3.2. C-FACS
A chemometrics FACS (c-FACS) method is developed and applied to differentiate between bovine, porcine and fish gelatin spectra. The method involves the representation of the FTIR spectra in the form of a FACS graph and its adjacency matrix for further analysis to identify the dominant output.
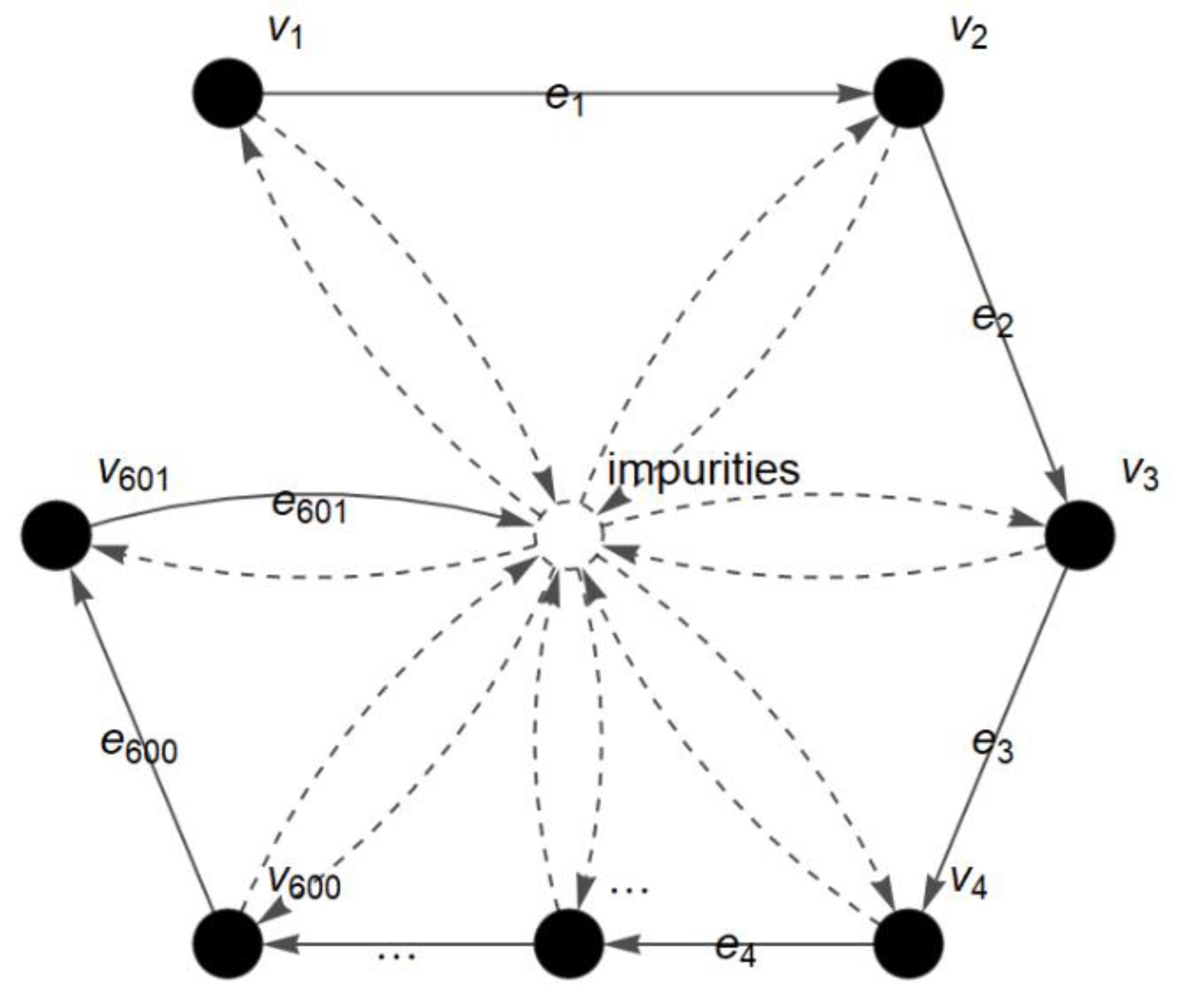
The process of infrared absorption by the gelatin molecule during FTIR analysis is represented in the form of vertices and edges. Each vertex in
represents the wavenumber (cm
−1) and an edge in
is the transitional path of a given molecule throughout the FTIR analysis (see
Figure 2).
In short, the FACS graph describes the flow process and movement of a molecule from one wavenumber of light to the next wavenumber during the FTIR analysis. The detected amount of infrared radiation absorbed by the molecule at each wavenumber (cm
−1) is represented as the absorbance value (A) and plotted in the FTIR spectrum [
26]. In addition, the presence of impurities during the analysis is also considered in the FACS graph. The obtained graph is then converted into a matrix for further analysis. The entries of the matrix are the membership values,
of the absorbance readings (
) at each wavenumber. The
is called fuzzy absorbance value and defined by:
The fuzzy absorbance value is substituted as an entry for the FTIR matrix. Further descriptions of the FTIR matrix are provided by the following definitions.
Definition 7. A square matrixis called a FTIR matrix such thatis the membership value of the absorbance reading,forand the dimension of matrix A is defined as follows:In other words, whenever, thenand whenever, then Definition 8. If the number of data isandis not a square matrix (andwithand), then the entriesand they are named as the ghost entries.
The FTIR matrices are further analyzed using graph dynamic procedures by Ahmad et al. [
11] to determine their dominances. The procedure then computes the eigenvalues and Perron Frobenius eigenvectors. Several new definitions and theorems are introduced to formalize the procedure. A reverse transformation matrix is defined since the FTIR spectra are conventionally presented in descending order of wavenumbers. Thus, the reverse transformation matrix of FACS is needed to show the equivalence of adjacency matrix and its reverse matrix. The new definitions and theorems for the transformation matrix are introduced as follows.
Definition 9. The row swap,is an operation whereby two rows in the matrix together with their entries are exchanged in their positions such that In short,
Replace row 1 with row n,
Replace row 2 with row n−1,
Replace row 3 with row n−2,
Replace row n−1 with row 2,
Replace row n with row 1.
Definition 10. The column swap,, is an operation whereby two columns in the matrix together with their entries are exchanged in their positions such that In short,
Replace column 1 with column n,
Replace column 2 with column n−1,
Replace column 3 with column n−2,
Replace column n−1 with column 2,
Replace column n with column 1.
Definition 11. Thetransformation of matrixtois a composition of two operations.wherebyis transformed to. In short, thetransformation is an operation to switch position of rows to columns simultaneously. The row swap, , and column swap, , operations are stated as mappings in the following two lemmas without proofs, respectively.
Lemma 1. Letbe a square matrix. Theis a mapping such thatandThe mappingis called row swapping; i.e.,.
Further properties on the row swapping are listed in the following theorems. Their proofs are also omitted since trivial.
Theorem 1. The row swappingis a function.
Theorem 2. The row swappingis linear.
Lemma 2. Letbe a square matrix.is a mapping such thatandThe mappingis called column swapping; i.e.,.
Further properties on the column swapping are presented without proofs as follows.
Theorem 3. The column swappingis a function.
Theorem 4. The column swappingis linear.
Theorems 2 and 4 lead to the following result.
Theorem 5. Thetransformation is linear.
Next, based on Definition 11, Lemmas 1 and 2, transformation of FACS to its reverse is stated in the following corollary.
Corollary 1. Letbe squareFACS matrix. The composition of linear transformationsyields the transformation ofto its reverse matrix,, such that.
The transformation of the FACS matrix, yields the reverse FACS matrix, . The relation for both matrices is presented in the following theorem.
Theorem 6. The eigenvalues of the FACS matrix are the same as the eigenvalues of its reverse.
Proof. By Corollary 1, the reverse of matrix FACS is .
Then, the characteristic equation of , is
by Corollary 1 and
by scalar multiplication of a matrix
by distributive property of matrices
by property of determinant,
commutativity for scalar multiplication
by property of determinant,
since
since .
Hence, characteristic equation for is identical to . In other words, the eigenvalues of matrix FACS and its reverse matrix are identical. □
Since , then is equivalent to . By Theorem 6, the matrix of FACS and its reverse produce the same characteristic equation; hence, its respective eigenvalues and eigenvectors are similar. Consequently, using the FACS graph dynamic procedure, it surely will produce the same output. In this paper, the reverse matrix is denoted as FTIR matrix, otherwise stated, and is solved using the modified FACS graphing dynamic procedure.
A new algorithm involving the FTIR matrix is constructed to identify the dominant matrix. The algorithm contains the FACS graph dynamic and coordinated FACS procedures. The algorithm is specifically designed in order to facilitate the chemometrics analysis involving FTIR spectroscopy and is called the chemometrics FACS (c-FACS) algorithm. The c-FACS algorithm is presented in Algorithm 1.
| Algorithm 1: The c-FACS algorithm |
Select the preferred variables from a system as inputs. Build FACS graph by representing the variables as vertices, and their links as edges, . Describe the membership value of the FACS graph. Determine the adjacency matrix of the graph with the membership values as the entries. For FTIR matrix, the entries or membership values are described by the fuzzy absorbance values at each wavenumber. Identify the Perron Frobenius eigenvectors (PFE) of the matrix. The smallest PFE and its corresponding vertices and edges are removed from the system to form matrix. Display the dominant output matrix together with its corresponding dominant variables, until matrix is obtained. Calculate the transition and Laplacian matrices to obtain the xy-coordinates. The y-coordinates of FACS are obtained by solving Ly = b using conjugate gradient method and the x-coordinates are determined using the Fiedler vector of Laplacian. Plot the obtained coordinates in the Euclidean space. Repeat step 1 to 9 to analyze another sample or system.
|
Step 2 and 9 have complexity of O(n). Step 4 has complexity of O(n2) and for Step 8, the overall computation takes O(n3). The others have O(1) complexities. The algorithm is used to analyze the FACS matrix of the FTIR gelatin spectra for selected regions to determine the dominant wavenumbers of each gelatin and the patterns of their nodes in Euclidean space. The implementation and analysis on gelatin using c-FACS are presented in the next subsection.
3.3. C-FACS Implementation and Results
The FTIR spectra of bovine, porcine and fish gelatins are analyzed using the c-FACS algorithm to identify their dominant wavenumbers and signatures. The gelatin spectra at Amide and 1600–1000 cm
−1 (fingerprint) wavenumber regions are represented in the form of a FACS graph and analyzed as described in the c-FACS algorithm. The FACS graph for spectra at fingerprint region that contains 601 wavenumbers (1600–1000 cm
−1) is shown in
Figure 3 with the set of vertices
and the set of edges
. The vertices in the graph represent the wavenumbers and the absorbance at each of the wavenumbers is the membership value of the vertex.
The graph is then converted into the FTIR matrix with dimension of
to illustrate all 601 entries or absorbance data in the fingerprint region and further analyzed to obtain the dominant matrix. The differences between the gelatins are observed based on their dominant obtained output matrices. The dominant output matrix represents the dominant wavenumbers of each gelatin at Amide II, Amide III and fingerprint regions. The dominant matrices of each gelatin at the fingerprint region are listed in
Table 1 and the overall results are presented in
Table 2.
At the fingerprint region, the differences between the gelatins are observed from the output matrix (
) such that the dominant wavenumbers for bovine, porcine and fish gelatins are identified at 1475–1470 cm
−1, 1450–1444 cm
−1 and 1500–1496 cm
−1, respectively. At the Amide II region, the dominant wavenumbers for bovine gelatin are in the range of 1480–1473 cm
−1, while dominant wavenumbers for porcine and fish gelatins are detected at 1448–1441 cm
−1 and 1496–1490 cm
−1, respectively. Dominant wavenumbers for the gelatins at the Amide III region are observed at 810–786 cm
−1, 1232–1228 cm
−1 and 1252–1249 cm
−1 for bovine, 680–678 cm
−1, 1077–1071 cm
−1 and 1096–1087 cm
−1 for porcine, and 844–837 cm
−1, 862–853 cm
−1 and 1303–1280 cm
−1 for fish gelatin. These results showed that each gelatin possesses distinct and unique dominant wavenumbers, particularly at the fingerprint and Amide II regions. Further analysis using coordinated FACS is performed to examine the pattern and signature of bovine, porcine and fish gelatins at the fingerprint region and displayed in Euclidean space. The transformation procedure involves the construction of transition and combinatorial Laplacian matrices based on Equations (1) and (2) to determine the
x-coordinates followed by solving the optimal arrangement
y* using conjugate gradient method to compute for y-coordinates. The
x-coordinates of the nodes of FACS are determined by identifying the Fiedler vector that is the eigenvector associated with the smallest positive eigenvalue of the Laplacian matrix. The Fiedler vector is obtained by solving the eigenvalue problem and the optimal arrangement
y* representing the y-coordinates are determined using the conjugate gradient method. All computations are performed using MATLAB. The coordinated FACS of the gelatins exhibits distinct patterns for bovine, porcine and fish (see
Figure 4).
Based on the figure, distinct patterns for bovine, porcine and fish gelatin are observed, particularly on the right area. The nodes of each gelatin are dispersed at different locations; particularly nodes for porcine gelatin are clearly separated and located a distance away from nodes of bovine and fish gelatins. The result indicates that porcine gelatin has a unique feature as compared to other gelatins since it is at farthest distance, while bovine and fish are closer to each other. The information indicates that the distance signifies the relationship and characteristic between the variables. The c-FACS method has successfully identified the differences between the gelatins, particularly non-halal porcine, based on the dominant wavenumbers’ identification and location of their nodes in the Euclidean space. The unique and different dominant wavenumbers signify the feature for each gelatin. The performance of c-FACS is then compared against principal component analysis (PCA). The PCA is a well-established method in chemometrics analysis. It is usually implemented with the aid of MATLAB and Minitab software [
17,
18], and the results are displayed in the form of score and loading plots. The clusters of bovine, porcine and fish gelatins are observed in the score plot. However, significant wavenumbers for porcine and fish gelatins failed to be identified in the loading plot (see
Figure 5).
The performances of c-FACS and PCA are summarized in
Table 3. The c-FACS is found to be faster, with elapsed time of 7.86 s than PCA (12.86 s) for analysis involving bovine, porcine and fish gelatin samples at the fingerprint region. Furthermore, the c-FACS was able to identify a particular range of wavenumbers as signature for each gelatin, while PCA failed to identify the corresponding wavenumbers for porcine and fish. Nevertheless, distinct classifications for the gelatins were observed using both methods, particularly porcine gelatin by c-FACS.
C-FACS on Published Data
The c-FACS method is applied on some published data to demonstrate its capability and efficiency. Several published data involving food products are analyzed using c-FACS and compared against PCA used by the respective researchers.
The first analysis involves a dataset of coffee products that is adopted from Briandet et al. [
27]. The two types of their coffee products are Arabica and Robusta. The researchers [
27] differentiated the two types of coffee products using FTIR and PCA. The FTIR spectra of 29 samples of Arabica and 27 samples of Robusta were obtained and further analyzed using PCA in their study. However, in this paper, the spectra of Arabica and Robusta at the 1600–1000 cm
−1 region are analyzed using c-FACS. The FACS graph of the coffee at the 1600–1000 cm
−1 region is shown in
Figure 6. The vertices
represent the position of wavenumbers for 1600 to 1000 cm
−1 and the edges
are the light that travels and passes through molecules during the FTIR analysis.
The graph is then converted into a matrix to determine its dominance and pattern in the Euclidean space. As a result, the dominant wavenumbers for Arabica and Robusta are identified and presented in
Table 4.
The dominant wavenumbers for Arabica are identified at 1447–1409 cm
−1 and 1096–1058 cm
−1. On the other hand, the dominant wavenumbers for Robusta are detected at 1297–1258 cm
−1. These results indicate that each type of coffee bean has distinct and unique dominant wavenumbers. Furthermore, coordinated FACS shows that the nodes for Arabica are mainly clustered in the right area while Robusta’s nodes are mainly observed in the left area of the plane (see
Figure 7).
The results of the c-FACS are compared against PCA. The PCA score plot of the coffee shows that Arabica is clustered mainly in the upper right area, while Robusta is clustered in the left area (see
Figure 8).
The PCA results by Briandet et al. [
27] also showed distinct clusters of Arabica and Robusta coffee beans with respect to second and third component scores. However, their PCA method failed to determine their significant wavenumbers. According to Briandet et al. [
27], Robusta coffee has higher content of caffeine and chlorogenic acid as compared to Arabica, and the bands that associated with caffeine and chlorogenic acid are in the range of 1750–1550 cm
−1 and 1300–1150 cm
−1 regions, respectively. Thus, the dominant wavenumber results obtained from c-FACS for Robusta at 1297–1258 cm
−1 were in agreement with the range of chlorogenic acid at 1300–1150 cm
−1.
The c-FACS method is also applied on a dataset of meat, which is obtained from Al–Jowder et al. [
28]. Three different types of meats, namely, chicken, pork and turkey, were used in their study. The spectra of the meats were obtained and PCA was used by the researchers [
28]. In this paper, the spectra of the samples at 1600–1000 cm
−1 region are analyzed using c-FACS. Its graph is shown in
Figure 9.
The vertices,
, are the wavenumbers and the edges,
, are the light that travels and passes through the molecules. The graph is then converted into a matrix to identify its dominance and pattern in the Euclidean space. As a result, the dominant wavenumbers for chicken, pork and turkey are identified and presented in
Table 5.
The dominant wavenumbers for pork are clearly identified at 1042–1071 cm−1, and dominant wavenumbers for both chicken and turkey are identified at 1409–1418 cm−1. In addition, the dominant wavenumbers for pork are also detected at 1005–1036 cm−1, and turkey showed dominance at 1216–1245 cm−1 in dominant matrix.
Furthermore, coordinated FACS showed distinct locations of nodes for each meat, particularly pork (see
Figure 10).
The results of the c-FACS are compared against PCA. The PCA score plot shows that the meats are clustered at different locations (see
Figure 11), which are in agreement with the report published by Al-Jowder et al. [
28].
However, their PCA method failed to determine the significant wavenumbers for the meats. In addition, the samples for chicken and turkey are often grouped in the same area and overlapped with each other in PCA score plot [
28,
29], hence justify the similarity in dominant wavenumbers obtained for the two meats when analyzed using c-FACS. Furthermore, the dominant wavenumbers for pork at 1005–1071 cm
−1 obtained using c-FACS is in agreement to the dominant wavenumbers of porcine gelatin listed in
Table 2 earlier. Therefore, it provides some sort of signature for pig derivative in related food products.
The c-FACS and PCA are two different chemometrics methods that have different approaches in dealing with the dataset. The c-FACS is a fuzzy graph-based method that converts a system to a graph with fuzzy vertices and edges, and PCA is a statistical method that transforms variables to components called principal components. The results for c-FACS are presented in the form of dominant matrices and coordinated nodes, while PCA displayed its result in the form of a score plot. The PCA method has been around for chemometrics analyses, particularly in food authentication. In this paper, a novel fuzzy graph-based chemometrics method is introduced and applied for gelatin authentication and the results show superior performance compared to PCA, with respect to computing time and identification of significant features. Every sample or system possesses some differences [
30]. The c-FACS is efficient in determining the signature and feature of samples via their dominant wavenumbers and nodes pattern in Euclidean space. The unique feature of the sample with respect to the dominant wavenumbers signifies the identity of the sample. The c-FACS method is able to extract the most dominant and signature features of each sample through graph representation and eigen-analysis of its matrix. Thus, c-FACS holds promise as the superior method for authentication purposes.