Knowledge, Attitude, and Practice of Intranasal Corticosteroid in Allergic Rhinitis Patients: Development of a New Questionnaire
Abstract
:1. Introduction
2. Materials and Methods
2.1. Questionnaire Development
2.2. Study Setting and Participants
2.3. Validation of Questionnaire
2.4. Statistical Analysis
3. Results
3.1. Content Validity
3.2. Face Validity
3.3. Construct Validity
3.4. Internal Consistency
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scadding, G.; Bousquet, J.; Bachert, C.; Fokkens, W.J.; Hellings, P.W.; Prokopakis, E.; Pfaar, O.; Price, D. Rhinology future trends: 2017 EUFOREA debate on allergic rhinitis. Rhinology 2019, 57, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Brozek, J.L.; Bousquet, J.; Agache, I.; Agarwal, A.; Bachert, C.; Bosnic-Anticevich, S.; Brignardello-Petersen, R.; Canonica, G.W.; Casale, T.; Chavannes, N.H.; et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. J. Allergy Clin. Immunol. 2017, 140, 950–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- May, J.R.; Dolen, W.K. Management of Allergic Rhinitis: A Review for the Community Pharmacist. Clin. Ther. 2017, 39, 2410–2419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scadding, G.K.; Kariyawasam, H.H.; Scadding, G.; Mirakian, R.; Buckley, R.J.; Dixon, T.; Durham, S.R.; Farooque, S.; Jones, N.; Leech, S.; et al. BSACI guidelines for the management of allergic and non-allergic rhinitis (Revised Edition 2017; First edition 2007). Clin. Exp. Allergy 2017, 47, 856–889. [Google Scholar] [CrossRef] [PubMed]
- Katelaris, C.H.; Sacks, R.; Theron, P.N. Allergic rhinoconjunctivitis in the Australian population: Burden of disease and attitudes to intranasal corticosteroid treatment. Am. J. Rhinol. Allergy 2013, 27, 506–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keith, P.K.; Desrosiers, M.; Laister, T.; Schellenberg, R.R.; Waserman, S. The burden of allergic rhinitis (AR) in Canada: Perspectives of physicians and patients. Allergy Asthma Clin. Immunol. 2012, 8, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro-Locsin, C.G.; Romualdez, J.A. Attitudes, practices on allergic rhinitis of three socioeconomic classes of Filipinos in the National Capital Region. Asia Pac. Allergy 2016, 6, 94–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cingi, C.; Songu, M. Nasal steroid perspective: Knowledge and attitudes. Eur. Arch. Otorhinolaryngol. 2010, 267, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Larenas Linnemann, D.E.; Medina Ávalos, M.A.; Lozano Sáenz, J. How an online survey on the treatment of allergic rhinitis and its impact on asthma (ARIA) detected specialty-specific knowledge-gaps. World Allergy Organ. J. 2015, 8, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamanzadeh, V.; Ghahramanian, A.; Rassouli, M.; Abbaszadeh, A.; Alavi-Majd, H.; Nikanfar, A.R. Design and implementation content validity study: Development of an instrument for measuring patient-centered communication. J. Caring Sci. 2015, 4, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Costello, A.B.; Osborne, J.W. Best practices in exploratory factor analysis: Four recommendations for getting the most from your analysis. Pract. Assess. Res. Eval. 2005, 10, 1–9. [Google Scholar] [CrossRef]
- Jackson, C.; Furnham, A. Designing and Analysing Questionnaires and Surveys: A Manual for Health Professionals and Administrators; Whurr Publishers: London, UK; Philadelphia, PA, USA, 2000. [Google Scholar]
- Brown, T.A. Confirmatory Factor Analysis for Applied Research; The Guilford Press: New York, NY, USA, 2015. [Google Scholar]
- Kheirollahpour, M.; Shohaimi, S. Dimensional model for estimating factors influencing childhood obesity: Path analysis based modeling. Sci. World J. 2014, 2014, 512148. [Google Scholar] [CrossRef] [PubMed]
- Hair, J.F.; Black, W.C.; Babin, B.J.; Anderson, R.E. Multivariate Data Analysis, 7th ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2010. [Google Scholar]
- Gupta, R.S.; Springston, E.E.; Smith, B.; Kim, J.S.; Pongracic, J.A.; Wang, X.; Holl, J. Food allergy knowledge, attitudes, and beliefs of parents with food-allergic children in the United States. Pediatr. Allergy Immunol. 2010, 21, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Carrillo Zuniga, G.; Kirk, S.; Mier, N.; Garza, N.I.; Lucio, R.L.; Zuniga, M.A. The impact of asthma health education for parents of children attending head start centers. J. Community Health 2012, 37, 1296–1300. [Google Scholar] [CrossRef] [PubMed]
- Hellings, P.W.; Dobbels, F.; Denhaerynck, K.; Piessens, M.; Ceuppens, J.L.; De Geest, S. Explorative study on patient’s perceived knowledge level, expectations, preferences and fear of side effects for treatment for allergic rhinitis. Clin. Transl. Allergy 2012, 2, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yonezaki, M.; Akiyama, K.; Karaki, M.; Goto, R.; Inamoto, R.; Samukawa, Y.; Kobayashi, R.; Kobayashi, E.; Hoshikawa, H. Preference evaluation and perceived sensory comparison of fluticasone furoate and mometasone furoate intranasal sprays in allergic rhinitis. Auris Nasus Larynx 2016, 43, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, E.O.; Andrews, C.; Journeay, G.E.; Lim, J.; Prillaman, B.A.; Garris, C.; Philpot, E. Comparison of patient preference for sensory attributes of fluticasone furoate or fluticasone propionate in adults with seasonal allergic rhinitis: A randomized, placebo-controlled, double-blind study. Ann. Allergy Asthma Immunol. 2010, 104, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Khanna, P.; Shah, A. Assessment of sensory perceptions and patient preference for intranasal corticosteroid sprays in allergic rhinitis. Am. J. Rhinol. 2005, 19, 316–321. [Google Scholar] [CrossRef] [PubMed]
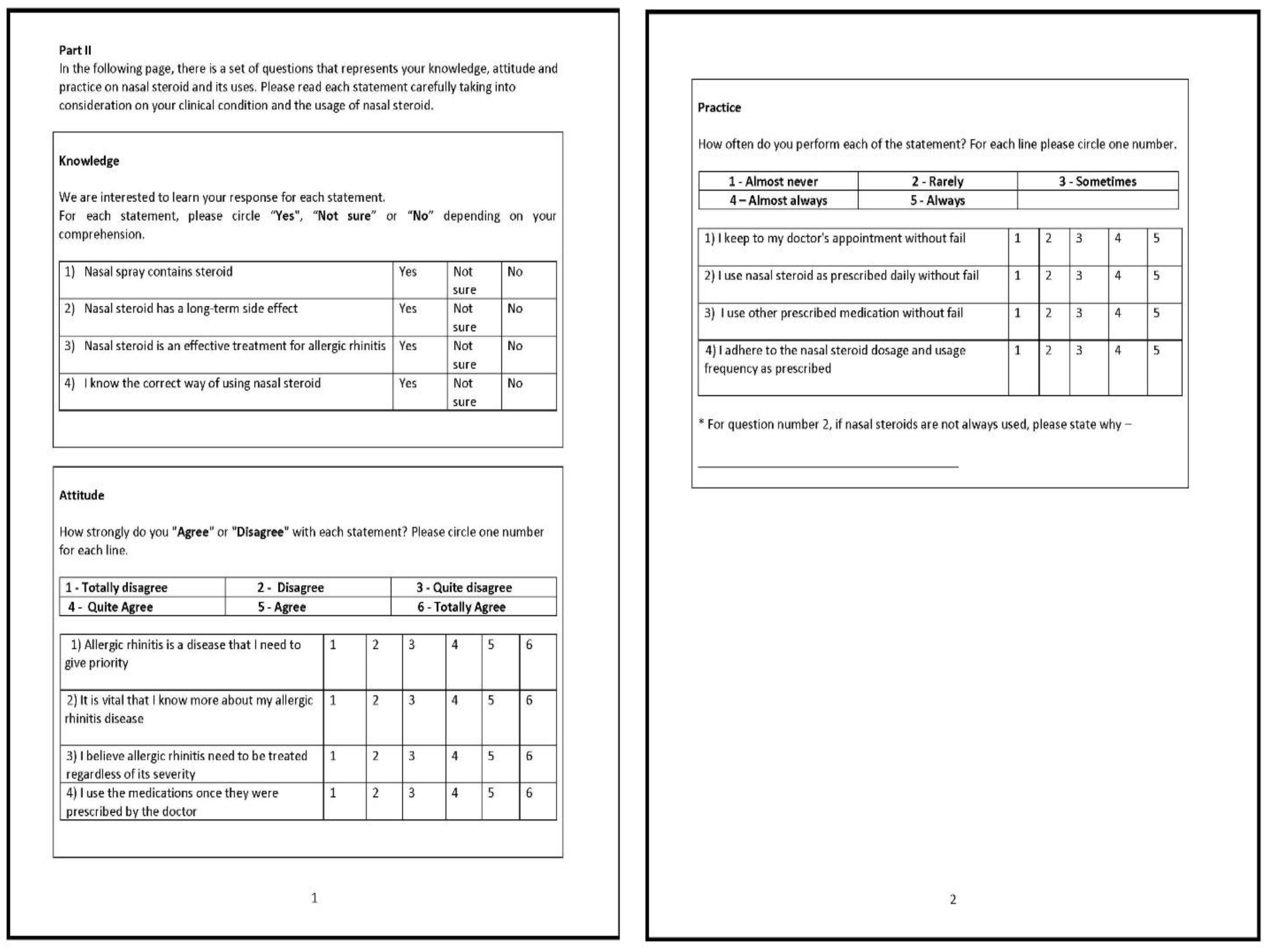
| Components | Item | Concepts Measured | Response Options |
|---|---|---|---|
| Knowledge (actual information from training or experience) | 5 questions | To gauge knowledge of INCS | Yes; No; Not sure |
| Attitude (a settled way of thinking or feeling about something) | 5 questions | To assess general attitude, behaviour, and cognitive factors towards INCS | Totally disagree; Disagree; Quite disagree; Quite agree; Agree; Totally agree |
| Practice (actual application of an idea, belief, or method) | 4 questions | To evaluate common practice of INCS | Almost never; Rarely; Sometimes; Almost always; Always |
| n (%) | |
|---|---|
| Gender | |
| Male | 38 (49.4) |
| Female | 39 (50.6) |
| Ethnicity | |
| Malay | 29 (37.7) |
| Chinese | 26 (33.8) |
| Indian | 18 (23.4) |
| Others | 4 (5.2) |
| Education | |
| Phd/Masters | 2 (2.6) |
| Bachelor degree | 42 (54.5) |
| Diploma | 9 (11.7) |
| Secondary | 24 (31.2) |
| Diagnosis | |
| Mild intermittent | 20 (26) |
| Mild persistent | 32 (41.6) |
| Moderate severe intermittent | 0 (0) |
| Moderate severe persistent | 25 (32.5) |
| Scale | Items | Mean (SD) | Yes (n %) | Not Sure (n %) | No (n %) |
|---|---|---|---|---|---|
| K-Q1 | I am aware of the importance of using nasal steroid | 1.62 (0.69) | 57 (74.0) | 11 (14.3) | 9 (11.7) |
| K-Q2 | Nasal spray contains steroid | 1.49 (0.64) | 44 (57.1) | 27 (35.1) | 6 (7.8) |
| K-Q3 | Nasal steroid has a long-term side effect | 1.39 (0.71) | 40 (51.9) | 27 (35.1) | 10 (13.0) |
| K-Q4 | Nasal steroid is an effective treatment for allergic rhinitis | 1.61 (0.59) | 51 (66.2) | 22 (28.6) | 4 (5.2) |
| K-Q5 | I know the correct method of using the nasal steroid | 1.65 (0.58) | 54 (70.1) | 19 (24.7) | 4 (5.2) |
| Scale | Items | Mean (SD) | Totally Disagree (%) | Disagree (%) | Quite Disagree (%) | Quite Agree (%) | Agree (%) | Totally Agree (%) |
|---|---|---|---|---|---|---|---|---|
| A-Q1 | Allergic rhinitis is a disease that I need to make a priority | 5.09 (1.03) | 2 (2.6) | 0 (0) | 2 (2.6) | 11 (14.3) | 32 (41.6) | 30 (39.0) |
| A-Q2 | My knowledge of allergic rhinitis is sufficient | 4.27 (0.93) | 1 (1.3) | 2 (2.6) | 9 (41.6) | 32 (41.6) | 29 (37.7) | 4 (5.2) |
| A-Q3 | It is vital that I know more about my allergic rhinitis disease | 5.08 (1.20) | 3 (3.9) | 0 (0) | 4 (5.2) | 10 (13.0) | 24 (31.2) | 36 (46.8) |
| A-Q4 | I believe allergic rhinitis need to be treated regardless of its severity | 5.27 (0.93) | 1 (1.3) | 1 (13) | 1 (1.3) | 6 (7.8) | 32 (41.6) | 32 (41.6) |
| A-Q5 | I use the medications once they are prescribed by the doctor | 5.06 (1.03) | 2 (2.6) | 0 (0) | 3 (3.9) | 9 (11.7) | 35 (45.5) | 28 (36.4) |
| Scale | Items | Mean (SD) | Almost Never (%) | Rarely (%) | Sometimes (%) | Almost Always (%) | Always (%) |
|---|---|---|---|---|---|---|---|
| P-Q1 | I keep my doctor’s appointment without fail | 4.03 (1.16) | 6 (7.8) | 2 (2.6) | 8 (10.4) | 29 (37.7) | 32 (41.6) |
| P-Q2 | I use nasal steroid as prescribed daily without fail | 3.73 (0.87) | 1 (1.3) | 4 (5.2) | 24 (31.2) | 34 (44.2) | 14 (18.2) |
| P-Q3 | I use other prescribed medication without fail | 3.49 (1.11) | 6 (7.8) | 6 (7.8) | 22 (28.6) | 30 (39) | 13 (16.9) |
| P-Q4 | I adhere to the nasal steroid dosage and usage frequency as prescribed | 3.9 (1.04) | 3 (3.9) | 4 (5.2) | 16 (20.8) | 30 (39) | 24 (31.2) |
| Factor | Item | Factor Loading a | Communality b | Cronbach’s Alpha c |
|---|---|---|---|---|
| 1. Attitude | A-Q1 | 0.719 | 0.563 | 0.809 |
| A-Q3 | 0.667 | 0.445 | ||
| A-Q4 | 0.845 | 0.674 | ||
| A-Q5 | 0.705 | 0.532 | ||
| 2. Practice | P-Q1 | 0.675 | 0.462 | 0.774 |
| P-Q2 | 0.824 | 0.634 | ||
| P-Q3 | 0.513 | 0.497 | ||
| P-Q4 | 0.717 | 0.564 | ||
| 3. Knowledge | K-Q2 | 0.690 | 0.467 | 0.735 |
| K-Q3 | 0.866 | 0.775 | ||
| 4. Knowledge 2 | K-Q4 | 0.527 | 0.478 | 0.614 |
| K-Q5 | 0.856 | 0.700 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Retinasekharan, S.; Md Shukri, N.; Ismail, A.F.; Abdullah, B. Knowledge, Attitude, and Practice of Intranasal Corticosteroid in Allergic Rhinitis Patients: Development of a New Questionnaire. Healthcare 2022, 10, 8. https://doi.org/10.3390/healthcare10010008
Retinasekharan S, Md Shukri N, Ismail AF, Abdullah B. Knowledge, Attitude, and Practice of Intranasal Corticosteroid in Allergic Rhinitis Patients: Development of a New Questionnaire. Healthcare. 2022; 10(1):8. https://doi.org/10.3390/healthcare10010008
Chicago/Turabian StyleRetinasekharan, Senthilraj, Norasnieda Md Shukri, Ahmad Filza Ismail, and Baharudin Abdullah. 2022. "Knowledge, Attitude, and Practice of Intranasal Corticosteroid in Allergic Rhinitis Patients: Development of a New Questionnaire" Healthcare 10, no. 1: 8. https://doi.org/10.3390/healthcare10010008
APA StyleRetinasekharan, S., Md Shukri, N., Ismail, A. F., & Abdullah, B. (2022). Knowledge, Attitude, and Practice of Intranasal Corticosteroid in Allergic Rhinitis Patients: Development of a New Questionnaire. Healthcare, 10(1), 8. https://doi.org/10.3390/healthcare10010008







