Chen, F.; Xu, B.Y.; Manyande, A.; et al. Development and validation of a nomogram for assessing survival in patients with COVID-19 pneumonia. Clin. Infect. Dis. 2021, 72, 652–660. [Google Scholar] [CrossRef]
- Sharma, A.; Mishra, P.K. Covid-manet: Multi-task attention network for explainable diagnosis and severity assessment of covid-19 from cxr images. Pattern Recognit. 2022, 131, 108826. [Google Scholar] [CrossRef]
- Kafieh, R.; Arian, R.; Saeedizadeh, N.; Amini, Z.; Serej, N.D.; Minaee, S.; Yadav, S.K.; Vaezi, A.; Rezaei, N.; Javanmard, S.H. COVID-19 in iran: Forecasting pandemic using deep learning. Comput. Math. Methods Med. 2021, 2021, 1–16. [Google Scholar] [CrossRef]
- Liang, W.; Yao, J.; Chen, A.; Lv, Q.; Zanin, M.; Liu, J.; Wong, S.; Li, Y.; Lu, J.; Liang, H.; et al. Early triage of critically ill COVID-19 patients using deep learning. Nat. Commun. 2020, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sidhom, J.W.; Baras, A.S. Deep learning identifies antigenic determinants of severe SARS-CoV-2 infection within t-cell repertoires. Sci. Rep. 2021, 11, 14275. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Yurtsever, E.; Renganathan, V.; Redmill, K.A.; Özgüner, Ü. A vision-based social distancing and critical density detection system for COVID-19. Sensors 2021, 21, 4608. [Google Scholar] [CrossRef]
- Sethi, S.; Kathuria, M.; Kaushik, T. Face mask detection using deep learning: An approach to reduce risk of coronavirus spread. J. Biomed. Inform. 2021, 120, 103848. [Google Scholar] [CrossRef]
- Jin, W.; Stokes, J.M.; Eastman, R.T.; Itkin, Z.; Zakharov, A.V.; Collins, J.J.; Jaakkola, T.S.; Barzilay, R. Deep learning identifies synergistic drug combinations for treating COVID-19. Proc. Natl. Acad. Sci. USA 2021, 118, e2105070118. [Google Scholar] [CrossRef]
- Zeng, Y.; Chen, X.; Luo, Y.; Li, X.; Peng, D. Deep drug-target binding affinity prediction with multiple attention blocks. Brief. Bioinform. 2021, 22, bbab117. [Google Scholar] [CrossRef]
- Zeng, X.; Song, X.; Ma, T.; Pan, X.; Zhou, Y.; Hou, Y.; Zhang, Z.; Li, K.; Karypis, G.; Cheng, F. Repurpose open data to discover therapeutics for COVID-19 using deep learning. J. Proteome Res. 2020, 19, 4624–4636. [Google Scholar] [CrossRef] [PubMed]
- Roh, H.; Shin, S.; Han, J.; Lim, S. A deep learning-based medication behavior monitoring system. Math. Biosci. Eng. 2021, 18, 1513–1528. [Google Scholar] [CrossRef]
- Wang, W.; Xu, Y.; Gao, R.; Lu, R.; Han, K.; Wu, G.; Tan, W. Detection of SARS-CoV-2 in different types of clinical specimens. Jama 2020, 323, 1843–1844. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Gao, J.-Y.; Hu, W.-M.; Zhang, X.-X.; Guo, L.; Liu, C.-Q.; Tang, Y.-W.; Lang, C.-H.; Mou, F.-Z.; Yi, Z.-J.; et al. Clinical characteristics of 51 patients discharged from hospital with COVID-19 in chongqing, China. medRxiv 2020. [Google Scholar] [CrossRef]
- Hu, Q.; Gois, F.N.B.; Costa, R.; Zhang, L.; Yin, L.; Magaia, N.; de Albuquerque, V.H.C. Explainable artificial intelligence-based edge fuzzy images for COVID-19 detection and identification. Appl. Soft Comput. 2022, 123, 108966. [Google Scholar] [CrossRef]
- Diaz-Escobar, J.; Ordóñez-Guillén, N.E.; Villarreal-Reyes, S.; Galaviz-Mosqueda, A.; Kober, V.; Rivera-Rodriguez, R.; Rizk, J.E.L. Deep-learning based detection of COVID-19 using lung ultrasound imagery. PLoS ONE 2021, 16, e0255886. [Google Scholar] [CrossRef]
- Fang, C.; Bai, S.; Chen, Q.; Zhou, Y.; Xia, L.; Qin, L.; Gong, S.; Xie, X.; Zhou, C.; Tu, D.; et al. Deep learning for predicting COVID-19 malignant progression. Med. Image Anal. 2021, 72, 102096. [Google Scholar] [CrossRef]
- Näppi, J.J.; Uemura, T.; Watari, C.; Hironaka, T.; Kamiya, T.; Yoshida, H. U-survival for prognostic prediction of disease progression and mortality of patients with COVID-19. Sci. Rep. 2021, 11, 9263. [Google Scholar] [CrossRef]
- Sun, C.; Hong, S.; Song, M.; Li, H.; Wang, Z. Predicting COVID-19 disease progression and patient outcomes based on temporal deep learning. BMC Med. Inform. Decis. Mak. 2021, 21, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Uemura, T.; Näppi, J.J.; Watari, C.; Hironaka, T.; Kamiya, T.; Yoshida, H. Weakly unsupervised conditional generative adversarial network for image-based prognostic prediction for COVID-19 patients based on chest ct. Med. Image Anal. 2021, 73, 102159. [Google Scholar] [CrossRef] [PubMed]
- Vaid, S.; Kalantar, R.; Bhandari, M. Deep learning COVID-19 detection bias: Accuracy through artificial intelligence. Int. Orthop. 2020, 44, 1539–1542. [Google Scholar] [CrossRef]
- Ikemura, K.; Bellin, E.; Yagi, Y.; Billett, H.; Saada, M.; Simone, K.; Stahl, L.; Szymanski, J.; Goldstein, D.Y.; Gil, M.R. Using automated machine learning to predict the mortality of patients with covid-19: Prediction model development study. J. Med. Internet Res. 2021, 23, e23458. [Google Scholar] [CrossRef]
- Meng, L.; Dong, D.; Li, L.; Niu, M.; Bai, Y.; Wang, M.; Qiu, X.; Zha, Y.; Tian, J. A deep learning prognosis model help alert for COVID-19 patients at high-risk of death: A multi-center study. IEEE J. Biomed. Health Inform. 2020, 24, 3576–3584. [Google Scholar] [CrossRef] [PubMed]
- Suppakitjanusant, P.; Sungkanuparph, S.; Wongsinin, T.; Virapongsiri, S.; Kasemkosin, N.; Chailurkit, L.; Ongphiphadhanakul, B. Identifying individuals with recent COVID-19 through voice classification using deep learning. Sci. Rep. 2021, 11, 1–7. [Google Scholar] [CrossRef]
- Gao, J.; Sharma, R.; Qian, C.; Glass, L.M.; Spaeder, J.; Romberg, J.; Sun, J.; Xiao, C. Stan: Spatio-temporal attention network for pandemic prediction using real-world evidence. J. Am. Med. Inform. Assoc. 2021, 28, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Dairi, A.; Harrou, F.; Zeroual, A.; Hittawe, M.M.; Sun, Y. Comparative study of machine learning methods for COVID-19 transmission forecasting. J. Biomed. Inform. 2021, 118, 103791. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Song, Y.; Ren, S.; Song, X.; Fan, X.; Liao, Z. Voc-dl: Deep learning prediction model for COVID-19 based on voc virus variants. Comput. Methods Programs Biomed. 2022, 224, 106981. [Google Scholar] [CrossRef]
- Mary, S.R.; Kumar, V.; Venkatesan, K.J.P.; Kumar, R.S.; Jagini, N.P.; Srinivas, A. Vulture-based adaboost-feedforward neural frame work for COVID-19 prediction and severity analysis system. Interdiscip Sci. 2022, 14, 582–595. [Google Scholar] [CrossRef]
- Mansour, R.F.; Escorcia-Gutierrez, J.; Gamarra, M.; Gupta, D.; Castillo, O.; Kumar, S. Unsupervised deep learning based variational autoencoder model for COVID-19 diagnosis and classification. Pattern Recognit. Lett. 2021, 151, 267–274. [Google Scholar] [CrossRef]
- Liao, Z.; Lan, P.; Fan, X.; Kelly, B.; Innes, A.; Liao, Z. Sirvd-dl: A COVID-19 deep learning prediction model based on time-dependent sirvd. Comput. Biol. Med. 2021, 138, 104868. [Google Scholar] [CrossRef]
- Zhang, T.; Li, J. Understanding and predicting the spatio-temporal spread of COVID-19 via integrating diffusive graph embedding and compartmental models. Trans GIS 2021, 25, 3025–3047. [Google Scholar] [CrossRef] [PubMed]
- Ottakath, N.; Elharrouss, O.; Almaadeed, N.; Al-Maadeed, S.; Mohamed, A.; Khattab, T.; Abualsaud, K. Vidmask dataset for face mask detection with social distance measurement. Displays 2022, 73, 102235. [Google Scholar] [CrossRef]
- Siah, C.R.; Lau, S.T.; Tng, S.S.; Chua, C.H.M. Using infrared imaging and deep learning in fit-checking of respiratory protective devices among healthcare professionals. J. Nurs. Sch. 2022, 54, 345–354. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Gao, K.; Chen, J.; Wang, R.; Wei, G.W. Unveiling the molecular mechanism of SARS-CoV-2 main protease inhibition from 137 crystal structures using algebraic topology and deep learning. Chem. Sci. 2020, 11, 12036–12046. [Google Scholar] [CrossRef]
- Santos, M.A.G.; Munoz, R.; Olivares, R.; Filho, P.P.R.; Ser, J.D.; de Albuquerque, V.H.C. Online heart monitoring systems on the internet of health things environments: A survey, a reference model and an outlook. Inf. Fusion 2020, 53, 222–239. [Google Scholar] [CrossRef]
- Parah, S.A.; Kaw, J.A.; Bellavista, P.; Loan, N.A.; Bhat, G.M.; Muhammad, K.; de Albuquerque, V.H.C. Efficient security and authentication for edge-based internet of medical things. IEEE Internet Things J. 2021, 8, 15652–15662. [Google Scholar] [CrossRef] [PubMed]
- Ching, T.; Himmelstein, D.S.; Beaulieu-Jones, B.K.; Kalinin, A.A.; Do, B.T.; Way, G.P.; Ferrero, E.; Agapow, P.M.; Zietz, M.; Hoffman, M.M.; et al. Opportunities and obstacles for deep learning in biology and medicine. J. R. Soc. Interface 2018, 15, 20170387. [Google Scholar] [CrossRef] [PubMed]
- Adak, A.; Pradhan, B.; Shukla, N. Sentiment analysis of customer reviews of food delivery services using deep learning and explainable artificial intelligence: Systematic review. Foods 2022, 11, 1500. [Google Scholar] [CrossRef] [PubMed]
- Miotto, R.; Wang, F.; Wang, S.; Jiang, X.; Dudley, J.T. Deep learning for healthcare: Review, opportunities and challenges. Brief. Bioinform. 2018, 19, 1236–1246. [Google Scholar] [CrossRef]
| Application | Dl Method | Reference | Description |
|---|---|---|---|
| Diagnosis | CNN | Wang et al. [18] | Training CNN on CXR to diagnose COVID-19. |
| CNN & transfer learning | Chouhan et al. [50] | By transfer learning, knowledge from one domain is transferred to the target domain, enabling better learning results in COVID-19 disease management. | |
| CNN & CapsNets | Afshar et al. [51] | Each layer of the capsule network (CapsNet) consists of several capsules, each representing a specific image instance in a specific location through multiple neurons. The transformation matrix distinguishes the training by backpropagating each EM expansion iteration between adjacent capsule layers. | |
| CNN & 2D/3D | Bayoudh et al. [52] | Uses the potential synergy between a pre-trained VGG16 model (i.e., 2D CNN) and a shallow 3D CNN. | |
| Semantic segmentation | Zhang et al. [53] | An improved dense GAN is developed for extended datasets and a multi-layer attention mechanism is proposed in conjunction with U-Net for COVID-19 lung CT image segmentation. | |
| Early warning system | Cox proportional risk model | Dong et al. [54] | Depends on regression analysis to confirm the association between predictive covariates (such as clinical features) and event occurrence risks (such as “death”). |
| Semantic segmentation | Sharma and Mishra [55] | The diagnosis is interpreted by activating the gradient-CAM mapping to generate a location-map survey model for each disease. | |
| Prediction | RNN | Kafieh et al. [56] | Information is extracted from COVID-19 data sources and used to train models to predict the time of the next occurrence. |
| LASSO | Liang et al. [57] | Regression analysis is performed to identify associations between predictive covariates and the risk of event occurrence, thereby predicting malignant progression of the disease. | |
| Novel coronavirus molecules | PrismNet | Sun et al. [12] | In vivo data is integrated to construct and train deep neural networks to simulate the interaction between RBP and RNA targets. |
| DeepTCR | Sidhom and Baras [58] | T cell receptor sequences in immune coding databases are analyzed to understand immune genomic differences and models are used to predict critically ill patients. | |
| Control | RL | Yang et al. [59] | Establishes a proactive surveillance system to slow the spread of COVID-19 by warning individuals in areas of interest. |
| CNN | Sethi et al. [60] | The initial face data is segmented by transfer learning technology and then sorted by mask classifier. | |
| Treatment | GCN | Jin et al. [61], Zeng et al. [62] | Combined molecular structure and biological target models predict synergistic drug combinations. |
| MLP | Zeng et al. [63] | Using Amazon Web Services computing resources and a deep-learning framework to identify reusable medications. | |
| MBMS | Roh et al. [64] | Using the Internet of Things and deep learning to detect patients’ medication behavior and feedback to doctors. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, S.; Liu, G.; Bai, Q. Deep Learning in COVID-19 Diagnosis, Prognosis and Treatment Selection. Mathematics 2023, 11, 1279. https://doi.org/10.3390/math11061279
Jin S, Liu G, Bai Q. Deep Learning in COVID-19 Diagnosis, Prognosis and Treatment Selection. Mathematics. 2023; 11(6):1279. https://doi.org/10.3390/math11061279
Chicago/Turabian StyleJin, Suya, Guiyan Liu, and Qifeng Bai. 2023. "Deep Learning in COVID-19 Diagnosis, Prognosis and Treatment Selection" Mathematics 11, no. 6: 1279. https://doi.org/10.3390/math11061279
APA StyleJin, S., Liu, G., & Bai, Q. (2023). Deep Learning in COVID-19 Diagnosis, Prognosis and Treatment Selection. Mathematics, 11(6), 1279. https://doi.org/10.3390/math11061279







