Automatic Image Characterization of Psoriasis Lesions
Abstract
:1. Introduction and Objectives
1.1. Introduction
1.2. Objectives
2. Materials and Methods
2.1. Mathematical Background
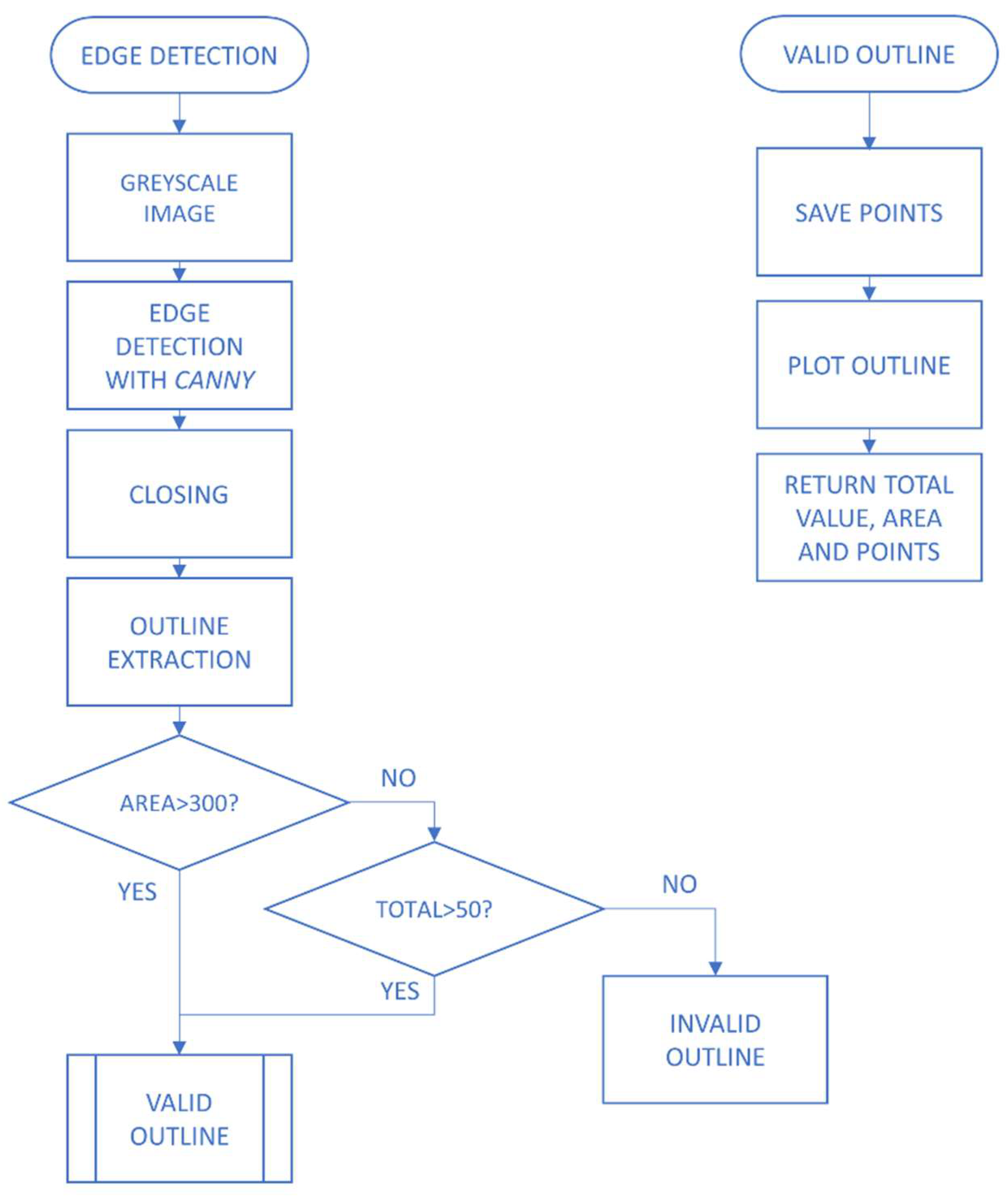
2.2. Methodology
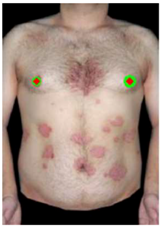
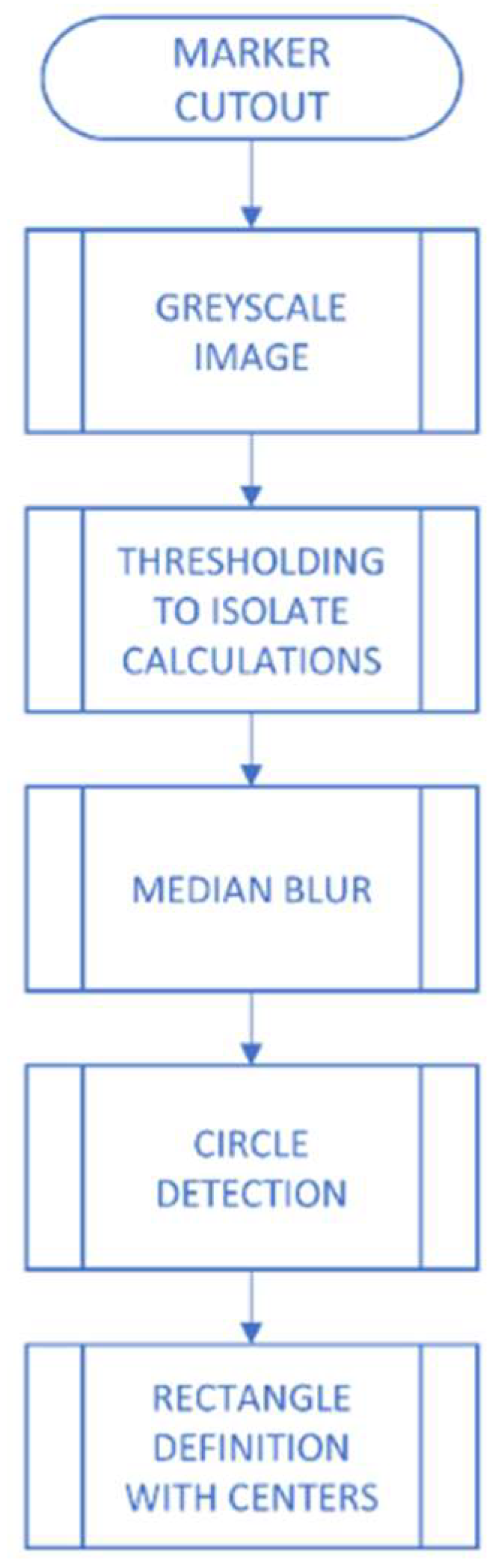
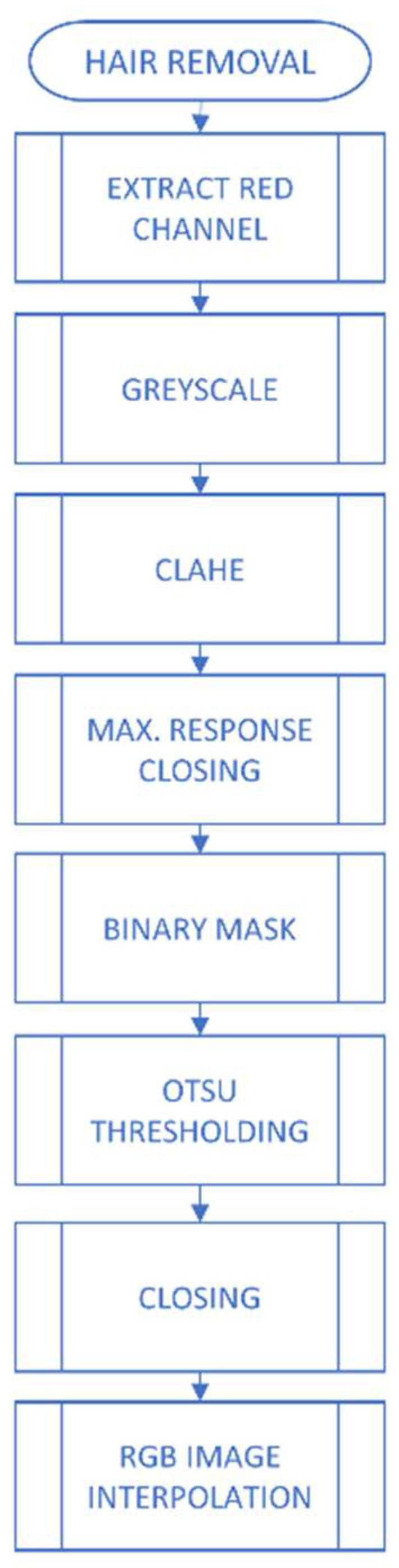
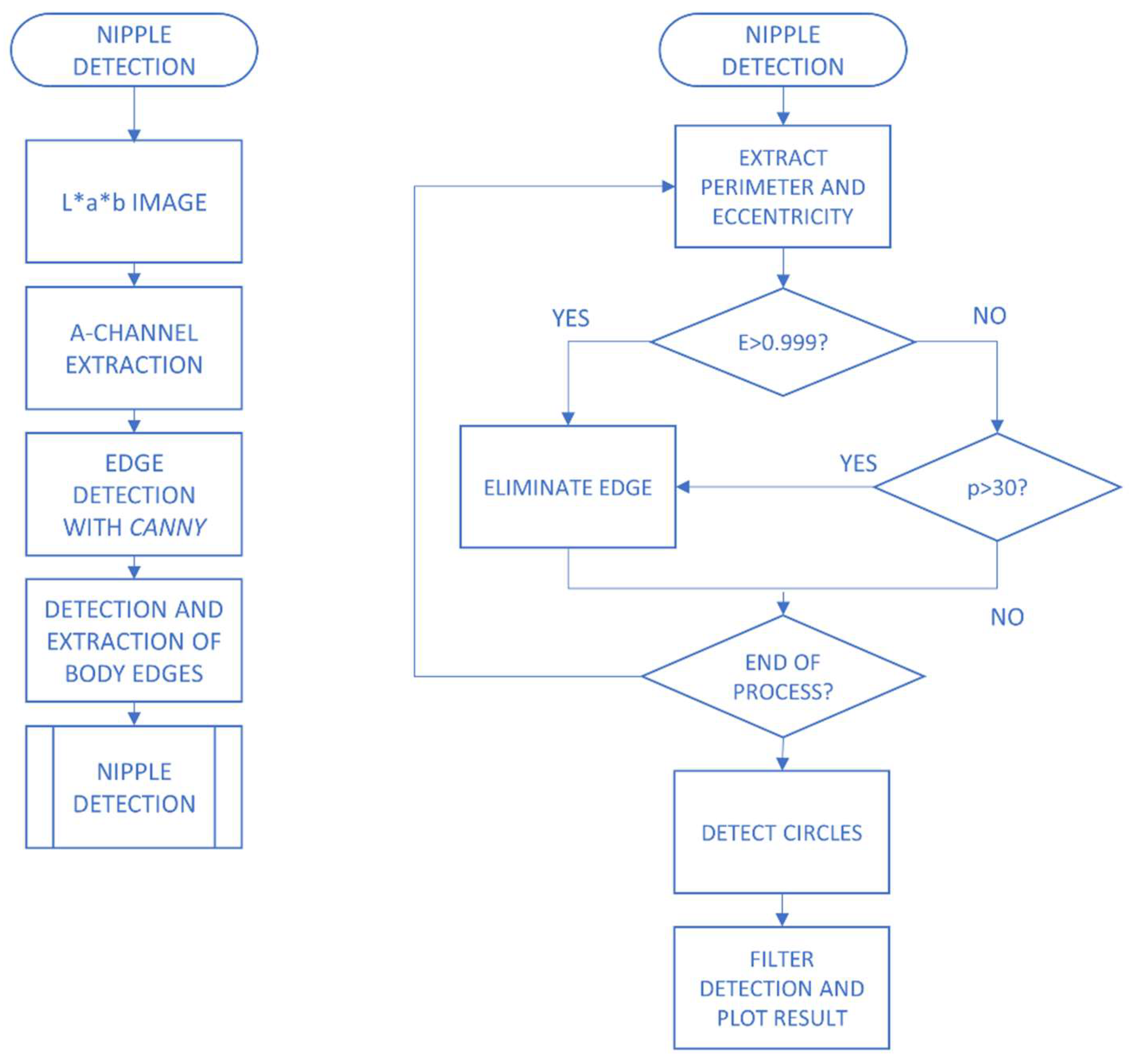
2.2.1. Image Pre-Processing
2.2.2. Extraction of Characteristics and Classification of Lesions
2.2.3. Parameter Estimation
2.3. Dataset
3. Results
4. Concluding Remarks and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sánchez, M.; Aldunce, M.J.; Belinchón, I.; Ribera, M.; Lafuente, R.F.; Carrascosa Carrillo, J.M.; Ferrándiz Foraster, C.; Puig Sanz, L.; Daudén Tello, E.; Vidal Sarró, D.; et al. Evidencebased guidelines of the spanish psoriasis group on the use of biological therapy in patients with psoriasis in difficult-treat sites (nails, scalp, palms and soles). Actas Dermo-Sifiliográficas 2014, 105, 923–934. [Google Scholar] [CrossRef]
- Kimball, A.; Gieler, U.; Linder, D.; Sampogna, F.; Warren, R.; Augustin, M. Psoriasis: Is the impairment to a patient’s life cumulative? J. Eur. Acad. Dermatol. Venereol. 2010, 24, 989–1004. [Google Scholar] [CrossRef] [PubMed]
- Boshle, M.J.; Kulkarni, A.; Feldman, S.R.; Balkrishnan, R. Quality of life in patients with psoriasis. Health Qual. Life Outcomes 2006, 4, 35. [Google Scholar]
- Raychaudhuri, S.K.; Maverakis, E.; Raychaudhuri, S.P. Diagnosis and classification of psoriasis. Autoimmun. Rev. 2014, 13, 490–495. [Google Scholar] [CrossRef]
- Shrivastava, V.K.; Londhe, N.D.; Sonawane, R.S.; Suri, J.S. First review on psoriasis severity risk stratification: An engineering perspective. Comput. Biol. Med. 2015, 63, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Güvenir, H.A.; Demiröz, G.; Ilter, N. Learning Differential Diagnosis of Erythemato-Squamos diseases using voting feature intervals. Artif. Intell. Med. 1998, 13, 147–165. [Google Scholar] [CrossRef] [Green Version]
- George, Y.; Aldeen, M.; Garnavi, R. Automatic Scale Severity Assessment Method in Psoriasis Skin Images using Local Descriptors. IEEE J. Biomed. Health Inform. 2020, 24, 577–585. [Google Scholar] [CrossRef]
- Lafuente, R.F.; Pérez, J. Impact of obesity on the effectiveness of adalimumab for the treatment of psoriasis: A retrospective study of 30 patients in daily practice. Eur. J. Derm. 2014, 24, 217–223. [Google Scholar] [CrossRef]
- Lasierra, N.; Alesanco, A.; Guillén, S.; García, J. A three stage ontology-driven solution to provide personalized care to chronic patients at home. J. Biomed. Inform. 2013, 46, 516–529. [Google Scholar] [CrossRef]
- Banu, S.M.; Toacşe, G. A mobile/desktop medical application for automatic differential diagnosis of psoriasis lesions. In Proceedings of the 2013 Second International Conference on E-Learning and E-Technologies in Education (ICEEE), Lodz, Poland, 23–25 September 2013. [Google Scholar]
- Lasierra, N.; Alesanco, A.; García, J. Designing an architecture for monitoring patients at home: Ontologies and web services for clinical and technical management integration. IEEE J. Biomed. Health Inform. 2014, 18, 896–906. [Google Scholar] [CrossRef]
- Shrivastava, V.K.; Londhe, N.D.; Sonawane, R.S.; Suri, J.S. Computer-aided diagnosis of psoriasis skin images with HOS, texture and color features: A first comparative study of its kind. Comput. Methods Prog. Biomed. 2016, 126, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, V.K.; Londhe, N.D.; Sonawane, R.S.; Suri, J.S. Reliable and accurate psoriasis disease classification in dermatology images using comprehensive feature space in machine learning paradigm. Expert Syst. Appl. 2015, 42, 6184–6195. [Google Scholar] [CrossRef]
- Le, N.Q.K.; Do, D.T.; Nguyen, T.T.D.; Nguyen, N.T.K.; Hung, T.N.K.; Trang, N.T.T. Identification of gene expression signatures for psoriasis classification using machine learning techniques. Med. Omics 2020, 1, 100001. [Google Scholar] [CrossRef]
- Tapak, L.; Afshar, S.; Afrasiabi, M.; Ghasemi, M.K.; Alirezaei, P. Application of Genetic Algorithm-Based Support Vector Machine in Identification of Gene Expression Signatures for Psoriasis Classification: A Hybrid Model. BioMed Res. Int. 2021, 2021, 5520710. [Google Scholar] [CrossRef] [PubMed]
- Han, S.S.; Kim, M.S.; Lim, W.; Park, G.H.; Park, I.; Chang, S.E. Classification of the clinical images for benign and malignant cutaneous tumors using a deep learning algorithm. J. Investig. Dermatol. 2018, 138, 1529–1538. [Google Scholar] [CrossRef] [Green Version]
- Pal, A.; Garain, U.; Chandra, A.; Chatterjee, R.; Senapati, S. Psoriasis skin biopsy image segmentation using deep convolutional neural network. Comput. Methods Programs Biomed. 2018, 159, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S. Noise Types and Various Removal Techniques. Int. J. Adv. Res. Electron. Commun. Eng. 2015, 4, 226–230. [Google Scholar]
- Manterola, H.; del Freson, M. Impainting digital aplicado a la reconstrucción de imágenes de ultrasonido. Mecánica Comput. 2013, XXXII, 3835–3848. [Google Scholar]
- Akbari, R.; Soryani, M.; Fathy, M. Image inpainting with prioritizing of hole’s pixels. In Proceedings of the 16th CSI International Symposium on Artificial Intelligence and Signal Processing (AISP 2012), Shiraz, Iran, 2–3 May 2012. [Google Scholar]
- Canny, J. A Computational Approach to Edge Detection. IEEE Trans. Pattern Anal. Mach. Intell. 1986, 8, 679–698. [Google Scholar] [CrossRef]
- Dougherty, G. Digital Image Processing for Medical Applications; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Bankman, I. Handbook of Medical Image Processing and Analysis; Academic Press: Cambridge, MA, USA, 2008. [Google Scholar]
- Sinha, G.; Bhagwati, B.; Patel, B. Medical Image Processing Concepts and Applications; PHI: Delhi, India, 2014. [Google Scholar]
- Toennies, K. Guide to Medical Image Analysis, Methods and Algorithms/K. Toennies; Springer: London, UK, 2012. [Google Scholar]
- Dhawan, A. Medical Image Analysis/A Dhawan; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2011. [Google Scholar]
- George, Y.; Aldeen, M.; Garnavi, R. Skin Hair Removal for 2D Psoriasis Images. In Proceedings of the 2015 International Conference on Digital Image Computing: Techniques and Applications (DICTA), Adelaide, SA, Australia, 23–25 November 2015. [Google Scholar]
- Lee, T.; Ng, V.; Gallagher, R.; Coldman, A.; Mclean, D. DullRazor: A Software Approach to Hair Removal from Images. Comput. Biol. Med. 1997, 27, 533–543. [Google Scholar] [CrossRef]
- Kiani, K.; Sharafat, A.R. E-shaver: An Improved DullRazor for Digitally Removing Dark and Light-colored Hairs in Dermoscopic Images. Comput. Biol. Med. 2011, 41, 139–145. [Google Scholar] [CrossRef]
- George, Y.; Aldeen, M.; Garnavi, R. Automatic Nipple Detection Method for Digital Skin Images with Psoriasis Lesions. In Proceedings of the 2019 Digital Image Computing: Techniques and Applications (DICTA), Perth, WA, Australia, 2–4 December 2019. [Google Scholar]
- Wang, Y.; Li, J.; Wang, H.; Hou, Z. Automatic nipple detection using shape and statistical skin color information. In International Conference on Multimedia Modeling; Springer: Berlin/Heidelberg, Germany, 2010; pp. 644–649. [Google Scholar]
- Al Abbadi, N.K.; Dahir, N.S.; Al-Dhalimi, M.A. Psoriasis Detection Using Skin Color and Texture Features. J. Comput. Sci. 2010, 6, 648–652. [Google Scholar] [CrossRef] [Green Version]
- Hassanein, A.S.; Mohammad, S.; Sameer, M.; Ragab, M.E. A Survey on Hough Transform, Theory, Techniques and Applications. Int. J. Comput. Sci. Issues (IJCSI) 2015, 12, 139–156. [Google Scholar]
- Lu, J.; Manton, J.H.; Kazmierczak, E.; Sinclair, R. Erythema Detection in Digital Skin Images. In Proceedings of the 2010 IEEE International Conference on Image Processing, Hong Kong, China, 26–29 September 2010. [Google Scholar]
- Lu, J.; Kazmierczak, E.; Manton, J.H.; Sinclair, R. Automatic Segmentation on Scaling in 2-D Psoriasis Skin Images. IEEE Trans. Med. Imaging 2013, 32, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Psoriasis 360. Más allá de la piel. Copyright: Janssen-Cilag, S.A. 2020. Available online: https://www.psoriasis360.es/ (accessed on 18 November 2021).
- Kumar, M.; Alshehri, M.; AlGhamdi, R.; Sharma, P.; Deep, V. A DE-ANN Inspired Skin Cancer Detection Approach Using Fuzzy C-Means Clustering. Mob. Netw. Appl. 2020, 25, 1319–1329. [Google Scholar] [CrossRef]
- Goyal, V.; Singh, G.; Tiwari, O.M.; Kumar Punia, S.; Kumar, M. Intelligent skin cancer detection mobile application using convolution neural network. J. Adv. Res. Dyn. Control Syst. 2019, 11, 253–259. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Torres, J.; Silva Piñeiro, A.; Alesanco, Á.; Pérez-Rey, I.; García, J. Automatic Image Characterization of Psoriasis Lesions. Mathematics 2021, 9, 2974. https://doi.org/10.3390/math9222974
Martínez-Torres J, Silva Piñeiro A, Alesanco Á, Pérez-Rey I, García J. Automatic Image Characterization of Psoriasis Lesions. Mathematics. 2021; 9(22):2974. https://doi.org/10.3390/math9222974
Chicago/Turabian StyleMartínez-Torres, Javier, Alicia Silva Piñeiro, Álvaro Alesanco, Ignacio Pérez-Rey, and José García. 2021. "Automatic Image Characterization of Psoriasis Lesions" Mathematics 9, no. 22: 2974. https://doi.org/10.3390/math9222974
APA StyleMartínez-Torres, J., Silva Piñeiro, A., Alesanco, Á., Pérez-Rey, I., & García, J. (2021). Automatic Image Characterization of Psoriasis Lesions. Mathematics, 9(22), 2974. https://doi.org/10.3390/math9222974