Stochastic Phase Model with Reflective Boundary and Induced Beating: An Approach for Cardiac Muscle Cells
Abstract
:1. Introduction
2. From the FitzHugh–Nagumo Model to the Phase Model
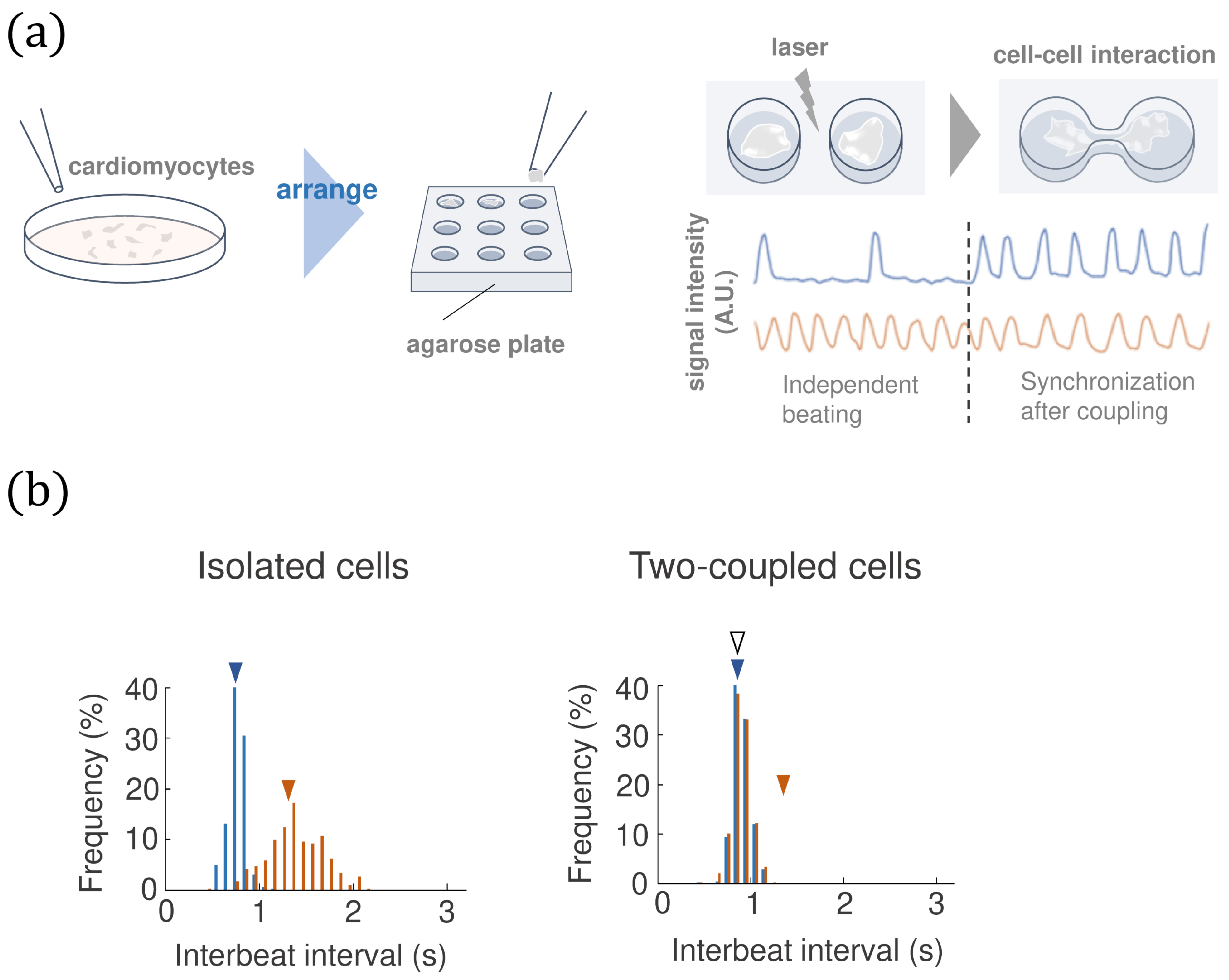
2.1. The Experimental Approach
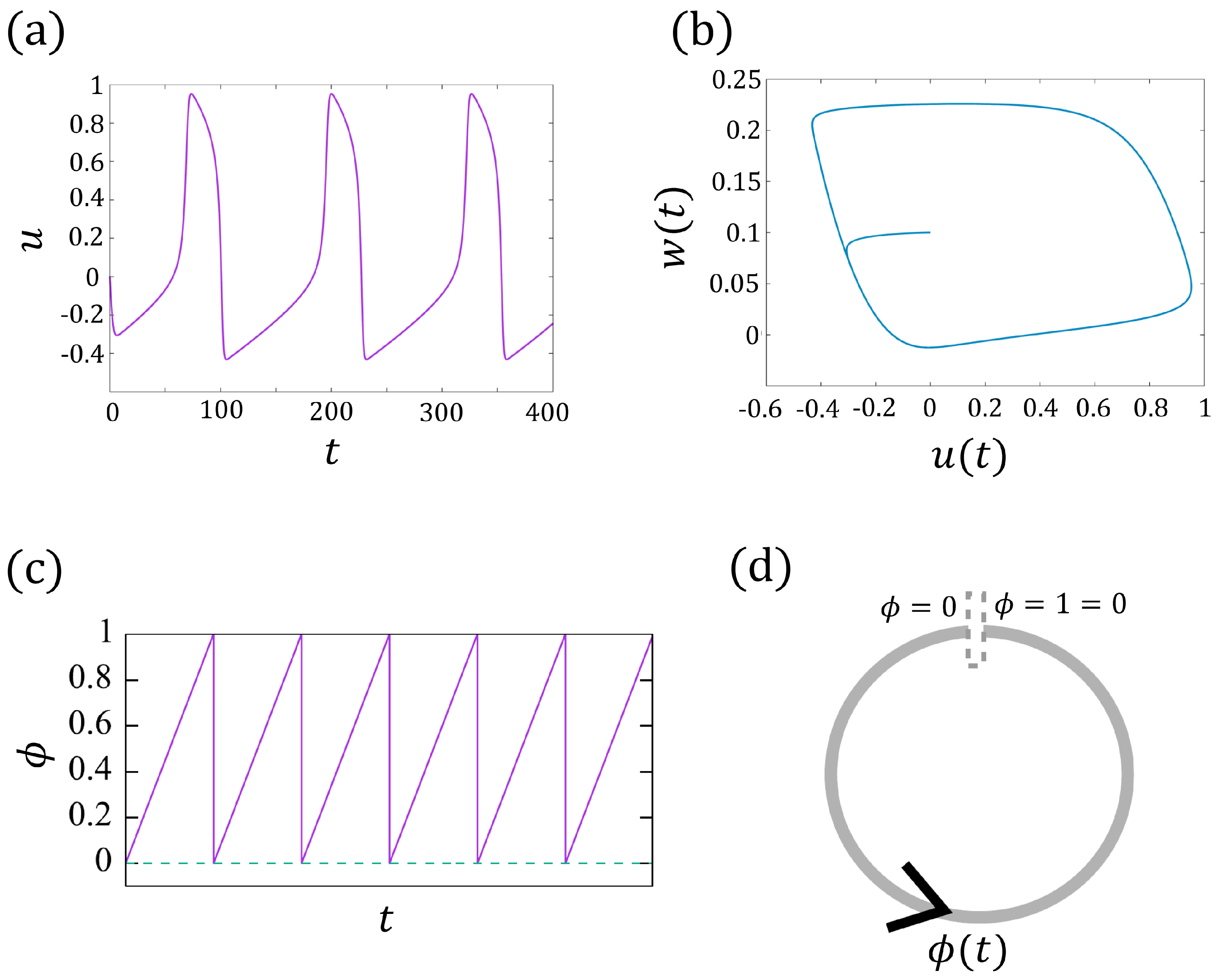
2.2. From the FitzHugh–Nagumo Model to the Phase Model
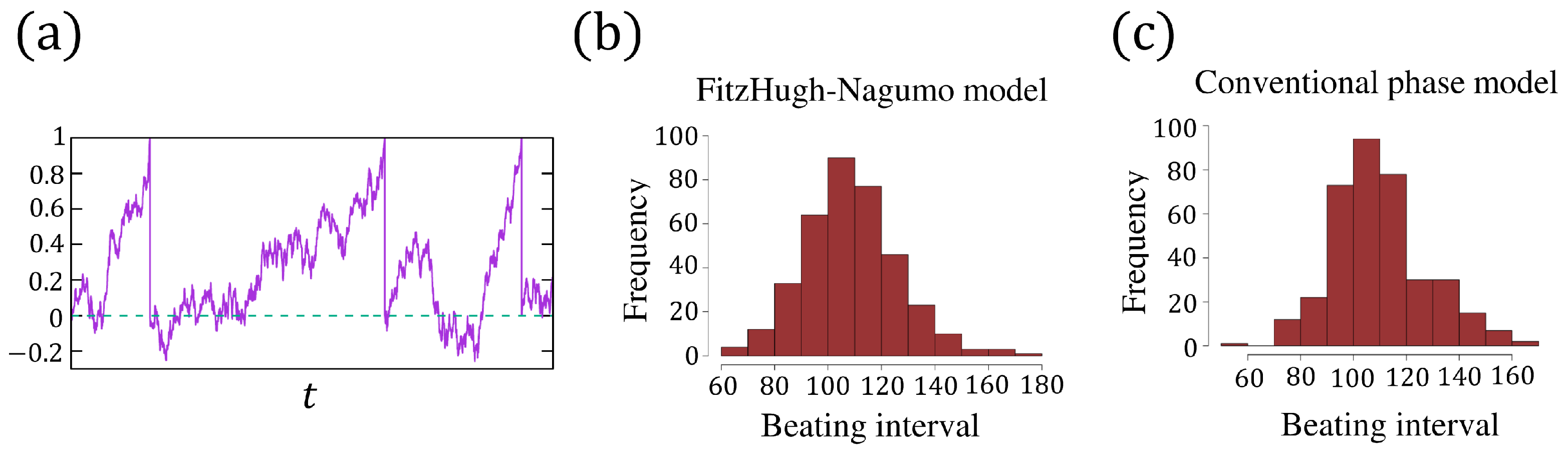
2.3. The FitzHugh–Nagumo and Phase Models with Noise
3. The Phase Models for an Isolated Cell
- (L1)
- , and is a nondecreasing, continuous process for such that ;
- (L2)
- increases only when .
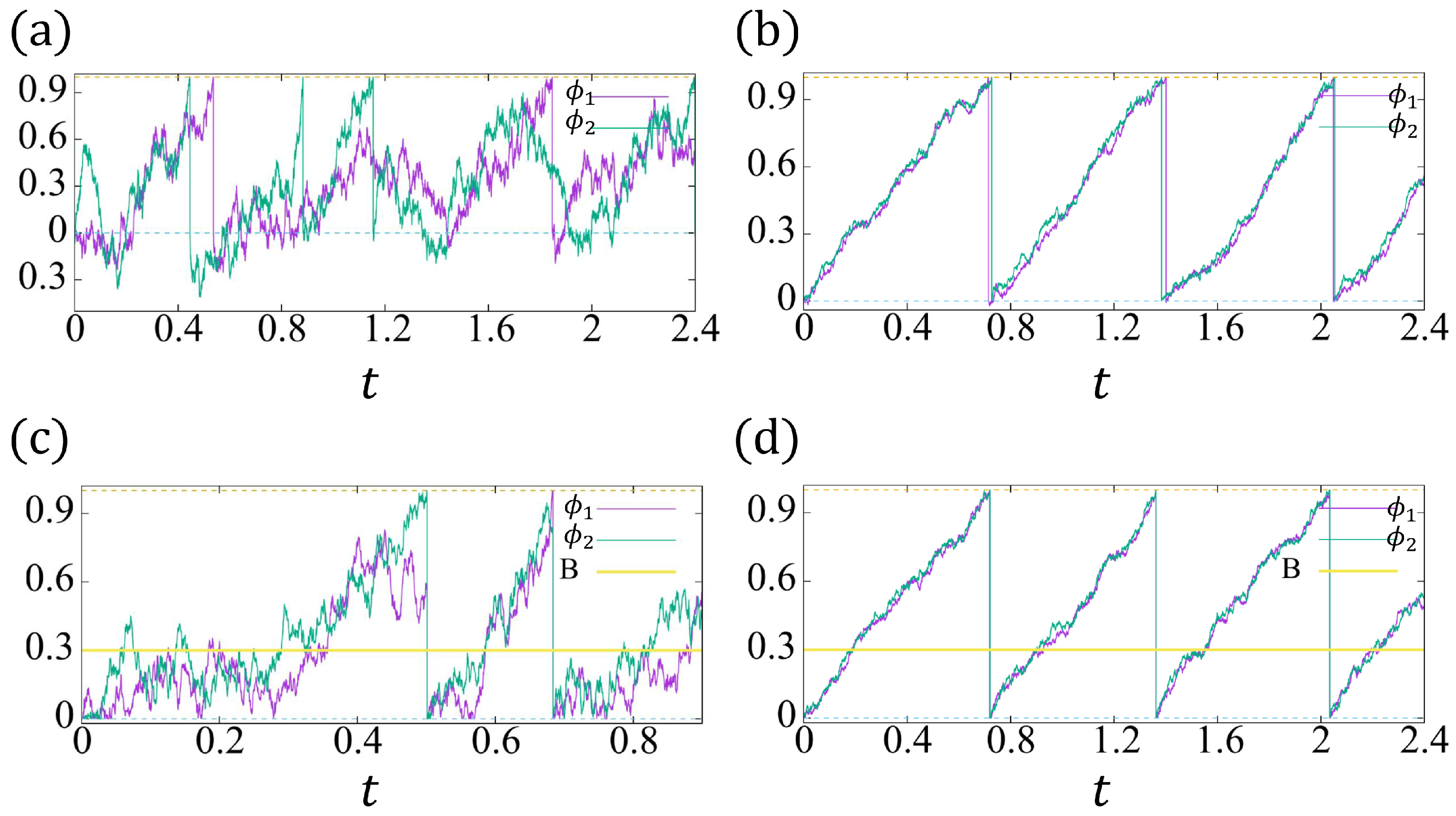
4. The Phase Models for Two Coupled Cells
- (L1)
- is continuous and nondecreasing during each beating interval of ;
- (L2)
- increases only when .
- (IND)
- If cell i is out of refractory, cell i beats promptly when the neighbor (cell j) beats spontaneously; in other words, if and , then (both two phases jump to 0 after beating to start a new oscillation cycle);
- (REF)
- If cell i is in refractory and the neighbor cell j beats spontaneously, then cell i will not be induced to beat, namely, if and , then and ( jumps to 0 but keeps going).
4.1. The Expectation and Variance in the Synchronized Beating Interval
4.2. The Synchronized Beating of the Conventional Model
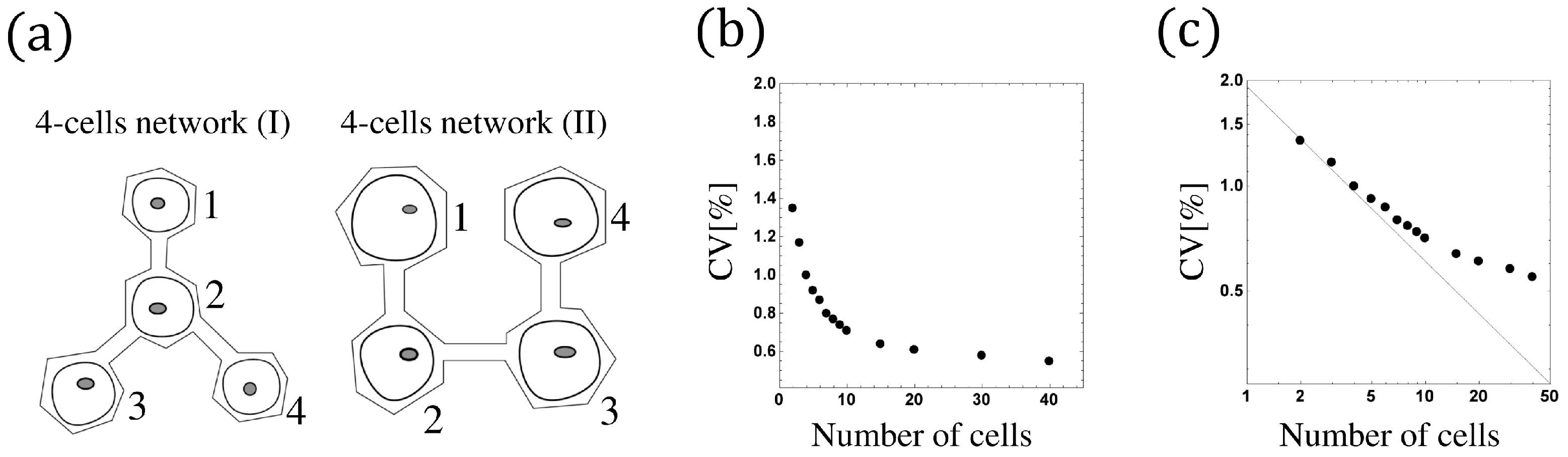
5. The Phase Model for the N-Cells Network
The Phase Model of the N-Cells Network
6. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
Appendix C
References
- Abramovich-Sivan, S.; Akselrod, S. A pacemaker cell pair model based on the phase response curve. Biol. Cybern. 1998, 79, 77–86. [Google Scholar] [CrossRef] [PubMed]
- DeHaan, R.L.; Hirakow, R. Numerical simulations of angiogenesis in the cornea. Exp. Cell Res. 1972, 70, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Goshima, K.; Tonomura, Y. Synchronized beating of embryonic mouse myocardial cells mediated by cells in monolayer culture. Exp. Cell Res. 1969, 56, 387–392. [Google Scholar] [CrossRef]
- Guevara, M.R.; Lewis, T.J. A minimal single-channel model for the regularity of beating in the sinoatrial node. Chaos 1995, 5, 174–183. [Google Scholar] [CrossRef]
- Harary, I.; Farley, B. In vitro studies on single beating rat heart cells. II. Intercellular communication. Exp. Cell Res. 1963, 29, 466–474. [Google Scholar] [CrossRef]
- Christoffels, V.M.; Smits, G.J.; Kispert, A.; Moorman, A.F. Development of the pacemaker tissues of the heart. Circ. Res. 2010, 106, 240–254. [Google Scholar] [CrossRef]
- Sakamoto, K.; Matsumoto, S.; Abe, N.; Sentoku, M.; Yasuda, K. Importance of Spatial Arrangement of Cardiomyocyte Network for Precise and Stable On-Chip Predictive Cardiotoxicity Measurement. Micromachines 2023, 14, 854. [Google Scholar] [CrossRef]
- Merks, R.M.H.; Koolwijk, P. Synchronization of electrically induced calcium firings in self-assembled cardiac cells. Biophys. Chem. 2005, 116, 33–39. [Google Scholar]
- Mitchell, C.C.; Schaeffer, D.G. A Two-Current Model for the Dynamics of Cardiac Membrane. Bull. Math. Biol. 2003, 65, 767–793. [Google Scholar] [CrossRef]
- Petrov, V.S.; Osipov, G.V.; Suykens, J.A.K. Influence of passive elements on the dynamics of oscillatory ensembles of cardiac cells. Phys. Rev. E 2009, 79, 046219. [Google Scholar] [CrossRef]
- Torre, V. A Theory of Synchronization of Heart Pace-maker Cell. J. Theor. Biol. 1976, 61, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, Y.; Harada, A.; Kawahara, K. Changes in the fluctuation of interbeat intervals in spontaneously beating cultured cardiac myocytes: Experimental and modeling studies. Biol. Cybern. 2002, 65, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Gomez, J.; Bashar, M.K.; Ji, J.; Can, U.I.; Chang, H.C.; Shukla, N.; Datta, S.; Zorlutuna, P. Cardiac Muscle Cell-Based Coupled Oscillator Network for Collective Computing. Adv. Intell. Syst. 2021, 3, 2000253. [Google Scholar] [CrossRef]
- Albanese, A.; Cheng, L.; Ursino, M.; Chbat, N.W. An integrated mathematical model of the human cardiopulmonary system: Model development. Am. J. Physiol.-Heart Circ. Physiol. 2016, 310, H899–H921. [Google Scholar] [CrossRef] [PubMed]
- Hatano, A.; Okada, J.; Washio, T.; Hisada, T.; Sugiura, S. A Three-Dimensional Simulation Model of Cardiomyocyte Integrating Excitation-Contraction Coupling and Metabolism. Biophys. J. 2011, 101, 2601–2610. [Google Scholar] [CrossRef] [PubMed]
- Hodgkin, A.L.; Huxley, A. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 1952, 117, 500–544. [Google Scholar] [CrossRef] [PubMed]
- FithHugh, R. Impulses and physiological states in theoretical models of nerve membrane. Biophys. J. 1961, 1, 445–466. [Google Scholar] [CrossRef]
- Nagumo, J.; Arimoto, S.; Yoshizawa, A. An active pulse transmission line simulating nerve axon. Proc. IRE 1962, 50, 2061–2072. [Google Scholar] [CrossRef]
- Keener, J.; Sneyd, J. Mathematical Physiology; Springer: New York, NY, USA, 1998. [Google Scholar]
- Murray, J.D. Mathematical Biology, 3rd ed.; Springer: Berlin/Heiderberg, Germany, 2002. [Google Scholar]
- Kaneko, T.; Kojima, K.; Yasuda, K. Dependence of the community effect of cultured cardiomyocytes on the cell network pattern. Biochem. Biophys. Res. Commun. 2007, 356, 494–498. [Google Scholar] [CrossRef]
- Kojima, K.; Kaneko, T.; Yasuda, K. Role of the community effect of cardiomyocyte in the in the entrainment and reestablishment of stable beating rhythms. Biochem. Biophys. Res. Commun. 2006, 351, 209–215. [Google Scholar] [CrossRef]
- Kori, H.; Kawamura, Y.; Masuda, N. Structure of cell networks critically determines oscillation regularity. J. Theor. Biol. 2012, 297, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Kuramoto, Y. Chemical Oscillations, Waves, and Turbulence; Springer: New York, NY, USA, 1984. [Google Scholar]
- Chang, Y.C.; Juang, J. Stable Synchrony in Globally Coupled Integrate-and-Fire Oscillators. SIAM J. Appl. Dyn. Syst. 2008, 7, 1445–1476. [Google Scholar] [CrossRef]
- Mirollo, R.E.; Strogatz, S.H. Synchronization of Pulse-Coupled Biological Oscillators. SIAM J. Appl. Math. 1990, 50, 1645–1662. [Google Scholar] [CrossRef]
- Burkitt, A.N. A review of the integrate-and-fire neuron model: I. Homogeneous synaptic input. Biol. Cybern. 2006, 95, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Keener, J.P.; Hoppensteadt, F.C.; Rinzel, J. Integrate-and-Fire Models of Nerve Membrane Response to Oscillatory Input. SIAM J. Appl. Math. 1981, 41, 503–517. [Google Scholar] [CrossRef]
- Peskin, C.S. Mathematical Aspects of Heart Physiology; Courant Institute of Mathematical Sciences, New York University: New York, NY, USA, 1975. [Google Scholar]
- Sacerdote, L.; Giraudo, M.T. Stochastic Integrable and Fire Models: A Review on Mathematical Methods and Their Applications. In Stochastic Biomathematical Models with Applications to Neuronal Modeling; Springer: Berlin/Heiderberg, Germany, 2013; pp. 99–148. [Google Scholar]
- Winfree, A.T. The Geometry of Biological Time; Springer: New York, NY, USA, 2001. [Google Scholar]
- Hayashi, T.; Tokihiro, T.; Kurihara, H.; Yasuda, K. Community effect of cardiomyocytes in beating rhythms is determined by stable cells. Sci. Rep. 2017, 7, 15450. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.M. Brownian Motion and Stochastic Flow Systems; John Wiley & Sons: Hoboken, NJ, USA, 1985. [Google Scholar]
- Lions, P.L.; Sznitman, A.S. Stochastic differential equations with reflecting boundary conditions. Comm. Pure. Appl. Math. 1984, 37, 511–537. [Google Scholar] [CrossRef]
- Skorokhod, A.V. Stochastic equations for diffusion processes in a bounded region. Theory Probab. Appl. 1961, 6, 264–274. [Google Scholar] [CrossRef]
- Slominski, L. Some remarks on approximation of solutions of SDE’s with reflecting boundary conditions. Math. Comp. Simulat. 1995, 38, 109–117. [Google Scholar] [CrossRef]
- Çinlar, E. Introduction to Stochastic Processes; Dover Publications, Inc.: Mineola, NY, USA, 2013. [Google Scholar]
- Mckean, H.P. Stochastic Integrals; Academic Press: Cambridge, MA, USA, 1969. [Google Scholar]
- Evans, L.C. An Introduction to Stochastic Differential Equations; American Mathematical Society: Providence, RI, USA, 2013. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, G.; Hayashi, T.; Tokihiro, T. Stochastic Phase Model with Reflective Boundary and Induced Beating: An Approach for Cardiac Muscle Cells. Mathematics 2024, 12, 2964. https://doi.org/10.3390/math12192964
Zhou G, Hayashi T, Tokihiro T. Stochastic Phase Model with Reflective Boundary and Induced Beating: An Approach for Cardiac Muscle Cells. Mathematics. 2024; 12(19):2964. https://doi.org/10.3390/math12192964
Chicago/Turabian StyleZhou, Guanyu, Tatsuya Hayashi, and Tetsuji Tokihiro. 2024. "Stochastic Phase Model with Reflective Boundary and Induced Beating: An Approach for Cardiac Muscle Cells" Mathematics 12, no. 19: 2964. https://doi.org/10.3390/math12192964






