Synchronization in a Three Level Network of All-to-All Periodically Forced Hodgkin–Huxley Reaction–Diffusion Equations
Abstract
:1. Introduction
2. Forced ODE HH Equations
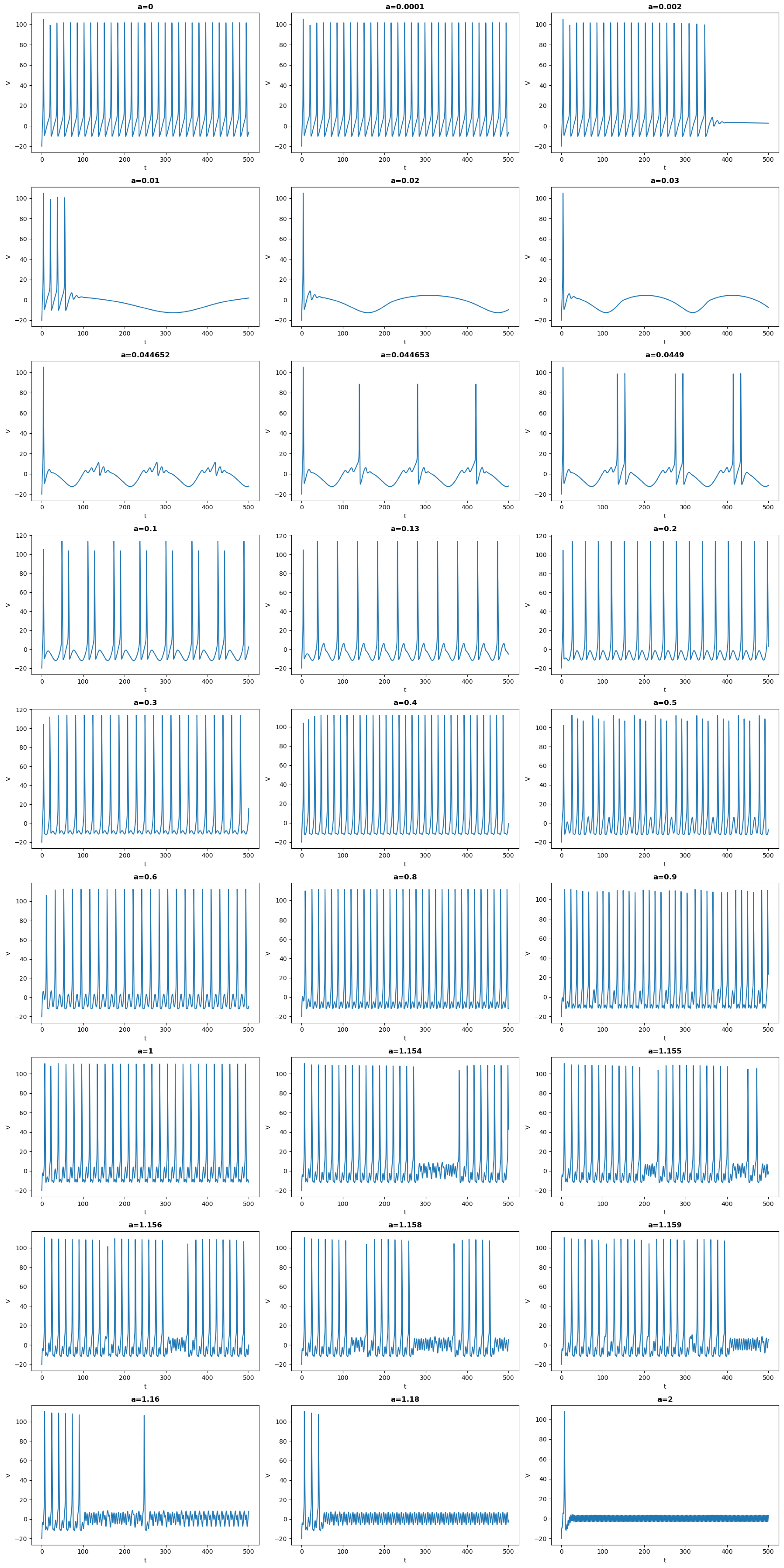
- When the frequency of the input is very slow (), then the behavior is akin to an autonomous HH with . For the initial conditions considered here, the system evolves toward a limit cycle and the frequency observed is intrinsic to the autonomous HH;
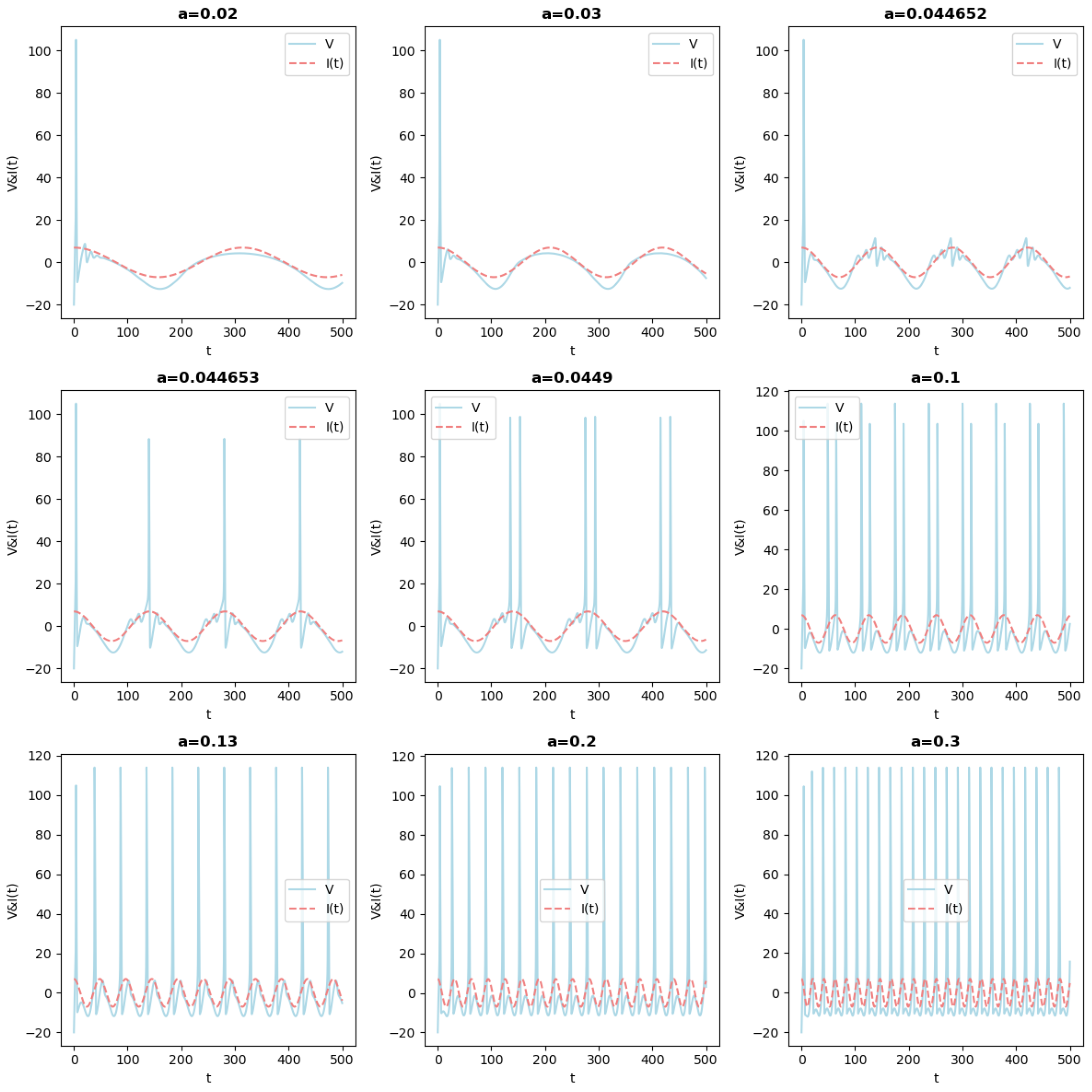
- When the frequency increases, for a range of , there are two frequencies that play a role. There is a recurrent pattern with a frequency of , which is imposed by , i.e., the time-periodicity of the global recurrent pattern is given by the periodicity of . Concurrently, within this period, the dynamics of HH appear. For example, for , the system stays at an equilibrium that varies with , but there is no spike. For other values, such as some spikes arise. For some values, one can observe the appearance of the so-called mixed-mode oscillations (MMOs), see, for example, references [13,14,15,16,17,18] and the references therein cited;
- It is worth emphasizing the qualitative difference between the output for and . Although the oscillatory frequency is the same, for , the oscillations correspond to the intrinsic frequency of the non-autonomous HH. In this case, one can clearly observe the characteristic difference of the trajectories in a slow manifold and a jump, see [19] and the references therein cited. For , however, the frequency is imposed by ;
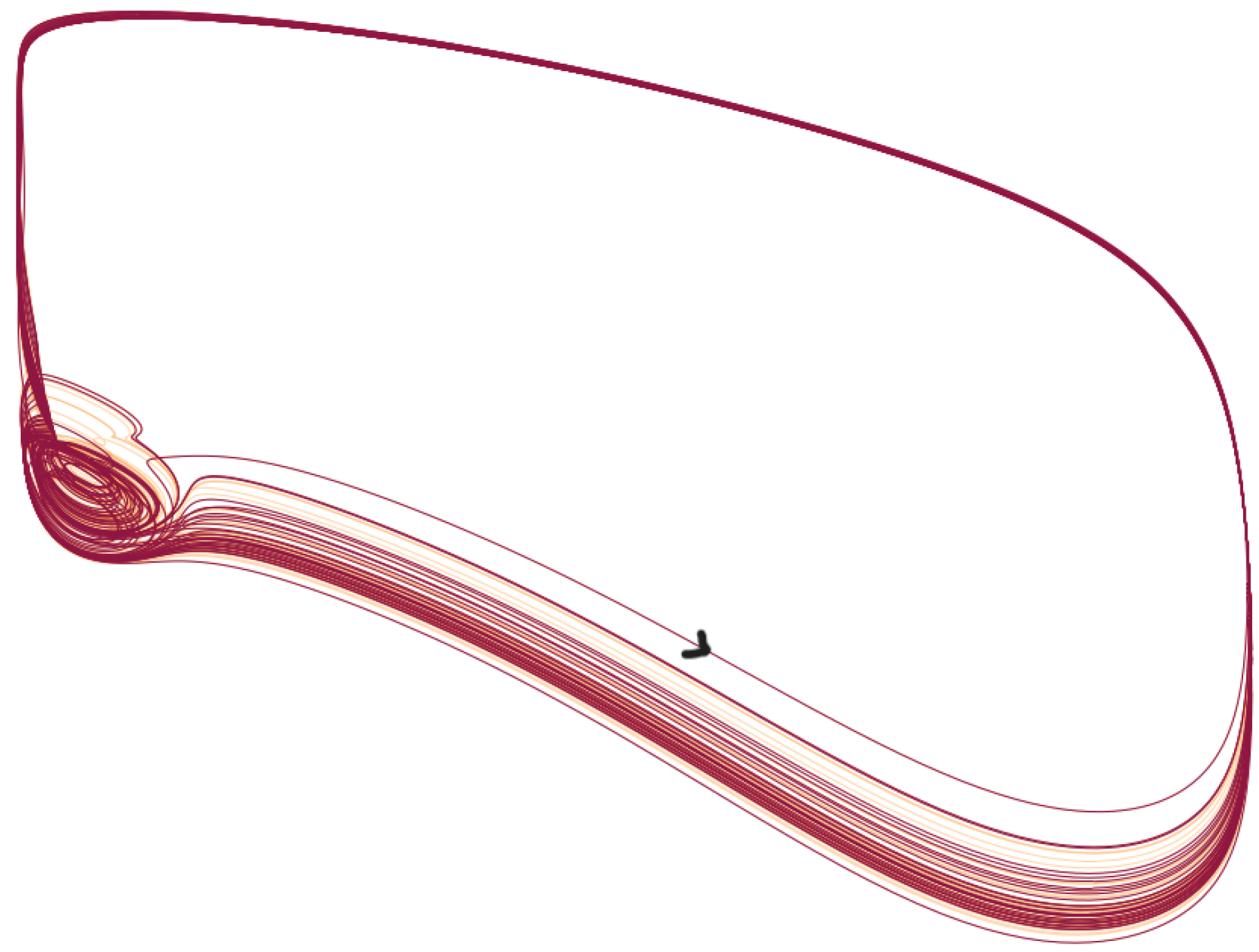
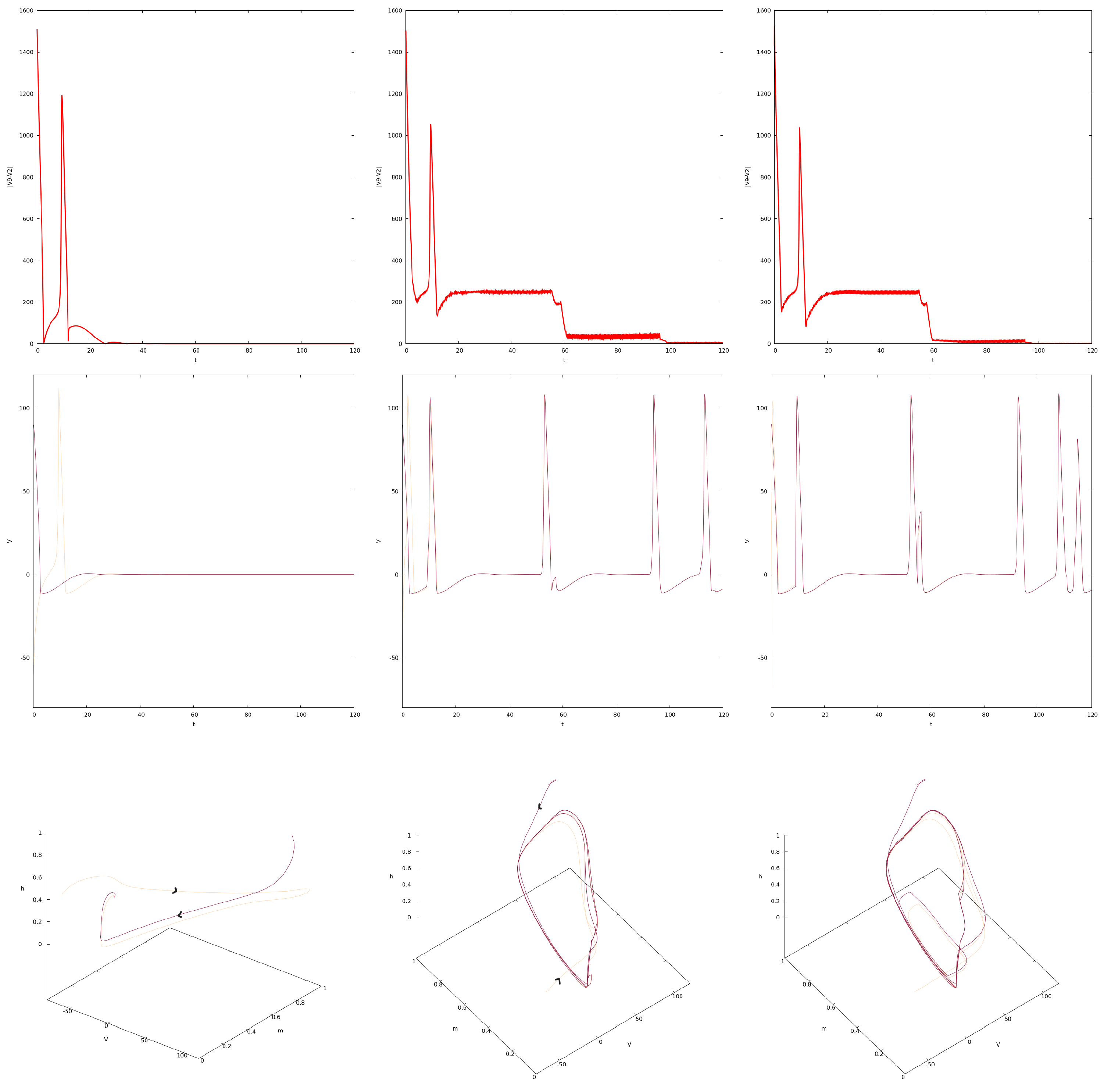
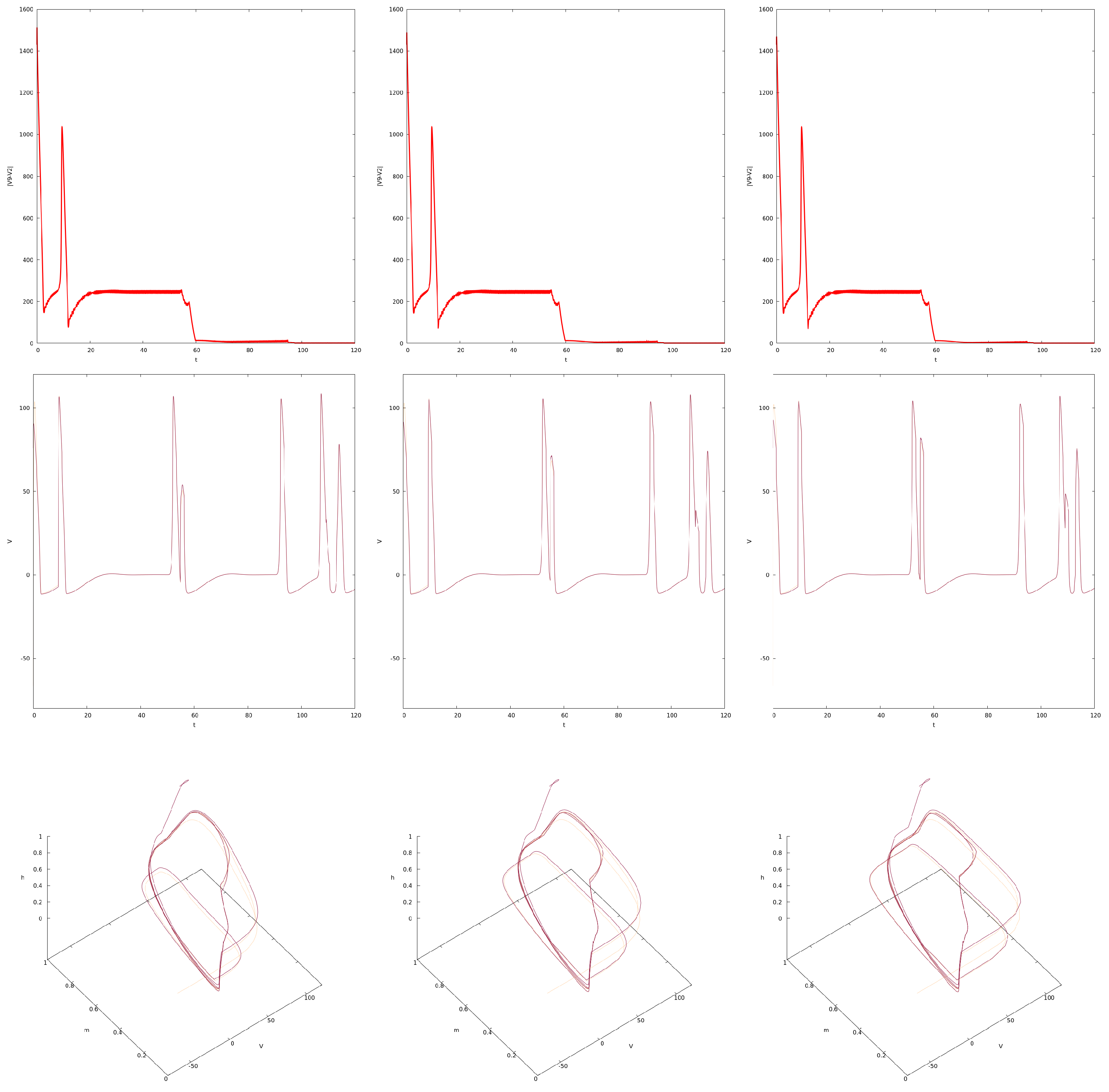
- After , the situation changes, and the period of becomes smaller than the one of the output, i.e., there is a periodic pattern, but its period results from a not straightforward interplay between the drive and the dynamics of HH. An analysis of such an interplay was carried out in [20] in the simpler case of a FitzHugh–Nagumo system that was kicked periodically. For some values of the frequency, the behavior is more complicated and difficult to predict. The neuron can, in this case, spike or not in an erratic way. This is the case, for example, for a = 1.156. See also Figure 4, in which the solution is represented in the phase space. In this case, the behavior is difficult to predict: the trajectories can switch between small and large oscillations in an unpredictable manner. This picture illustrates a geometry appearing in some slow–fast systems, in which the switching between the small and large oscillations occurs as canard solutions and in a tiny space region. We refer to [16,21] for such systems derivated from the FitzHugh–Nagumo system and with three time scales. Although there is no small parameter in the HH equation, its hidden slow–fast nature has been studied for a long time, see [5,22,23];
- When the frequency is too high, for example, for , only small oscillations persist.
3. Theoretical Framework and Analysis
- 1.
- there exists a unique solution of Equation (1) in .
- 2.
- For all , for all , and ,
- 3.
- .
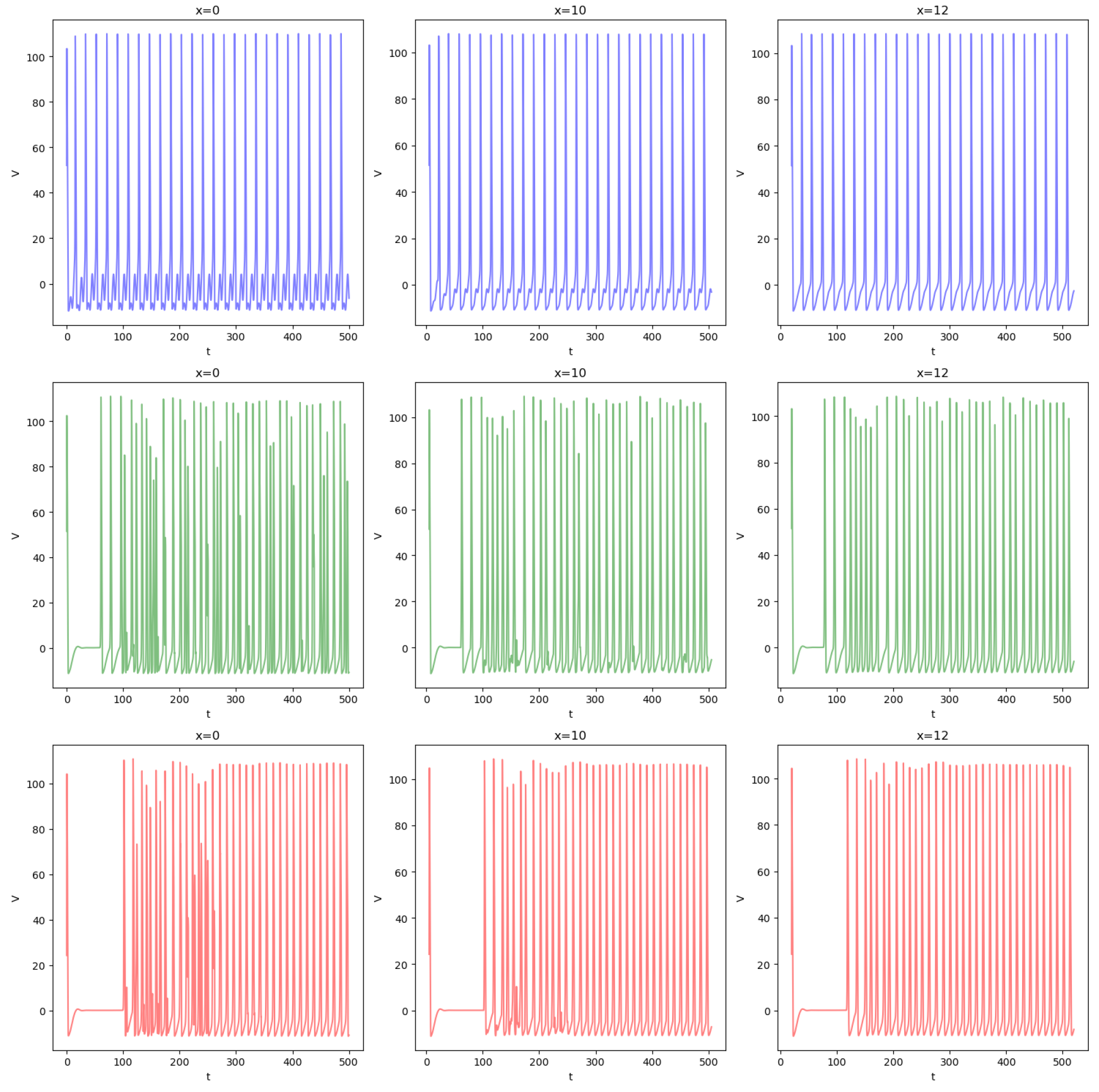
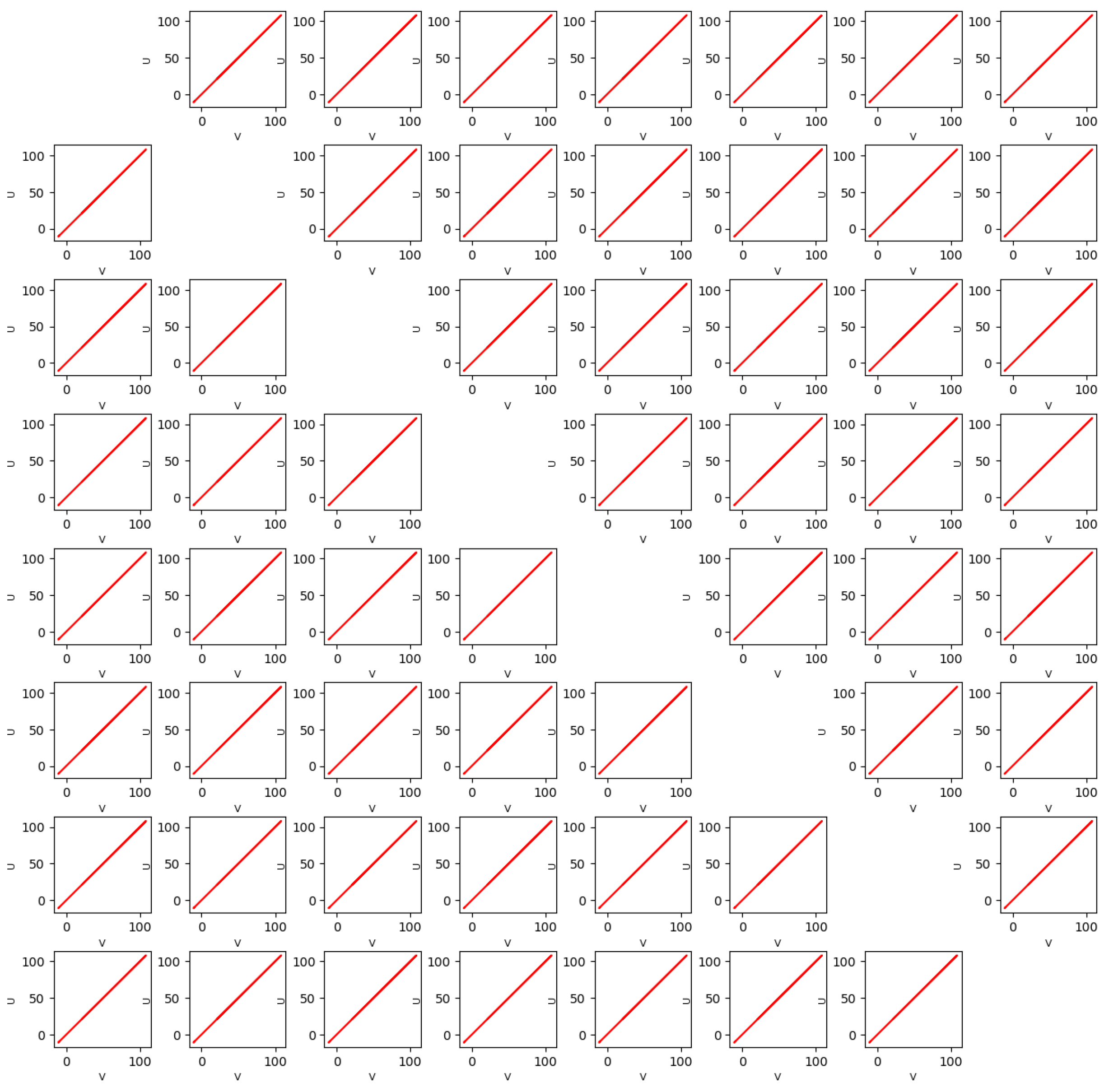
4. Synchronization in the Forced Network of PDE HH Equations
4.1. Synchronization for
4.2. Variation in c and Synchronization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Coombes, S.; Wedgwood, K.C.A. Neurodynamics, 1st ed.; Texts in applied mathematics; Springer International Publishing: Cham, Switzerland, 2023. [Google Scholar]
- Ermentrout, G.; Terman, D. Mathematical Foundations of Neuroscience; Interdisciplinary applied mathematics; Springer: New York, NY, USA, 2010. [Google Scholar]
- Ambrosio, B.; Aziz-Alaoui, M.A.; Balti, A. Propagation of bursting oscillations in coupled non-homogeneous Hodgkin–Huxley reaction–diffusion systems. Differ. Equ. Dyn. Syst. 2021, 29, 841–855. [Google Scholar] [CrossRef]
- Hodgkin, A.L.; Huxley, A.F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 1952, 117, 500–544. [Google Scholar] [CrossRef] [PubMed]
- Izhikevich, E.M. Dynamical Systems in Neuroscience: The Geometry of Excitability and Bursting (Computational Neuroscience); The MIT Press: Cambridge, MA, USA, 2006. [Google Scholar]
- Belykh, I.; de Lange, E.; Hasler, M. Synchronization of Bursting Neurons: What Matters in the Network Topology. Phys. Rev. Lett. 2005, 94, 188101. [Google Scholar] [CrossRef] [PubMed]
- Corson, N.; Aziz-Alaoui, M.A. Complex emergent properties in synchronized neuronal oscillations. In From System Complexity to Emergent Properties; Springer: Berlin/Heidelberg, Germany, 2009; pp. 243–259. [Google Scholar] [CrossRef]
- Wischnewski, M.; Alekseichuk, I.; Opitz, A. Neurocognitive, physiological, and biophysical effects of transcranial alternating current stimulation. Trends Cogn. Sci. 2023, 27, 189–205. [Google Scholar] [CrossRef] [PubMed]
- Saturnino, G.B.; Puonti, O.; Nielsen, J.D.; Antonenko, D.; Madsen, K.H.; Thielscher, A. SimNIBS 2.1: A Comprehensive Pipeline for Individualized Electric Field Modelling for Transcranial Brain Stimulation. In Brain and Human Body Modeling; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 3–25. [Google Scholar] [CrossRef]
- Volpert, V.; Xu, B.; Tchechmedjiev, A.; Harispe, S.; Aksenov, A.; Mesnildrey, Q.; Beuter, A. Characterization of spatiotemporal dynamics in EEG data during picture naming with optical flow patterns. Math. Biosci. Eng. 2023, 20, 11429–11463. [Google Scholar] [CrossRef] [PubMed]
- Volpert, V.; Sadaka, G.; Mesnildrey, Q.; Beuter, A. Modelling EEG Dynamics with Brain Sources. Symmetry 2024, 16, 189. [Google Scholar] [CrossRef]
- Rho, Y.A.; Sherfey, J.; Vijayan, S. Emotional Memory Processing during REM Sleep with Implications for Post-Traumatic Stress Disorder. J. Neurosci. 2023, 43, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio, B.; Françoise, J.P. Propagation of bursting oscillations. Philos. Trans. R. Soc. Math. Phys. Eng. Sci. 2009, 367, 4863–4875. [Google Scholar] [CrossRef]
- Ambrosio, B. Hopf Bifurcation in an Oscillatory-Excitable Reaction–Diffusion Model with Spatial Heterogeneity. Int. J. Bifurc. Chaos 2017, 27, 1750065. [Google Scholar] [CrossRef]
- Brøns, M.; Kaper, T.J.; Rotstein, H.G. Introduction to Focus Issue: Mixed Mode Oscillations: Experiment, Computation, and Analysis. Chaos Interdiscip. J. Nonlinear Sci. 2008, 18, 015101. [Google Scholar] [CrossRef]
- Krupa, M.; Popović, N.; Kopell, N. Mixed-Mode Oscillations in Three Time-Scale Systems: A Prototypical Example. SIAM J. Appl. Dyn. Syst. 2008, 7, 361–420. [Google Scholar] [CrossRef]
- Krupa, M.; Ambrosio, B.; Aziz-Alaoui, M.A. Weakly coupled two-slow–two-fast systems, folded singularities and mixed mode oscillations. Nonlinearity 2014, 27, 1555–1574. [Google Scholar] [CrossRef]
- Rubin, J.; Wechselberger, M. The selection of mixed-mode oscillations in a Hodgkin-Huxley model with multiple timescales. Chaos Interdiscip. J. Nonlinear Sci. 2008, 18, 015105. [Google Scholar] [CrossRef]
- Kuehn, C. Multiple Time Scale Dynamics; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Ambrosio, B.; Mintchev, S.M. Periodically kicked feedforward chains of simple excitable FitzHugh–Nagumo neurons. Nonlinear Dyn. 2022, 110, 2805–2829. [Google Scholar] [CrossRef]
- Ambrosio, B.; Aziz-Alaoui, M.A.; Mondal, A.; Mondal, A.; Sharma, S.K.; Upadhyay, R.K. Non-Trivial Dynamics in the FizHugh–Rinzel Model and Non-Homogeneous Oscillatory-Excitable Reaction-Diffusions Systems. Biology 2023, 12, 918. [Google Scholar] [CrossRef] [PubMed]
- Rubin, J.; Wechselberger, M. Giant squid-hidden canard: The 3D geometry of the Hodgkin–Huxley model. Biol. Cybern. 2007, 97, 5–32. [Google Scholar] [CrossRef] [PubMed]
- Maama, M.; Ambrosio, B.; Aziz-Alaoui, M.; Mintchev, S.M. Emergent properties in a V1-inspired network of Hodgkin–Huxley neurons. Math. Model. Nat. Phenom. 2024, 19, 3. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambrosio, B.; Aziz-Alaoui, M.A.; Oujbara, A. Synchronization in a Three Level Network of All-to-All Periodically Forced Hodgkin–Huxley Reaction–Diffusion Equations. Mathematics 2024, 12, 1382. https://doi.org/10.3390/math12091382
Ambrosio B, Aziz-Alaoui MA, Oujbara A. Synchronization in a Three Level Network of All-to-All Periodically Forced Hodgkin–Huxley Reaction–Diffusion Equations. Mathematics. 2024; 12(9):1382. https://doi.org/10.3390/math12091382
Chicago/Turabian StyleAmbrosio, B., M. A. Aziz-Alaoui, and A. Oujbara. 2024. "Synchronization in a Three Level Network of All-to-All Periodically Forced Hodgkin–Huxley Reaction–Diffusion Equations" Mathematics 12, no. 9: 1382. https://doi.org/10.3390/math12091382






