Extension of SEIR Compartmental Models for Constructive Lyapunov Control of COVID-19 and Analysis in Terms of Practical Stability
Abstract
:1. Introduction
1.1. Historical Development of Compartmental Models for Epidemics
1.2. Motivation
1.3. Literature Review
1.4. Structure of the Paper
2. Methodology
2.1. Generalized SEIR Model
- : The number of susceptible individuals who could be potentially subjected to the infection;
- : The number of exposed hosts in the latent state, modeled to be not yet infectious for this model variant;
- : The number of infected individuals after the latent period with symptoms;
- : The number of removed individuals, either recovered or dead;
- : The infection rate, at which susceptible individuals get infected each day. quantifies how many people are transitioned from to due to contact with . Since this parameter should not depend on the population size, ratio is used in the model, so has the same physical dimension as the other transition rates.
- : Inverse of the average duration of the latent state, which turns the exposed into the infected;
- : The recovery rate, at which infected individuals recover ( is the average recovery time);
- : The death rate, at which infected individuals die ( being the average death time);
- : Vaccination control, showing the number of susceptible individuals vaccinated per day (represented using negative signal values);
- : Quarantine control, showing the number of susceptible individuals quarantined per day (represented using positive signal values).
2.2. Analysis and Control of the Generalized SEIR Model
2.3. Advanced SPEIQRD Model
- : The number of exposed hosts in the latent state, modeled to already be infectious for this model variant;
- : The number of protected individuals who have been vaccinated;
- : The number of quarantined individuals who get quarantined when they are infectious;
- : The number of recovered individuals;
- : The number of dead individuals;
- : The protection rate, at which susceptible individuals get vaccinated;
- : The recurrence rate, at which recovered people become susceptible again. Since the probability is tiny, we assume this parameter to be zero;
- : The quarantine rate, at which infected people get quarantined;
- : The recovery rate, at which quarantined individuals recover;
- : At this recovery rate, those infected individuals recover before quarantine. We assume the parameter to be zero since the probability is much smaller than ;
- : The death rate, at which quarantined individuals die;
- : At this death rate, those infected individuals die before quarantine. We assume the parameter also to be zero since the probability is much smaller than .
2.4. Analysis and Control of the Advanced SPEIQRD Model
3. Model Validation
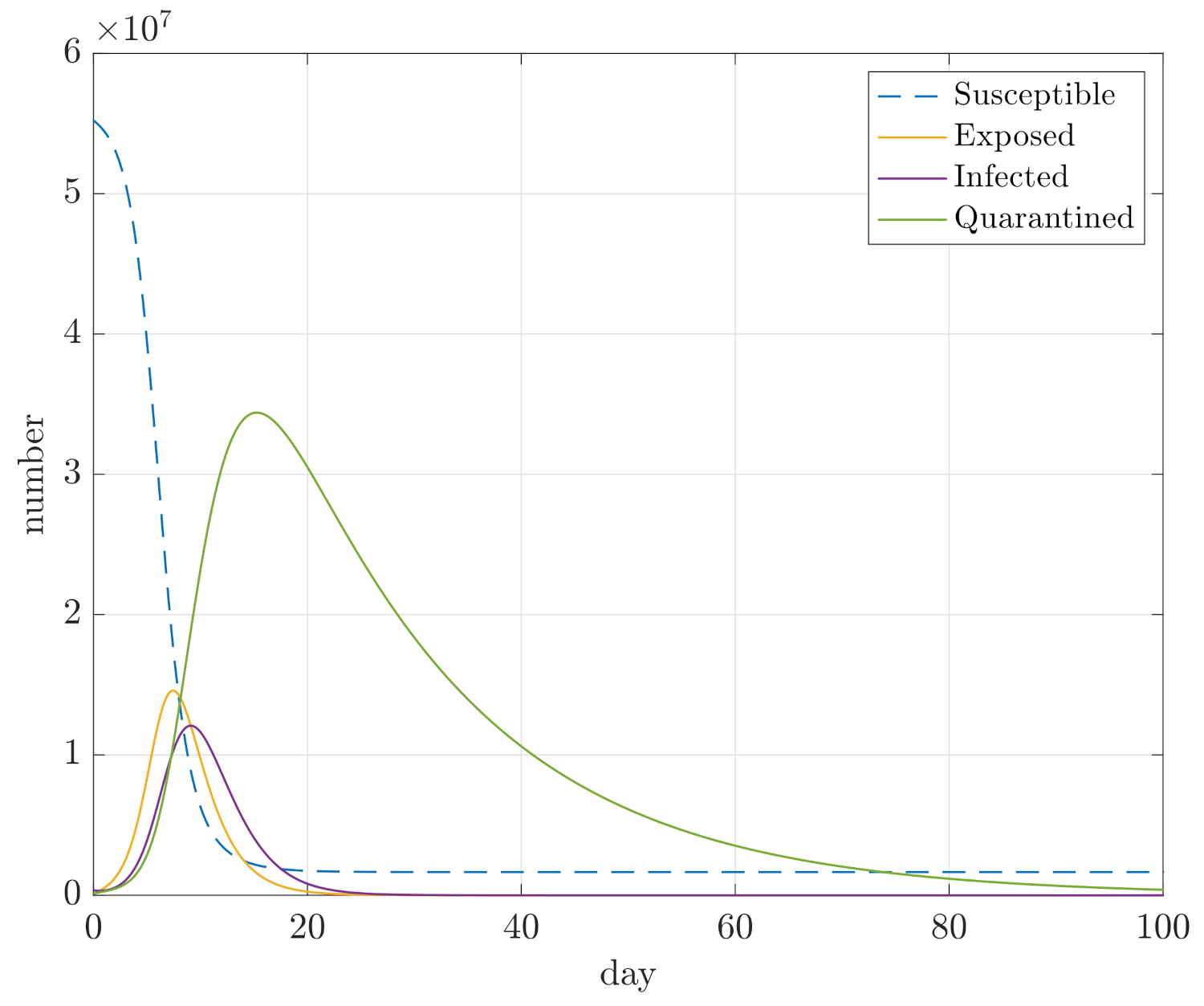
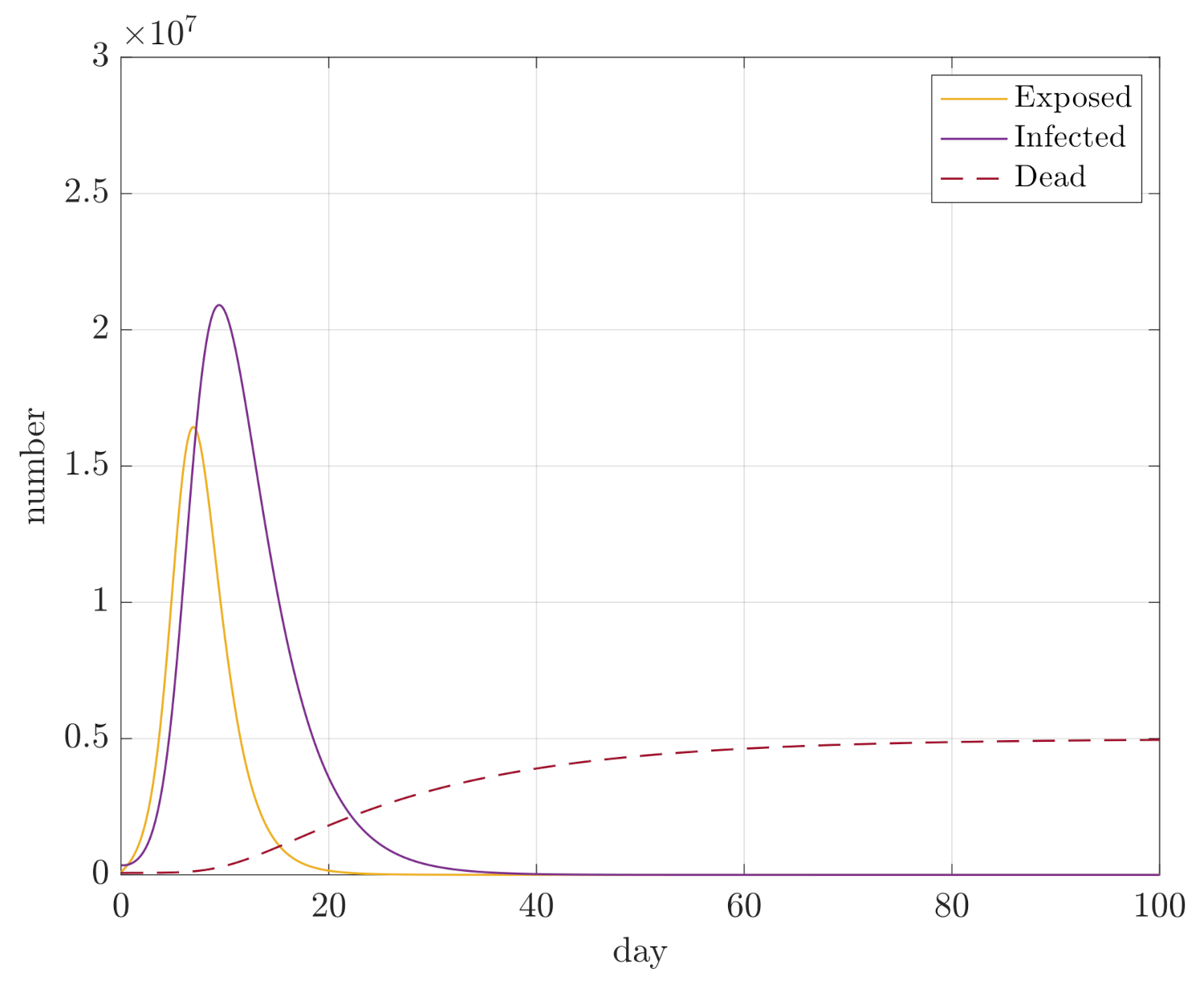
4. Simulation Results
- Maximum sizes of compartments D, E, and I;
- Integral of E and I for the duration of the simulation, so and , in order to assess how quickly the compartments converge.
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bernoulli, D. Réflexions sur les avantages de l’inoculation. Mercur. Fr. 1760, 173–190. [Google Scholar] [CrossRef]
- Bernoulli, D. Essai d’une nouvelle analyse de la mortalite causee par la petite vérole. In Mémoires de Mathématique et de Physique, Presentés à l’Académie Royale des Sciences, par Divers Sçavans & lûs dans ses Assemblées; Complutense University of Madrid: Madrid, Spain, 1760–1766; pp. 1–45. [Google Scholar]
- Alembert, J.D. Onzième mémoire, Sur l’application du calcul des probabilités à l’inoculation de la petite vérole. In Opuscules Mathématiques, Tome Second; David: Paris, France, 1761; pp. 26–95. [Google Scholar]
- Duvillard, E. Analyse et tableaux de l’influence de la petite vérole sur la mortalité à chaque age; Impr. ImpÉRiale: Paris, France, 1806. [Google Scholar]
- Kermack, W.O.; McKendrick, A.G. A contribution to the mathematical theory of epidemics. Proc. R. Soc. London. Ser. A 1927, 115, 700–721. [Google Scholar] [CrossRef] [Green Version]
- Lotka, A. Elements of Physical Biology; Williams and Wilking Company: Baltimora, MD, USA, 1925. [Google Scholar]
- Volterra, V. Variazioni e fluttuazioni del numero d’individui in specie animali conviventi. In Memoria della Reale Accademia Nazionale dei Lincei; Accademia Nazionale dei Lincei: Rome, Italy, 1926; pp. 31–113. [Google Scholar]
- Bahloul, M.A.; Chahid, A.; Laleg-Kirati, T.M. Fractional-Order SEIQRDP Model for Simulating the Dynamics of COVID-19 Epidemic. IEEE Open J. Eng. Med. Biol. 2020, 1, 249–256. [Google Scholar] [CrossRef]
- Postnikov, E.B. Estimation of COVID-19 dynamics “on a back-of-envelope”: Does the simplest SIR model provide quantitative parameters and predictions? Chaos Solitons Fractals 2020, 135, 109841. [Google Scholar] [CrossRef]
- Wan, H.; Cui, J.A.; Yang, G.J. Risk estimation and prediction of the transmission of coronavirus disease-2019 (COVID-19) in the mainland of China excluding Hubei province. Infect. Dis. Poverty 2020, 9, 116. [Google Scholar] [CrossRef]
- Zu, J.; Li, M.L.; Li, Z.F.; Shen, M.W.; Xiao, Y.N.; Ji, F.P. Transmission patterns of COVID-19 in the mainland of China and the efficacy of different control strategies: A data- and model-driven study. Infect. Dis. Poverty 2020, 9, 83. [Google Scholar] [CrossRef]
- Haus, B.; Mercorelli, P.; Aschemann, H. Gain Adaptation in Sliding Mode Control Using Model Predictive Control and Disturbance Compensation with Application to Actuators. Information 2019, 10, 182. [Google Scholar] [CrossRef] [Green Version]
- Aschemann, H.; Haus, B.; Mercorelli, P. Second-Order SMC with Disturbance Compensation for Robust Tracking Control in PMSM Applications. IFAC-PapersOnLine 2020, 53, 6225–6231. [Google Scholar] [CrossRef]
- Lakshmikantham, V.; Martyniuk, A.A.; Leela, S. Practical Stability of Nonlinear Systems; World Scientific Pub. Co.: Singapore, 1990. [Google Scholar]
- Moreau, L.; Aeyels, D. Practical stability and stabilization. IEEE Trans. Autom. Control 2000, 45, 1554–1558. [Google Scholar] [CrossRef]
- Thompson, R.N. Epidemiological models are important tools for guiding COVID-19 interventions. BMC Med. 2020, 18. [Google Scholar] [CrossRef]
- Tenreiro Machado, J.A. An Evolutionary Perspective of Virus Propagation. Mathematics 2020, 8, 779. [Google Scholar] [CrossRef]
- Avram, F.; Adenane, R.; Ketcheson, D.I. A Review of Matrix SIR Arino Epidemic Models. Mathematics 2021, 9. [Google Scholar] [CrossRef]
- Mwalili, S.; Kimathi, M.; Ojiambo, V.; Gathungu, D.; Mbogo, R. SEIR model for COVID-19 dynamics incorporating the environment and social distancing. BMC Res. Notes 2020, 13. [Google Scholar] [CrossRef] [PubMed]
- Gosak, M.; Duh, M.; Markovič, R.; Perc, M. Community lockdowns in social networks hardly mitigate epidemic spreading. New J. Phys. 2021, 23, 043039. [Google Scholar] [CrossRef]
- Batista, B.; Dickenson, D.; Gurski, K.; Kebe, M.; Rankin, N. Minimizing disease spread on a quarantined cruise ship: A model of COVID-19 with asymptomatic infections. Math. Biosci. 2020, 329, 108442. [Google Scholar] [CrossRef]
- Das, A.; Dhar, A.; Goyal, S.; Kundu, A.; Pandey, S. COVID-19: Analytic results for a modified SEIR model and comparison of different intervention strategies. Chaos Solitons Fractals 2021, 144, 110595. [Google Scholar] [CrossRef] [PubMed]
- Aronna, M.; Guglielmi, R.; Moschen, L. A model for COVID-19 with isolation, quarantine and testing as control measures. Epidemics 2021, 34, 100437. [Google Scholar] [CrossRef]
- Roda, W.C.; Varughese, M.B.; Han, D.; Li, M.Y. Why is it difficult to accurately predict the COVID-19 epidemic? Infect. Dis. Model. 2020, 5, 271–281. [Google Scholar] [CrossRef]
- Zhu, H.; Li, Y.; Jin, X.; Huang, J.; Liu, X.; Qian, Y.; Tan, J. Transmission dynamics and control methodology of COVID-19: A modeling study. Appl. Math. Model. 2021, 89, 1983–1998. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, X.; Sun, H.; Tick, G.R.; Wei, W.; Jin, B. Applicability of time fractional derivative models for simulating the dynamics and mitigation scenarios of COVID-19. Chaos Solitons Fractals 2020, 138, 109959. [Google Scholar] [CrossRef]
- Omar, O.A.; Elbarkouky, R.A.; Ahmed, H.M. Fractional stochastic models for COVID-19: Case study of Egypt. Results Phys. 2021, 23, 104018. [Google Scholar] [CrossRef]
- Noeiaghdam, S.; Micula, S.; Nieto, J.J. A Novel Technique to Control the Accuracy of a Nonlinear Fractional Order Model of COVID-19: Application of the CESTAC Method and the CADNA Library. Mathematics 2021, 9, 1321. [Google Scholar] [CrossRef]
- Dong, N.P.; Long, H.V.; Khastan, A. Optimal control of a fractional order model for granular SEIR epidemic with uncertainty. Commun. Nonlinear Sci. Numer. Simul. 2020, 88, 105312. [Google Scholar] [CrossRef]
- Razzaq, O.A.; Rehman, D.U.; Khan, N.A.; Ahmadian, A.; Ferrara, M. Optimal surveillance mitigation of COVID-19 disease outbreak: Fractional order optimal control of compartment model. Results Phys. 2021, 20, 103715. [Google Scholar] [CrossRef] [PubMed]
- Piovella, N. Analytical solution of SEIR model describing the free spread of the COVID-19 pandemic. Chaos Solitons Fractals 2020, 140, 110243. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Chen, J.; Ruan, S. Estimating asymptomatic, undetected and total cases for the COVID-19 outbreak in Wuhan: A mathematical modeling study. BMC Infect. Dis. 2021, 21. [Google Scholar] [CrossRef] [PubMed]
- Memon, Z.; Qureshi, S.; Memon, B.R. Assessing the role of quarantine and isolation as control strategies for COVID-19 outbreak: A case study. Chaos Solitons Fractals 2021, 144, 110655. [Google Scholar] [CrossRef]
- ud Din, R.; Algehyne, E.A. Mathematical analysis of COVID-19 by using SIR model with convex incidence rate. Results Phys. 2021, 23, 103970. [Google Scholar] [CrossRef]
- Efimov, D.; Ushirobira, R. On an interval prediction of COVID-19 development based on a SEIR epidemic model. Annu. Rev. Control 2021. [Google Scholar] [CrossRef]
- López, L.; Rodó, X. A modified SEIR model to predict the COVID-19 outbreak in Spain and Italy: Simulating control scenarios and multi-scale epidemics. Results Phys. 2021, 21, 103746. [Google Scholar] [CrossRef]
- Ghezzi, L.L.; Piccardi, C. PID control of a chaotic system: An application to an epidemiological model. Automatica 1997, 33, 181–191. [Google Scholar] [CrossRef]
- Jiao, H.; Shen, Q. Dynamics Analysis and Vaccination-Based Sliding Mode Control of a More Generalized SEIR Epidemic Model. IEEE Access 2020, 8, 174507–174515. [Google Scholar] [CrossRef]
- Wang, B.; Sun, Y.; Duong, T.Q.; Nguyen, L.D.; Hanzo, L. Risk-Aware Identification of Highly Suspected COVID-19 Cases in Social IoT: A Joint Graph Theory and Reinforcement Learning Approach. IEEE Access 2020, 8, 115655–115661. [Google Scholar] [CrossRef]
- Vrabac, D.; Shang, M.; Butler, B.; Pham, J.; Stern, R.; Paré, P.E. Capturing the Effects of Transportation on the Spread of COVID-19 with a Multi-Networked SEIR Model. IEEE Control Syst. Lett. 2021, 6, 103–108. [Google Scholar] [CrossRef]
- Small, M.; Cavanagh, D. Modelling Strong Control Measures for Epidemic Propagation With Networks—A COVID-19 Case Study. IEEE Access 2020, 8, 109719–109731. [Google Scholar] [CrossRef] [PubMed]
- Giamberardino, P.D.; Iacoviello, D.; Papa, F.; Sinisgalli, C. Dynamical Evolution of COVID-19 in Italy With an Evaluation of the Size of the Asymptomatic Infective Population. IEEE J. Biomed. Health Inform. 2021, 25, 1326–1332. [Google Scholar] [CrossRef]
- Ó Náraigh, L.; Byrne, A. Piecewise-constant optimal control strategies for controlling the outbreak of COVID-19 in the Irish population. Math. Biosci. 2020, 330, 108496. [Google Scholar] [CrossRef]
- Sasmita, N.R.; Ikhwan, M.; Suyanto, S.; Chongsuvivatwong, V. Optimal control on a mathematical model to pattern the progression of coronavirus disease 2019 (COVID-19) in Indonesia. Glob. Health Res. Policy 2020, 5. [Google Scholar] [CrossRef]
- Deressa, C.T.; Mussa, Y.O.; Duressa, G.F. Optimal control and sensitivity analysis for transmission dynamics of Coronavirus. Results Phys. 2020, 19, 103642. [Google Scholar] [CrossRef]
- Cao, B.; Kang, T. Nonlinear adaptive control of COVID-19 with media campaigns and treatment. Biochem. Biophys. Res. Commun. 2021. [Google Scholar] [CrossRef]
- Nicho, J. The SIR Epidemiology Model in Predicting Herd Immunity. Undergrad. J. Math. Model. ONE+ Two 2010, 2. [Google Scholar] [CrossRef] [Green Version]
- Britton, T.; Ball, F.; Trapman, P. A mathematical model reveals the influence of population heterogeneity on herd immunity to SARS-CoV-2. Science 2020, 369, 846–849. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Peng, Y.; Sun, K. SEIR modeling of the COVID-19 and its dynamics. Nonlinear Dyn. 2020, 101, 1667–1680. [Google Scholar] [CrossRef] [PubMed]
- Godio, A.; Pace, F.; Vergnano, A. SEIR Modeling of the Italian Epidemic of SARS-CoV-2 Using Computational Swarm Intelligence. Int. J. Environ. Res. Public Health 2020, 17, 3535. [Google Scholar] [CrossRef] [PubMed]














| Parameter or Variable | Value |
|---|---|
| M | 60,359,546 |
| 55,223,559 | |
| 2,215,504 | |
| 100,000 | |
| 355,983 | |
| 118,661 | |
| 2,274,400 | |
| 71,439 | |
| 1 | |
| 0 | |
| 0 | |
| 0 | |
| 10 | |
| 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Haus, B.; Mercorelli, P. Extension of SEIR Compartmental Models for Constructive Lyapunov Control of COVID-19 and Analysis in Terms of Practical Stability. Mathematics 2021, 9, 2076. https://doi.org/10.3390/math9172076
Chen H, Haus B, Mercorelli P. Extension of SEIR Compartmental Models for Constructive Lyapunov Control of COVID-19 and Analysis in Terms of Practical Stability. Mathematics. 2021; 9(17):2076. https://doi.org/10.3390/math9172076
Chicago/Turabian StyleChen, Haiyue, Benedikt Haus, and Paolo Mercorelli. 2021. "Extension of SEIR Compartmental Models for Constructive Lyapunov Control of COVID-19 and Analysis in Terms of Practical Stability" Mathematics 9, no. 17: 2076. https://doi.org/10.3390/math9172076







