Dynamic Evolution of Coal Pore-Fracture Structure and Its Fractal Characteristics under the Action of Salty Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coal Sample Preparation
2.2. Salt Solution Preparation and Test Procedures
2.3. The NMR Theory
3. Results
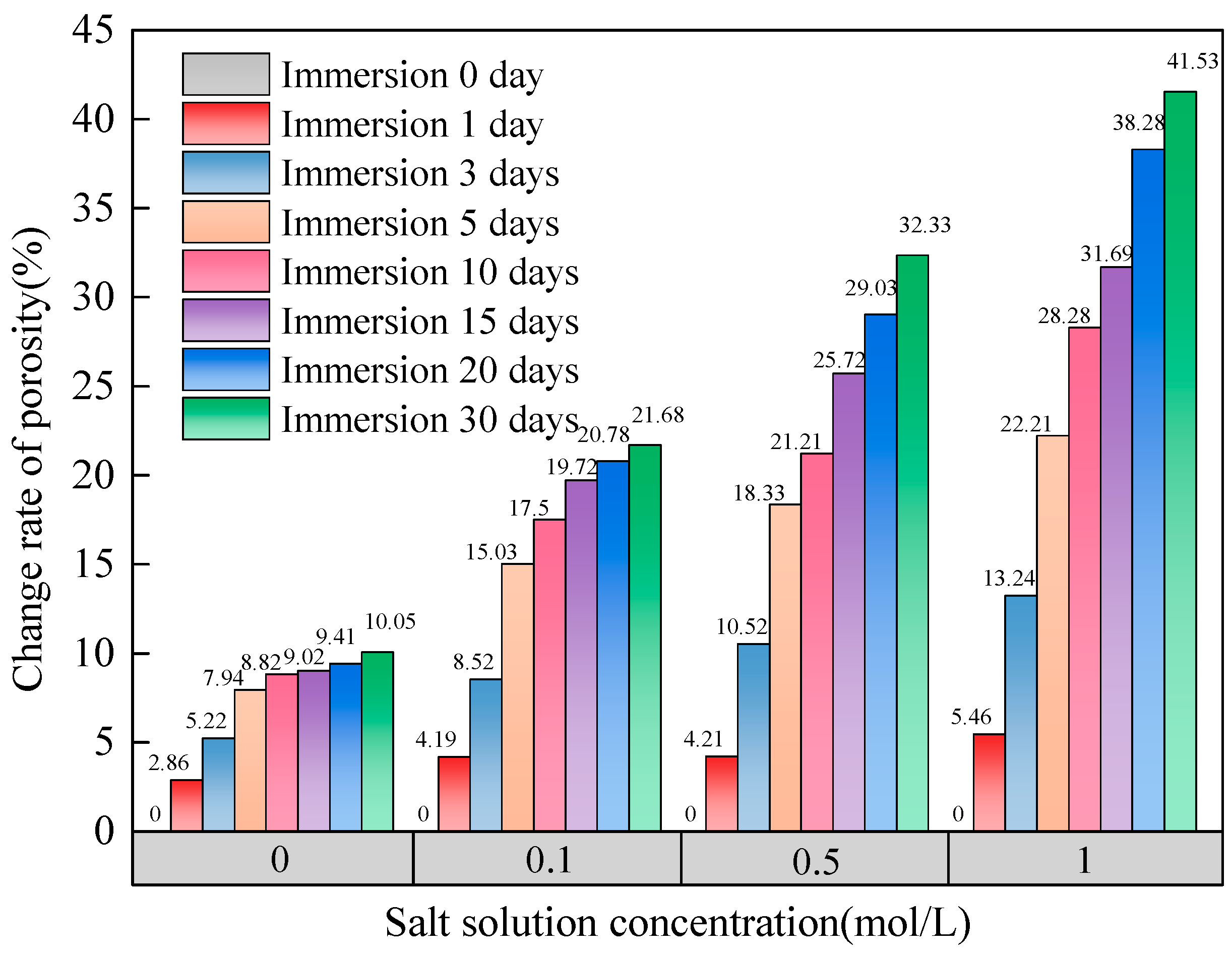
3.1. Time-Dependent Changes in Porosity
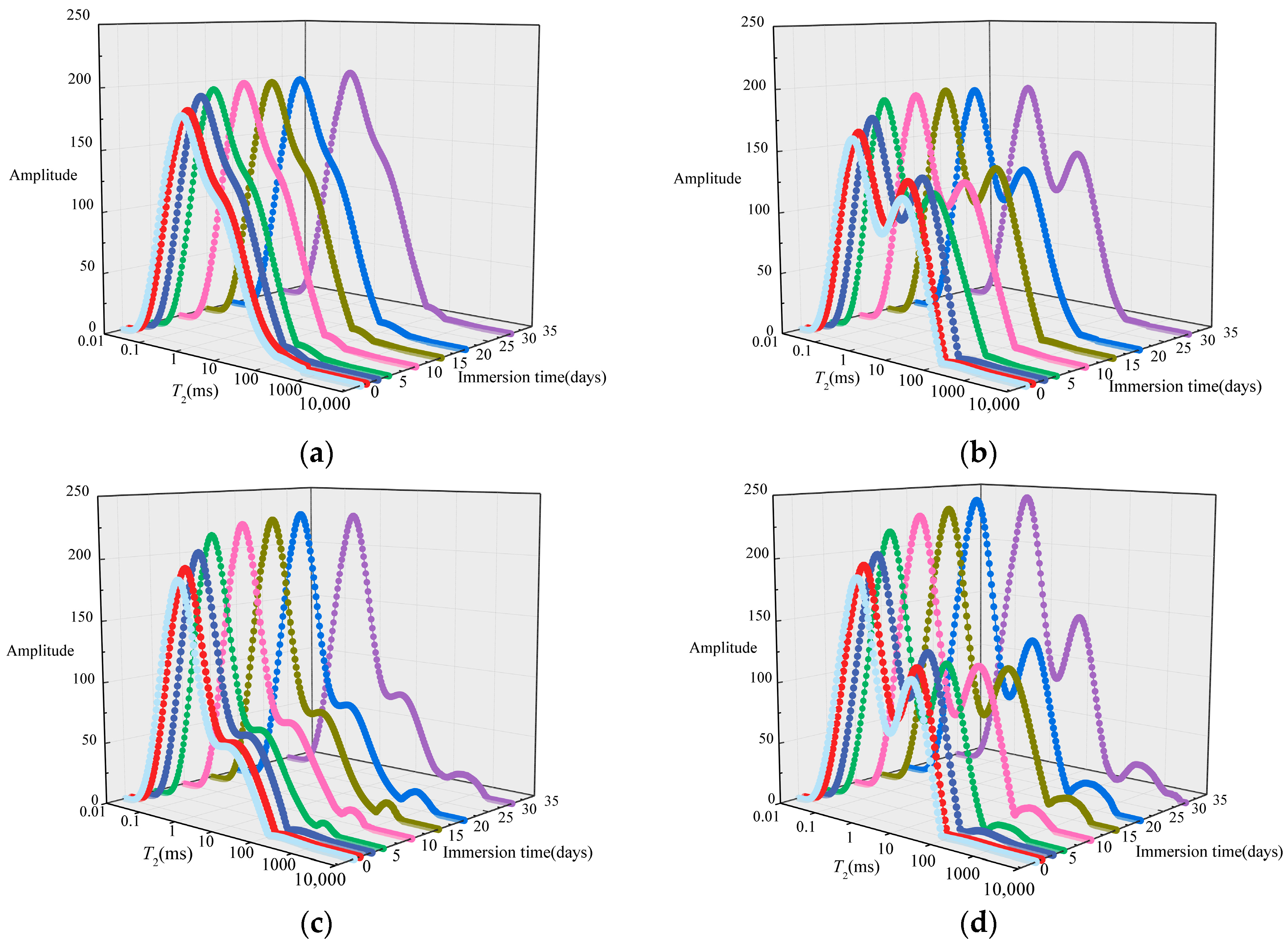
3.2. Evolution of Pore-Fracture Structure
3.2.1. Evolution Characteristics of Pore Size
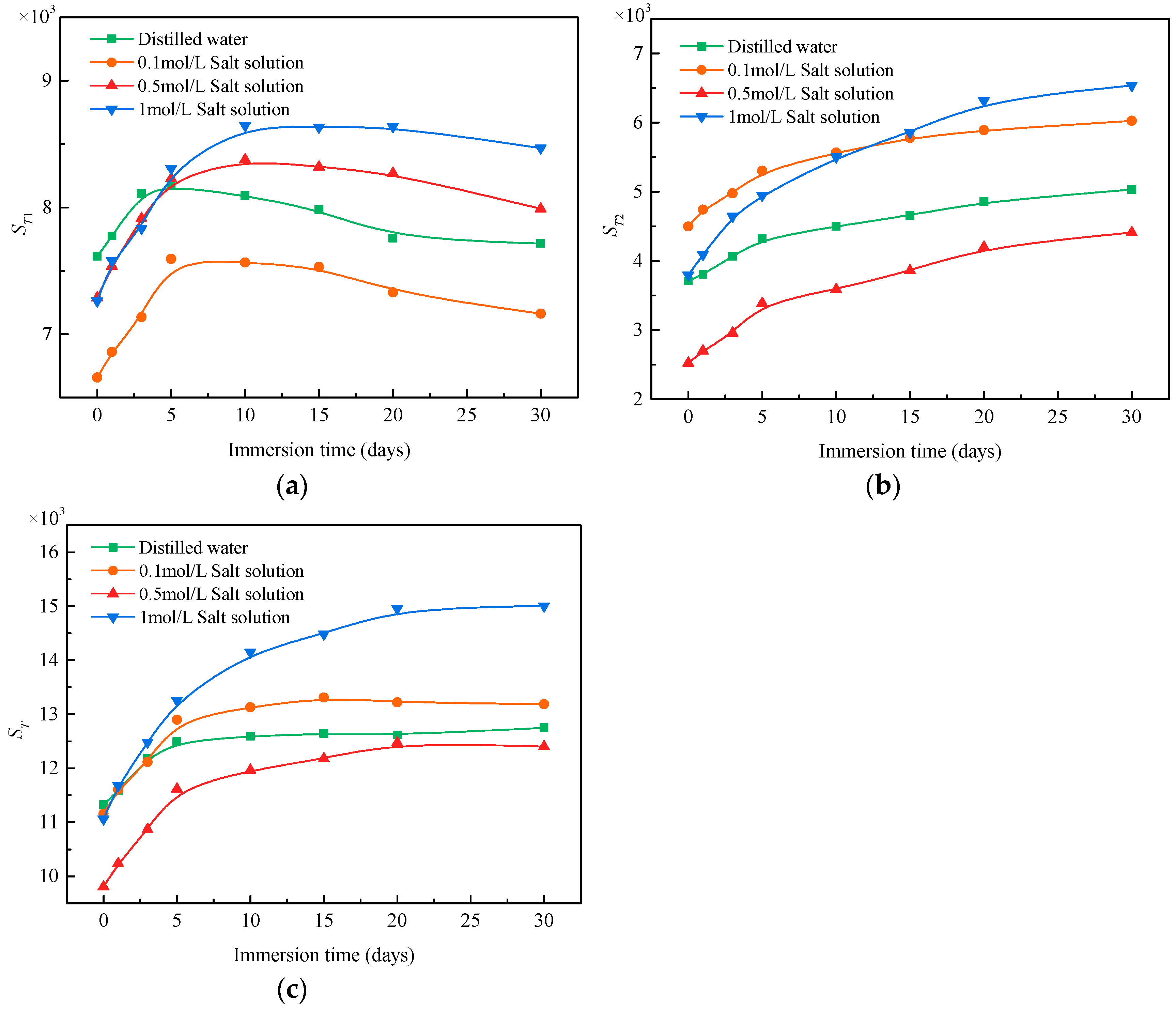
3.2.2. Variation in Pore Number
4. Discussion
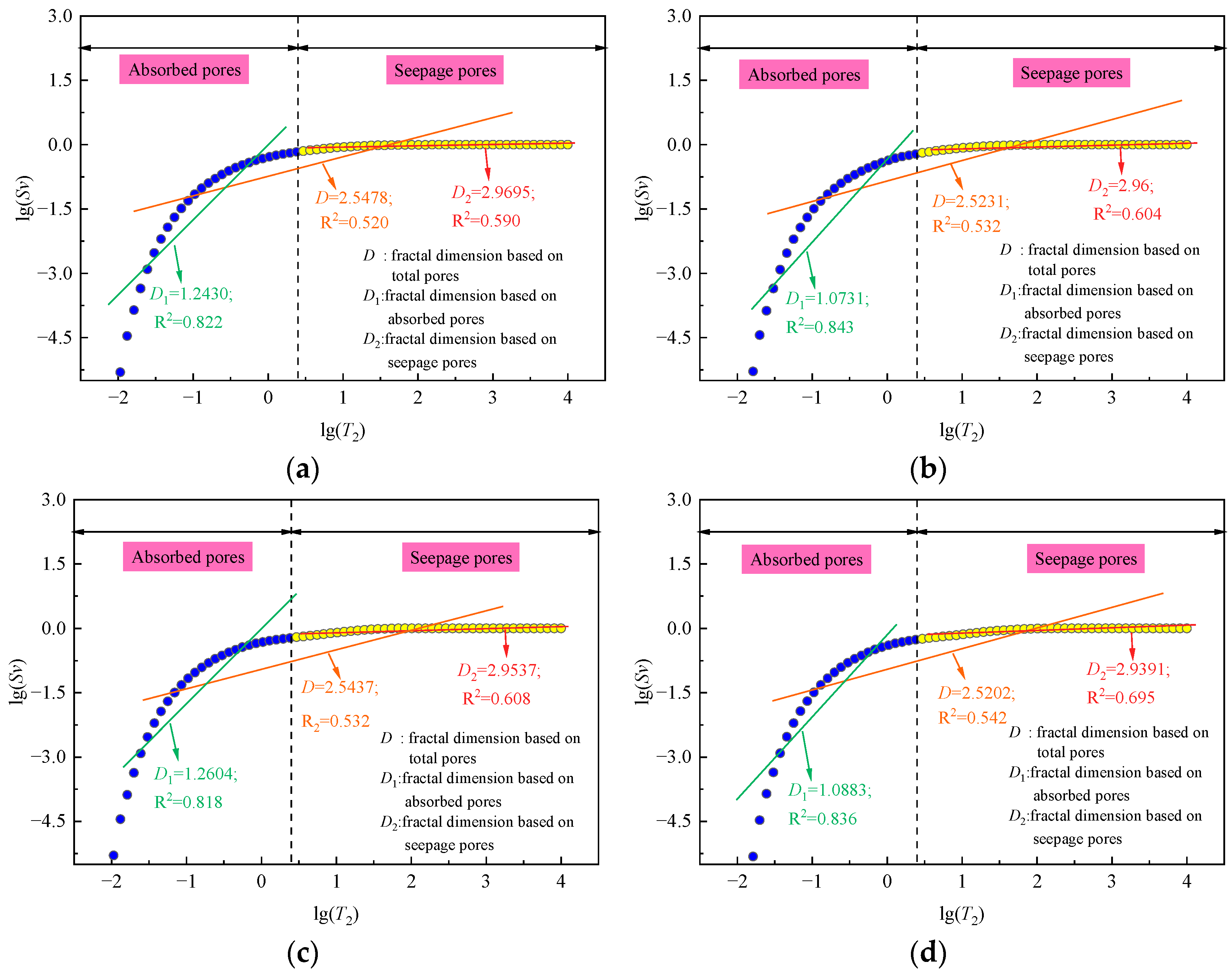
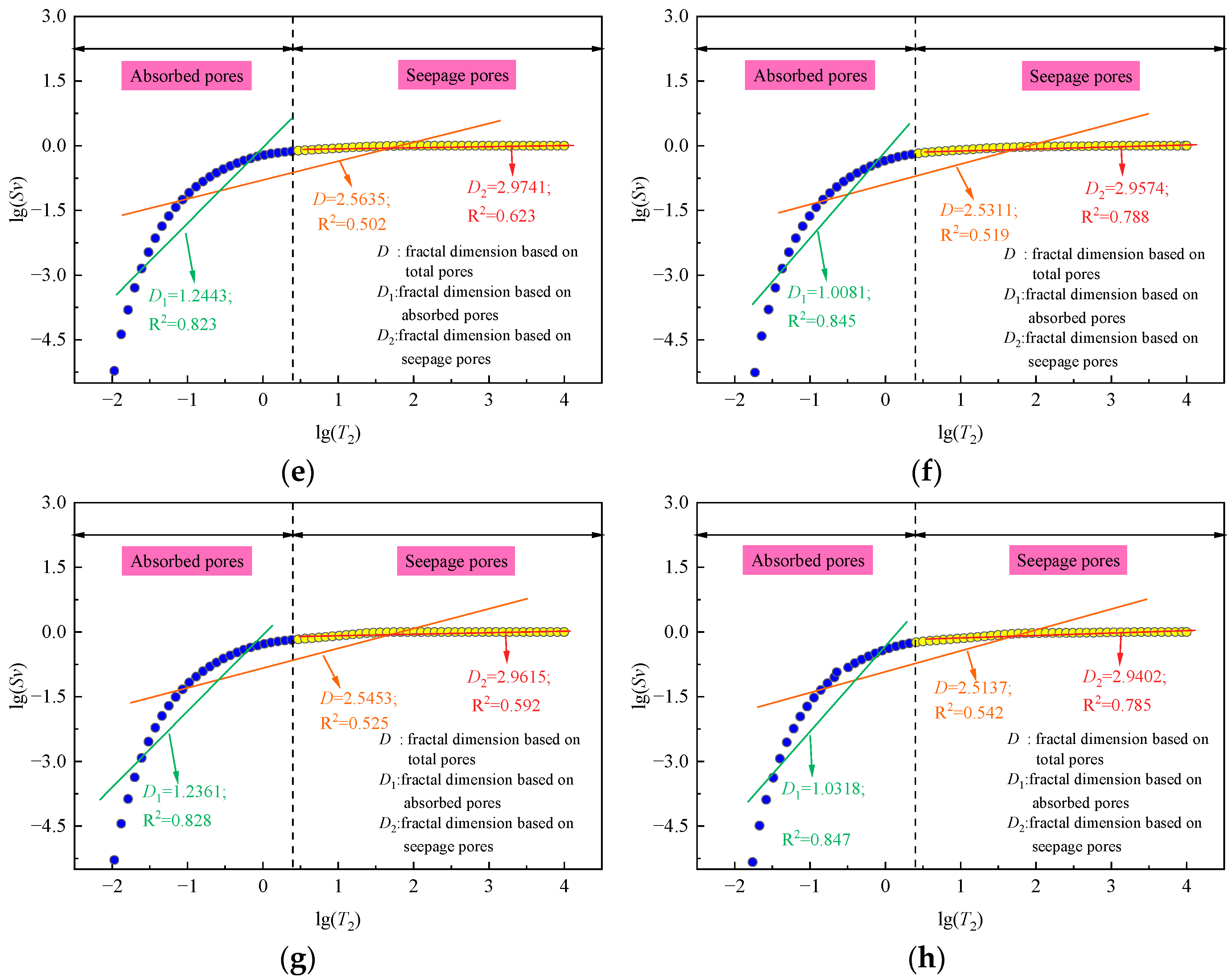
4.1. Fractal Characteristics
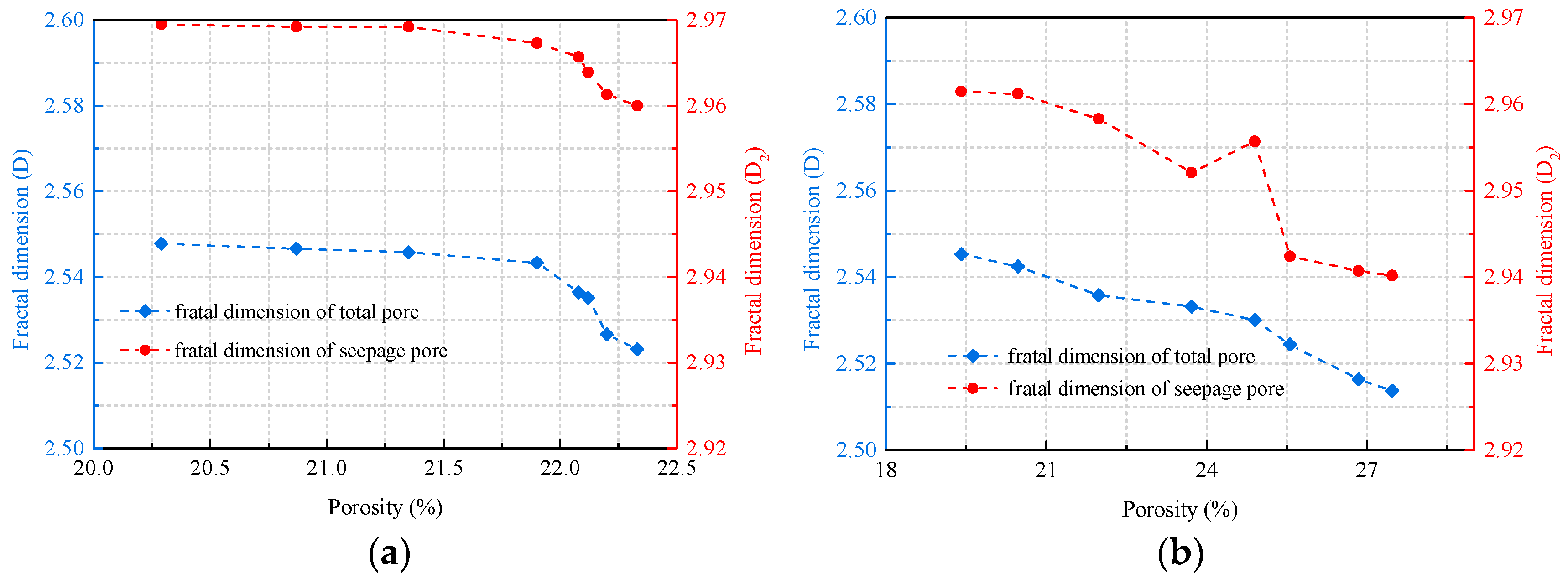
4.2. Relationship between Porosity and Fractal Dimension
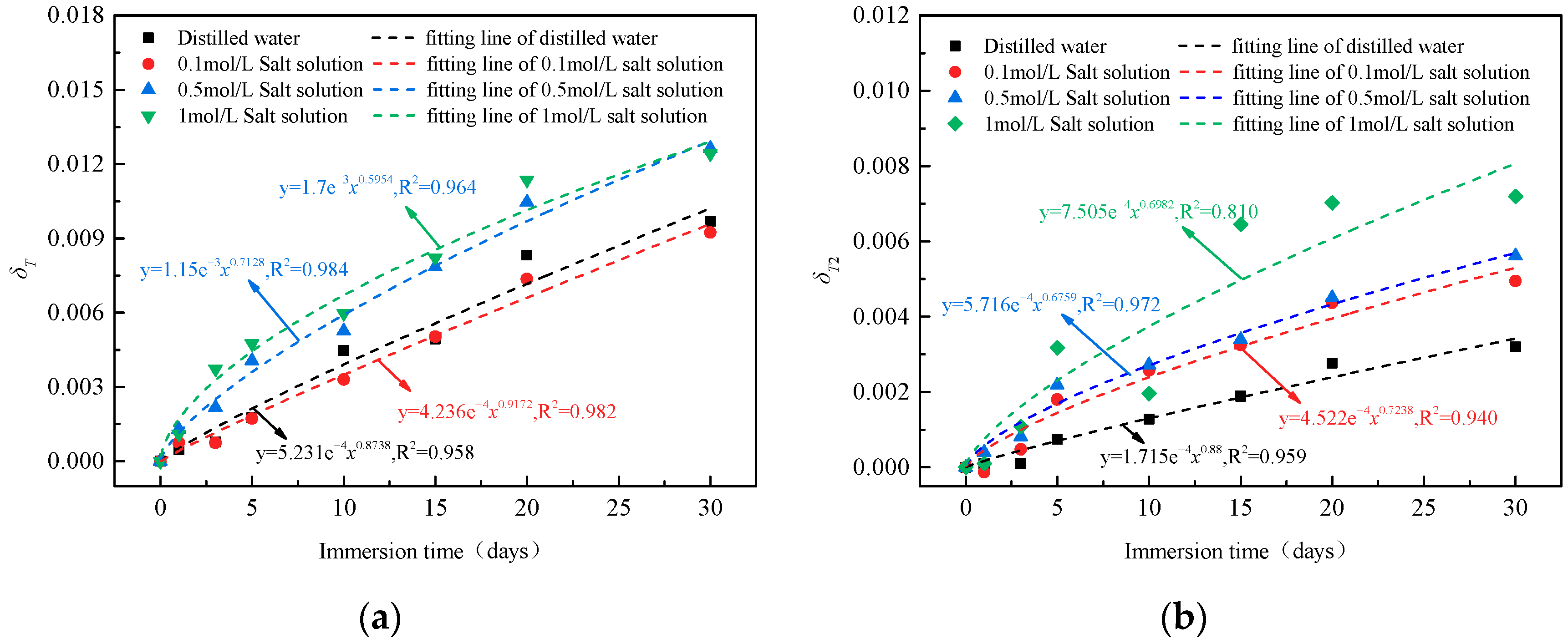
4.3. Diachronic Evolution of Damage
4.4. Damage Mechanism
4.4.1. Mechanism of Water Weakening
4.4.2. Mechanism of Chemical Reaction between Coal and H+ or OH− in Solution
4.4.3. Interaction Mechanism between Coal and Salt Solution
5. Conclusions
- (1)
- With the effect of salt solution immersion, the porosity of coal increased with soaking time. The change rate of porosity increased nonlinearly with soaking time and linearly with salt solution concentration.
- (2)
- Under the action of salt solution immersion, the NMR transverse relaxation time range of coal becomes wider, and more pores with larger or smaller size were gradually formed. The number of seepage pores and total pores increased with soaking time. while the number of adsorption pores increased first and then decreased with soaking time. At the later stage of immersion, the pores with small size gradually connect to form larger pores.
- (3)
- The relationship between fractal dimension and soaking time is linearly decreasing. Under the same immersion time, the pore fractal dimension of coal immersed in salt solution decreased more rapidly than that of coal immersed in distilled water. The damage effect of salt solution soaking on coal matrix is greater than that of distilled water. There is a negative correlation between fractal dimension and porosity.
- (4)
- The diachronic damage variables based on pore fractal dimension was defined. The damage variables have a nonlinear increasing relationship with the soaking time, indicating that the longer the soaking time, the more obvious the pore structure changes and the greater the deterioration of coal. The damage mechanism of coal properties was described from three aspects: water corrosion, acid or alkali chemical reaction and interaction between salt and coal.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shi, J.; Huang, W.; Han, H.; Xu, C. Review on treatment technology of salt wastewater in coal chemical industry of China. Desalination 2020, 493, 114640. [Google Scholar] [CrossRef]
- Hamawand, I.; Yusaf, T.; Hamawand, S. Coal seam gas and associated water: A review paper. Renew. Sustain. Energy Rev. 2013, 22, 550–560. [Google Scholar] [CrossRef]
- Qiao, L.; Wang, Z.; Huang, A. Alteration of mesoscopic properties and mechanical behavior of sandstone due to hydro-physical and hydro-chemical effects. Rock Mech. Rock Eng. 2017, 50, 255–267. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, P.; Cai, X.; Cao, W. Influence of Water Content on Energy Partition and Release in Rock Failure: Implications for Water-Weakening on Rock-burst Proneness. Rock Mech. Rock Eng. 2023, 56, 6189–6205. [Google Scholar] [CrossRef]
- Noël, C.; Baud, P.; Violay, M. Effect of water on sandstone’s fracture toughness and frictional parameters: Brittle strength constraints. Int. J. Rock Mech. Min. 2021, 147, 104916. [Google Scholar] [CrossRef]
- Feng, X.; Chen, S.; Li, S. Effects of water chemistry on microcracking and compressive strength of granite. Int. J. Rock Mech. Min. 2001, 38, 557–568. [Google Scholar] [CrossRef]
- Zhou, Z.; Cai, X.; Cao, W.; Li, X.; Xiong, C. Influence of Water Content on Mechanical Properties of Rock in Both Saturation and Drying Processes. Rock Mech. Rock Eng. 2016, 49, 3009–3025. [Google Scholar] [CrossRef]
- Farquharson, J.; Kushnir, A.; Wild, B.; Baud, P. Physical property evolution of granite during experimental chemical stimulation. Geotherm. Energy 2020, 8, 14. [Google Scholar] [CrossRef]
- Erguler, Z.; Ulusay, R. Water-induced variations in mechanical properties of clay-bearing rocks. Int. J. Rock Mech. Min. 2009, 46, 355–370. [Google Scholar] [CrossRef]
- Chen, S.; Du, Z.; Zhang, Z.; Zhang, H.; Xia, Z.; Feng, F. Effects of chloride on the early mechanical properties and microstruc-ture of gangue-cemented paste backfill. Constr. Build. Mater. 2020, 235, 117504. [Google Scholar] [CrossRef]
- Ai, T.; Wu, S.; Zhang, R.; Gao, M.; Zhou, J.; Xie, J.; Ren, L.; Zhang, Z. Changes in the structure and mechanical properties of a typical coal induced by water immersion. Int. J. Rock Mech. Min. 2021, 138, 104597. [Google Scholar] [CrossRef]
- Gu, H.; Tao, M.; Cao, W.; Zhou, J.; Li, X. Dynamic fracture behaviour and evolution mechanism of soft coal with different porosities and water contents. Theor. Appl. Fract. Mech. 2019, 103, 102265. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, S.; Jiang, Y.; Wang, K.; Huang, Y. Dynamic Tensile Strength of Coal under Dry and Saturated Conditions. Rock Mech. Rock Eng. 2016, 49, 1709–1720. [Google Scholar] [CrossRef]
- Liu, P.; Fan, L.; Fan, J.; Zhong, F. Effect of water content on the induced alteration of pore morphology and gas sorption/diffusion kinetics in coal with ultrasound treatment. Fuel 2021, 306, 121752. [Google Scholar] [CrossRef]
- Zhang, X.; Gamage, R.; Perera, M.; Ranathunga, A. Effects of Water and Brine Saturation on Mechanical Property Alterations of Brown Coal. Energies 2018, 11, 1116. [Google Scholar] [CrossRef]
- Yao, Q.; Chen, T.; Ju, M.; Liang, S.; Liu, Y.; Li, X. Effects of water intrusion on mechanical properties of and crack propagation in coal. Rock Mech. Rock Eng. 2016, 49, 4699–4709. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, G.; Li, M.; Zhang, D.; Huang, G.; Liu, P.; Liu, C.; Zhao, H.; Yu, B. Mechanical properties and failure behavior of dry and water-saturated anisotropic coal under true-triaxial loading conditions. Rock Mech. Rock Eng. 2020, 53, 4799–4818. [Google Scholar] [CrossRef]
- Yao, Q.; Zheng, C.; Tang, C.; Xu, Q.; Chong, Z.; Li, X. Experimental Investigation of the Mechanical Failure Behavior of Coal Specimens With Water Intrusion. Front. Earth Sci. 2020, 7, 348. [Google Scholar] [CrossRef]
- Wang, S.; Elsworth, D.; Liu, J. Permeability evolution in fractured coal: The roles of fracture geometry and water-content. Int. J. Coal Geol. 2011, 87, 13–25. [Google Scholar] [CrossRef]
- Larsen, J. The effects of dissolved CO2 on coal structure and properties. Int. J. Coal Geol. 2004, 57, 63–70. [Google Scholar] [CrossRef]
- Balucan, R.; Turner, L.; Steel, K. Acid-induced mineral alteration and its influence on the permeability and compressibility of coal. J. Nat. Gas. Sci. Eng. 2016, 33, 973–987. [Google Scholar] [CrossRef]
- Xu, J.; Zhai, C.; Liu, S.; Qin, L.; Wu, S. Pore variation of three different metamorphic coals by multiple freezing-thawing cycles of liquid CO2 injection for coalbed methane recovery. Fuel 2017, 208, 41–51. [Google Scholar] [CrossRef]
- Xie, J.; Ni, G.; Xie, H.; Li, S.; Sun, Q.; Dong, K. The effect of adding surfactant to the treating acid on the chemical properties of an acid-treated coal. Powder Technol. 2019, 356, 263–272. [Google Scholar]
- Li, S.; Ni, G.; Wang, H.; Xun, M.; Xu, Y. Effects of acid solution of different components on the pore structure and mechanical properties of coal. Adv. Powder Technol. 2020, 31, 1736–1747. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, R.; Yang, H. Effect of acid and alkali solutions on micro-components of coal. J. Mol. Liq. 2021, 329, 115518. [Google Scholar] [CrossRef]
- Yang, H.; Yu, Y.; Cheng, W.; Rui, J.; Xu, Q. Influence of acetic acid dissolution time on evolution of coal phase and surface morphology. Fuel 2021, 286, 119464. [Google Scholar] [CrossRef]
- Li, Y.; Cui, H.; Zhang, P.; Wang, D.; Wei, J. Three-dimensional visualization and quantitative characterization of coal fracture dynamic evolution under uniaxial and triaxial compression based on μCT scanning. Fuel 2020, 262, 116568. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, D.; Cai, Y.; Yao, Y.; Pan, Z.; Zhou, Y. Application of nuclear magnetic resonance (NMR) in coalbed methane and shale reservoirs: A review. Int. J. Coal Geol. 2020, 218, 103261. [Google Scholar] [CrossRef]
- Nie, B.; Liu, X.; Yang, L.; Meng, J.; Li, X. Pore structure characterization of different rank coals using gas adsorption and scanning electron microscopy. Fuel 2015, 158, 908–917. [Google Scholar] [CrossRef]
- Li, Y.; Song, D.; Liu, S.; Ji, X.; Hao, H. Evaluation of pore properties in coal through compressibility correction based on mercury intrusion porosimetry: A practical approach. Fuel 2021, 291, 120130. [Google Scholar] [CrossRef]
- Ni, G.; Li, S.; Rahman, S.; Xun, M.; Wang, H.; Xu, Y.; Xie, H. Effect of nitric acid on the pore structure and fractal characteristics of coal based on the low-temperature nitrogen adsorption method. Powder Technol. 2020, 367, 506–516. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, D.; Cai, Y.; Yao, Y. Fractal characterization of pore-fracture in low-rank coals using a low-field NMR relaxation method. Fuel 2016, 181, 218–226. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, H.; Wang, W.; Cheng, W.; Xin, L. Experimental Study on the Pore Structure Fractals and Seepage Characteristics of a Coal Sample Around a Borehole in Coal Seam Water Infusion. Transp. Porous Media 2018, 125, 289–309. [Google Scholar] [CrossRef]
- Chen, S.; Tang, D.; Tao, S.; Ji, X.; Xu, H. Fractal analysis of the dynamic variation in pore-fracture systems un-der the action of stress using a low-field NMR relaxation method: An experimental study of coals from western Guizhou in China. J. Pet. Sci. Eng. 2019, 173, 617–629. [Google Scholar] [CrossRef]
- Qin, L.; Zhai, C.; Liu, S.; Xu, J.; Wu, S.; Dong, R. Fractal dimensions of low rank coal subjected to liquid nitrogen freeze-thaw based on nuclear magnetic resonance applied for coalbed methane recovery. Powder Technol. 2018, 325, 11–20. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, D.; Tang, D.; Tang, S.; Huang, W.; Liu, Z.; Che, Y. Fractal characterization of seepage-pores of coals from China: An in-vestigation on permeability of coals. Comput. Geosci. 2009, 35, 1159–1166. [Google Scholar] [CrossRef]
- Wang, M.; Guo, Q.; Tian, Y.; Dai, B. Physical and Mechanical Properties Evolution of Coal Subjected to Salty Solution and a Damage Constitutive Model under Uniaxial Compression. Mathematics 2021, 9, 3264. [Google Scholar] [CrossRef]
- Ni, G.; Xie, H.; Li, S.; Sun, Q.; Huang, D.; Cheng, Y.; Wang, N. The effect of anionic surfactant (SDS) on pore-fracture evolution of acidified coal and its significance for coalbed methane extraction. Adv. Powder Technol. 2019, 30, 940–951. [Google Scholar]
- Liu, R.; Li, B.; Jiang, Y. A fractal model based on a new governing equation of fluid flow in fractures for characterizing hydraulic properties of rock fracture networks. Comput. Geotech. 2016, 75, 57–68. [Google Scholar] [CrossRef]
- Li, B.; Liu, R.; Jiang, Y. A multiple fractal model for estimating permeability of dual-porosity media. J. Hydrol. 2016, 540, 659–669. [Google Scholar] [CrossRef]
- Li, P.; Cai, M.; Gao, Y.; Wang, P.; Miao, S.; Wang, Y.; Xi, X. Dynamic mechanical behavior and cracking mechanism of cross-jointed granite containing a hole. J. Mater. Res. Technol. 2023, 22, 1572–1594. [Google Scholar] [CrossRef]
- Cai, X.; Zhou, Z.; Tan, L.; Zang, H.; Song, Z. Fracture behavior and damage mechanisms of sandstone subjected to wetting-drying cycles. Eng. Fract. Mech. 2020, 234, 107109. [Google Scholar] [CrossRef]
- Smoliński, A.; Malashkevych, D.; Petlovanyi, M.; Rysbekov, K.; Lozynskyi, V.; Sai, K. Research into Impact of Leaving Waste Rocks in the Mined-Out Space on the Geomechanical State of the Rock Mass Surrounding the Longwall Face. Energies 2022, 15, 9522. [Google Scholar] [CrossRef]
- Gu, H.; Tao, M.; Wang, J.; Jiang, H.; Li, Q.; Wang, W. Influence of water content on dynamic mechanical properties of coal. Geomech. Eng. 2018, 16, 85–95. [Google Scholar]
- Wang, M.; Xi, X.; Guo, Q. Deterioration Mechanism and Constitutive Model of Coal Subjected to Dry-ing-Wetting Cycles of Salty Mine Water. Lithosphere 2022, 2022, 7935979. [Google Scholar] [CrossRef]
- Pitois, O.; Moucheront, P.; Chateau, X. Liquid Bridge between Two Moving Spheres: An Experimental Study of Viscosity Effects. J. Colloid Interface Sci. 2000, 231, 26–31. [Google Scholar] [CrossRef]
- Ni, G.; Sun, Q.; Xun, M.; Wang, H.; Wang, H.; Cheng, W.; Wang, G. Effect of NaCl-SDS compound solution on the wettability and functional groups of coal. Fuel 2019, 257, 11607. [Google Scholar]





| Immersion Time (Days) | Distilled Water | 0.1 mol/L Salt Solution | 0.5 mol/L Salt Solution | 1 mol/L Salt Solution | ||||
|---|---|---|---|---|---|---|---|---|
| φp (%) | Δφp (%) | φp (%) | Δφp (%) | φp (%) | Δφp (%) | φp (%) | Δφp (%) | |
| 0 | 20.29 | 19.83 | 16.64 | 19.41 | ||||
| 1 | 20.87 | 2.86 | 20.66 | 4.19 | 17.34 | 4.21 | 20.47 | 5.46 |
| 3 | 21.35 | 5.22 | 21.52 | 8.52 | 18.39 | 10.52 | 21.98 | 13.24 |
| 5 | 21.90 | 7.94 | 22.81 | 15.03 | 19.69 | 18.33 | 23.72 | 22.21 |
| 10 | 22.08 | 8.82 | 23.30 | 17.50 | 20.17 | 21.21 | 24.90 | 28.28 |
| 15 | 22.12 | 9.02 | 23.74 | 19.72 | 20.92 | 25.72 | 25.56 | 31.69 |
| 20 | 22.20 | 9.41 | 23.95 | 20.78 | 21.47 | 29.03 | 26.84 | 38.28 |
| 30 | 22.33 | 10.05 | 24.13 | 21.68 | 22.02 | 32.33 | 27.47 | 41.53 |
| Concentration of Salt Solution (mol/L) | Fit Function | a | b | R2 |
|---|---|---|---|---|
| 0 | y = a × (1 − e−b×x) | 9.513 | 0.3065 | 0.986 |
| 0.1 | 21.17 | 0.2015 | 0.988 | |
| 0.5 | 31.30 | 0.1358 | 0.979 | |
| 1 | 40.79 | 0.1285 | 0.983 |
| Immersion Time (Days) | Fit Function | a | b | R2 |
|---|---|---|---|---|
| 1 | y = a + b × x | 3.344 | 2.090 | 0.895 |
| 3 | 6.567 | 7.019 | 0.838 | |
| 5 | 11.06 | 12.05 | 0.730 | |
| 10 | 12.30 | 16.63 | 0.806 | |
| 15 | 13.76 | 19.45 | 0.755 | |
| 20 | 14.13 | 25.60 | 0.849 | |
| 30 | 15.03 | 28.43 | 0.858 |
| Immersion Solution Type | Immersion Time (Days) | Adsorption Pore | Seepage Pore | Total Pores | |||
|---|---|---|---|---|---|---|---|
| D1 | R2 | D2 | R2 | D | R2 | ||
| Distilled water | 0 | 1.2430 | 0.822 | 2.9695 | 0.590 | 2.5478 | 0.520 |
| 1 | 1.2475 | 0.822 | 2.9692 | 0.595 | 2.5466 | 0.520 | |
| 3 | 1.2218 | 0.824 | 2.9692 | 0.575 | 2.5458 | 0.521 | |
| 5 | 1.1989 | 0.827 | 2.9673 | 0.583 | 2.5433 | 0.522 | |
| 7 | 1.1626 | 0.832 | 2.9657 | 0.587 | 2.5364 | 0.526 | |
| 15 | 1.1398 | 0.832 | 2.9639 | 0.592 | 2.5352 | 0.525 | |
| 20 | 1.1033 | 0.840 | 2.9613 | 0.605 | 2.5266 | 0.531 | |
| 30 | 1.0731 | 0.843 | 2.9600 | 0.604 | 2.5231 | 0.532 | |
| 0.1 mol/L salt solution | 0 | 1.2604 | 0.818 | 2.9537 | 0.608 | 2.5437 | 0.532 |
| 1 | 1.2590 | 0.820 | 2.9541 | 0.591 | 2.5418 | 0.534 | |
| 3 | 1.2315 | 0.822 | 2.9523 | 0.608 | 2.5418 | 0.534 | |
| 5 | 1.2136 | 0.821 | 2.9484 | 0.686 | 2.5393 | 0.530 | |
| 7 | 1.1820 | 0.826 | 2.9461 | 0.687 | 2.5353 | 0.535 | |
| 15 | 1.1514 | 0.828 | 2.9441 | 0.680 | 2.5309 | 0.536 | |
| 20 | 1.1163 | 0.832 | 2.9408 | 0.707 | 2.5250 | 0.539 | |
| 30 | 1.0883 | 0.836 | 2.9391 | 0.695 | 2.5202 | 0.542 | |
| 0.5 mol/L salt solution | 0 | 1.2443 | 0.823 | 2.9741 | 0.623 | 2.5635 | 0.502 |
| 1 | 1.2166 | 0.825 | 2.9729 | 0.636 | 2.5602 | 0.503 | |
| 3 | 1.1934 | 0.827 | 2.9717 | 0.646 | 2.5579 | 0.504 | |
| 5 | 1.1394 | 0.830 | 2.9676 | 0.710 | 2.5531 | 0.505 | |
| 7 | 1.1098 | 0.832 | 2.9660 | 0.734 | 2.5500 | 0.506 | |
| 15 | 1.0773 | 0.838 | 2.9640 | 0.731 | 2.5434 | 0.511 | |
| 20 | 1.0398 | 0.842 | 2.9607 | 0.766 | 2.5367 | 0.505 | |
| 30 | 1.0081 | 0.845 | 2.9574 | 0.788 | 2.5311 | 0.519 | |
| 1 mol/L salt solution | 0 | 1.2361 | 0.828 | 2.9615 | 0.592 | 2.5453 | 0.525 |
| 1 | 1.2119 | 0.829 | 2.9612 | 0.588 | 2.5425 | 0.525 | |
| 3 | 1.1856 | 0.833 | 2.9583 | 0.606 | 2.5358 | 0.531 | |
| 5 | 1.1507 | 0.835 | 2.9521 | 0.703 | 2.5332 | 0.530 | |
| 7 | 1.1296 | 0.836 | 2.9557 | 0.721 | 2.5301 | 0.534 | |
| 15 | 1.0944 | 0.841 | 2.9424 | 0.816 | 2.5244 | 0.537 | |
| 20 | 1.0587 | 0.846 | 2.9407 | 0.800 | 2.5164 | 0.542 | |
| 30 | 1.0318 | 0.847 | 2.9402 | 0.785 | 2.5137 | 0.542 | |
| Immersion Time (Days) | Distilled Water | 0.1 mol/L Salt Solution | 0.5 mol/L Salt Solution | 1 mol/L Salt Solution | ||||
|---|---|---|---|---|---|---|---|---|
| δT (%) | δT2 (%) | δT (%) | δT2 (%) | δT (%) | δT2 (%) | δT (%) | δT2 (%) | |
| 0 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 1 | 0.05 | 0.01 | 0.07 | −0.01 | 0.13 | 0.04 | 0.11 | 0.01 |
| 3 | 0.08 | 0.01 | 0.07 | 0.05 | 0.22 | 0.08 | 0.37 | 0.11 |
| 5 | 0.18 | 0.07 | 0.17 | 0.18 | 0.41 | 0.22 | 0.48 | 0.32 |
| 10 | 0.45 | 0.13 | 0.33 | 0.26 | 0.53 | 0.27 | 0.60 | 0.20 |
| 15 | 0.49 | 0.19 | 0.50 | 0.33 | 0.78 | 0.34 | 0.82 | 0.64 |
| 20 | 0.83 | 0.28 | 0.74 | 0.44 | 1.05 | 0.45 | 1.14 | 0.70 |
| 30 | 0.97 | 0.32 | 0.92 | 0.49 | 1.26 | 0.56 | 1.24 | 0.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Tian, Y.; Zhang, Z.; Guo, Q.; Wu, L. Dynamic Evolution of Coal Pore-Fracture Structure and Its Fractal Characteristics under the Action of Salty Solution. Mathematics 2024, 12, 72. https://doi.org/10.3390/math12010072
Wang M, Tian Y, Zhang Z, Guo Q, Wu L. Dynamic Evolution of Coal Pore-Fracture Structure and Its Fractal Characteristics under the Action of Salty Solution. Mathematics. 2024; 12(1):72. https://doi.org/10.3390/math12010072
Chicago/Turabian StyleWang, Min, Yakun Tian, Zhijun Zhang, Qifeng Guo, and Lingling Wu. 2024. "Dynamic Evolution of Coal Pore-Fracture Structure and Its Fractal Characteristics under the Action of Salty Solution" Mathematics 12, no. 1: 72. https://doi.org/10.3390/math12010072





