Effects of Cardiac Rehabilitation in Cardiopulmonary Fitness with High-Risk Myocardial Infarction
Abstract
:1. Introduction
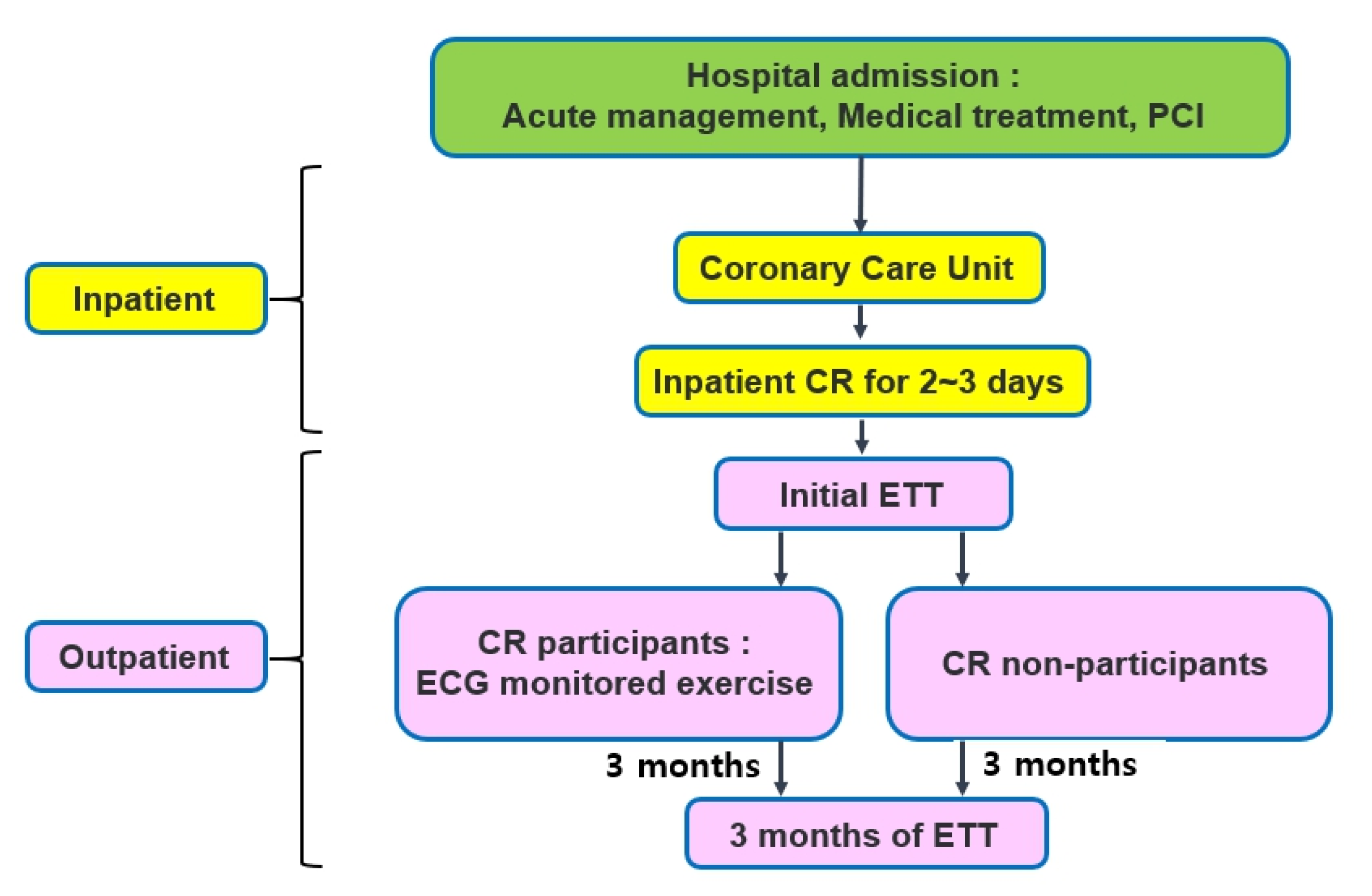
2. Materials and Methods
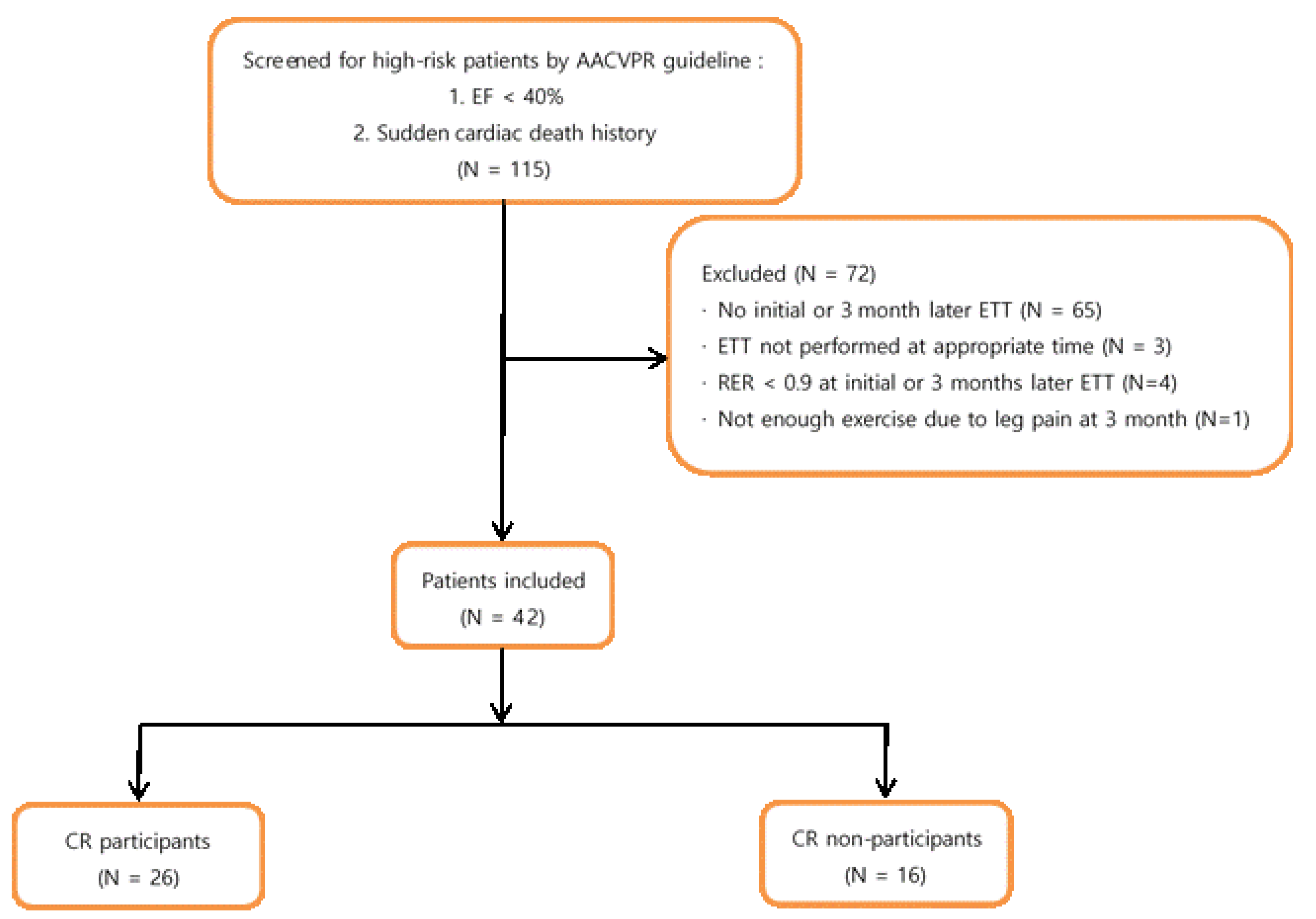
2.1. Subjects
- LVEF < 40%;
- A survivor of cardiac arrest or sudden death;
- Having complex ventricular dysrhythmias (ventricular tachycardia, frequent [>6/min] multiform premature ventricular contractions) at rest or during exercise;
- Having undergone MI or cardiac surgery that was complicated by cardiogenic shock, congestive heart failure, and/or signs or symptoms of post-procedure ischemia;
- Having an abnormal hemodynamic profile with exercise, especially flat or decreasing systolic blood pressure or chronotropic incompetence with increasing workload;
- Having significant silent ischemia (ST depression ≥ 2 mm without symptoms) with exercise or recovery;
- Having signs/symptoms including angina pectoris, dizziness, light headedness, or dyspnoea at low levels of exercise (<5.0 METs) or in recovery;
- Having a maximal functional capacity that is less than 5.0 METs;
- Having clinically significant depression or depressive symptoms.
2.2. Data Abstraction
2.3. Exercise Tolerance Test
2.4. Echocardiography
2.5. Statistical Analysis
3. Results
Demographic Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dagres, N.; Hindricks, G. Sudden Cardiac Death in Acute Coronary Syndromes. Card. Electrophysiol. Clin. 2017, 9, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Jenca, D.; Melenovsky, V.; Stehlik, J.; Stanek, V.; Kettner, J.; Kautzner, J.; Adamkova, V.; Wohlfahrt, P. Heart failure after myocardial infarction: Incidence and predictors. ESC Heart Fail. 2021, 8, 222–237. [Google Scholar] [CrossRef] [PubMed]
- Jordaens, L.; Tavernier, R.; Investigators, M. Determinants of sudden death after discharge from hospital for myocardial infarction in the thrombolytic era. Eur. Heart J. 2001, 22, 1214–1225. [Google Scholar] [CrossRef] [PubMed]
- Benzer, W.; Rauch, B.; Schmid, J.P.; Zwisler, A.D.; Dendale, P.; Davos, C.H.; Kouidi, E.; Simon, A.; Abreu, A.; Pogosova, N.; et al. Exercise-based cardiac rehabilitation in twelve European countries results of the European cardiac rehabilitation registry. Int. J. Cardiol. 2017, 228, 58–67. [Google Scholar] [CrossRef]
- Kim, S.H.; Ro, J.S.; Kim, Y.; Leigh, J.H.; Kim, W.S. Underutilization of Hospital-based Cardiac Rehabilitation after Acute Myocardial Infarction in Korea. J. Korean Med. Sci. 2020, 35, e262. [Google Scholar] [CrossRef]
- Im, H.W.; Baek, S.; Jee, S.; Ahn, J.M.; Park, M.W.; Kim, W.S. Barriers to Outpatient Hospital-Based Cardiac Rehabilitation in Korean Patients With Acute Coronary Syndrome. Ann. Rehabil. Med. 2018, 42, 154–165. [Google Scholar] [CrossRef]
- Anderson, L.; Thompson, D.R.; Oldridge, N.; Zwisler, A.D.; Rees, K.; Martin, N.; Taylor, R.S. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane. Database Syst. Rev. 2016, CD001800. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Corra, U.; Adamopoulos, S.; Benzer, W.; Bjarnason-Wehrens, B.; Cupples, M.; Dendale, P.; Doherty, P.; Gaita, D.; Hofer, S.; et al. Secondary prevention in the clinical management of patients with cardiovascular diseases. Core components, standards and outcome measures for referral and delivery: A policy statement from the cardiac rehabilitation section of the European Association for Cardiovascular Prevention & Rehabilitation. Endorsed by the Committee for Practice Guidelines of the European Society of Cardiology. Eur. J. Prev. Cardiol. 2014, 21, 664–681. [Google Scholar] [CrossRef]
- Kim, C.; Choi, I.; Cho, S.; Han, J.Y.; Kim, A.R.; Kim, W.S.; Jee, S.; Lee, J.H.; Joo, M.C.; Bang, H.J.; et al. Cardiac rehabilitation and 5-year mortality after acute myocardial infarction. Report from 11 tertiary hospitals in Korea (ETHIK Study). Eur. J. Phys. Rehabil. Med. 2020, 56, 489–495. [Google Scholar] [CrossRef]
- Cao, R.Y.; Zheng, H.; Hong, Y.; Zheng, Y.; Ding, Y.; Zhao, L.; Li, H.; Li, Q.; Yuan, W.; Liu, S.; et al. Cardiac Rehabilitation with Targeted Intensity Improves Cardiopulmonary Functions Accompanying with Reduced Copeptin Level in Patients with Coronary Artery Disease. J. Cardiovasc. Transl. Res. 2021, 14, 317–326. [Google Scholar] [CrossRef]
- Guidry, U.C.; Evans, J.C.; Larson, M.G.; Wilson, P.W.; Murabito, J.M.; Levy, D. Temporal trends in event rates after Q-wave myocardial infarction: The Framingham Heart Study. Circulation 1999, 100, 2054–2059. [Google Scholar] [CrossRef]
- Ottervanger, J.P.; Misier, A.R.R.; Dambrink, J.H.; de Boer, M.J.; Hoorntje, J.C.; Gosselink, A.T.; Suryapranata, H.; Reiffers, S.; van’t Hof, A.W.; Zwolle Myocardial Infarction Study Group. Mortality in patients with left ventricular ejection fraction </=30% after primary percutaneous coronary intervention for ST-elevation myocardial infarction. Am. J. Cardiol. 2007, 100, 793–797. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Ades, P.A.; Keteyian, S.J.; Wright, J.S.; Hamm, L.F.; Lui, K.; Newlin, K.; Shepard, D.S.; Thomas, R.J. Increasing Cardiac Rehabilitation Participation From 20% to 70%: A Road Map From the Million Hearts Cardiac Rehabilitation Collaborative. Mayo Clin. Proc. 2017, 92, 234–242. [Google Scholar] [CrossRef]
- Wang, L.; Ai, D.; Zhang, N. Exercise Benefits Coronary Heart Disease. Adv. Exp. Med. Biol. 2017, 1000, 3–7. [Google Scholar] [CrossRef]
- Giannuzzi, P.; Temporelli, P.L.; Corra, U.; Tavazzi, L.; Group, E.-C.S. Antiremodeling effect of long-term exercise training in patients with stable chronic heart failure: Results of the Exercise in Left Ventricular Dysfunction and Chronic Heart Failure (ELVD-CHF) Trial. Circulation 2003, 108, 554–559. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Lu, Y.; Tang, Y.; Yang, D.; Wu, H.F.; Bian, Z.P.; Xu, J.D.; Gu, C.R.; Wang, L.S.; Chen, X.J. The effects of different initiation time of exercise training on left ventricular remodeling and cardiopulmonary rehabilitation in patients with left ventricular dysfunction after myocardial infarction. Disabil. Rehabil. 2016, 38, 268–276. [Google Scholar] [CrossRef]
- Klecha, A.; Kawecka-Jaszcz, K.; Bacior, B.; Kubinyi, A.; Pasowicz, M.; Klimeczek, P.; Banys, R. Physical training in patients with chronic heart failure of ischemic origin: Effect on exercise capacity and left ventricular remodeling. Eur. J. Prev. Cardiol. 2007, 14, 85–91. [Google Scholar] [CrossRef]
- Mone, P.; Izzo, R.; Marazzi, G.; Manzi, M.V.; Gallo, P.; Campolongo, G.; Cacciotti, L.; Tartaglia, D.; Caminiti, G.; Varzideh, F.; et al. L-Arginine Enhances the Effects of Cardiac Rehabilitation on Physical Performance: New Insights for Managing Cardiovascular Patients During the COVID-19 Pandemic. J. Pharmacol. Exp. Ther. 2022, 381, 197–203. [Google Scholar] [CrossRef]
- Bittner, V.; Weiner, D.H.; Yusuf, S.; Rogers, W.J.; McIntyre, K.M.; Bangdiwala, S.I.; Kronenberg, M.W.; Kostis, J.B.; Kohn, R.M.; Guillotte, M.; et al. Prediction of mortality and morbidity with a 6-minute walk test in patients with left ventricular dysfunction. SOLVD Investigators. JAMA 1993, 270, 1702–1707. [Google Scholar] [CrossRef]
- Kodama, S.; Saito, K.; Tanaka, S.; Maki, M.; Yachi, Y.; Asumi, M.; Sugawara, A.; Totsuka, K.; Shimano, H.; Ohashi, Y.; et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: A meta-analysis. JAMA 2009, 301, 2024–2035. [Google Scholar] [CrossRef]
- do Nascimento, D.M.; Machado, K.C.; Bock, P.M.; Saffi, M.A.L.; Goldraich, L.A.; Silveira, A.D.; Clausell, N.; Schaan, B.D. Cardiopulmonary exercise capacity and quality of life of patients with heart failure undergoing a functional training program: Study protocol for a randomized clinical trial. BMC Cardiovasc. Disord. 2020, 20, 200. [Google Scholar] [CrossRef]
- Kamiya, K.; Sato, Y.; Takahashi, T.; Tsuchihashi-Makaya, M.; Kotooka, N.; Ikegame, T.; Takura, T.; Yamamoto, T.; Nagayama, M.; Goto, Y.; et al. Multidisciplinary Cardiac Rehabilitation and Long-Term Prognosis in Patients With Heart Failure. Circ. Heart Fail. 2020, 13, e006798. [Google Scholar] [CrossRef]
- Pedersen, B.K. Anti-inflammatory effects of exercise: Role in diabetes and cardiovascular disease. Eur. J. Clin. Invest. 2017, 47, 600–611. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, L.; Yang, Y.J.; Ge, R.K.; Zhou, M.; Hu, H.; Liu, H.; Cui, J.; Li, L.L.; Dong, Y.F.; et al. Aerobic exercise improves endothelial function and serum adropin levels in obese adolescents independent of body weight loss. Sci. Rep. 2017, 7, 17717. [Google Scholar] [CrossRef]
- Borjesson, M.; Onerup, A.; Lundqvist, S.; Dahlof, B. Physical activity and exercise lower blood pressure in individuals with hypertension: Narrative review of 27 RCTs. Br. J. Sports Med. 2016, 50, 356–361. [Google Scholar] [CrossRef]
- Avogaro, A.; Bonora, E.; Consoli, A.; Del Prato, S.; Genovese, S.; Giorgino, F. Glucose-lowering therapy and cardiovascular outcomes in patients with type 2 diabetes mellitus and acute coronary syndrome. Diab. Vasc. Dis. Res. 2019, 16, 399–414. [Google Scholar] [CrossRef]
- Lewis, E.F.; Velazquez, E.J.; Solomon, S.D.; Hellkamp, A.S.; McMurray, J.J.; Mathias, J.; Rouleau, J.L.; Maggioni, A.P.; Swedberg, K.; Kober, L.; et al. Predictors of the first heart failure hospitalization in patients who are stable survivors of myocardial infarction complicated by pulmonary congestion and/or left ventricular dysfunction: A VALIANT study. Eur. Heart J. 2008, 29, 748–756. [Google Scholar] [CrossRef]
- Arnold, S.V.; Bhatt, D.L.; Barsness, G.W.; Beatty, A.L.; Deedwania, P.C.; Inzucchi, S.E.; Kosiborod, M.; Leiter, L.A.; Lipska, K.J.; Newman, J.D.; et al. Clinical Management of Stable Coronary Artery Disease in Patients With Type 2 Diabetes Mellitus: A Scientific Statement From the American Heart Association. Circulation 2020, 141, e779–e806. [Google Scholar] [CrossRef]
- Jimenez-Navarro, M.F.; Lopez-Jimenez, F.; Perez-Belmonte, L.M.; Lennon, R.J.; Diaz-Melean, C.; Rodriguez-Escudero, J.P.; Goel, K.; Crusan, D.; Prasad, A.; Squires, R.W.; et al. Benefits of Cardiac Rehabilitation on Cardiovascular Outcomes in Patients With Diabetes Mellitus After Percutaneous Coronary Intervention. J. Am. Heart Assoc. 2017, 6, e006404. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.E.; Kim, C.; Sohn, Y. Cardiac Rehabilitation Exercise Training for High-Risk Cardiac Patients. Ann. Rehabil. Med. 2017, 41, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Sugita, Y.; Ito, K.; Sakurai, S.; Sakai, S.; Kuno, S. Epicardial adipose tissue is associated with cardiorespiratory fitness and hemodynamics among Japanese individuals of various ages and of both sexes. PLoS ONE 2021, 16, e0254733. [Google Scholar] [CrossRef]
- Fanget, M.; Bayle, M.; Labeix, P.; Roche, F.; Hupin, D. Effects of Cardiac Telerehabilitation During COVID-19 on Cardiorespiratory Capacities in Patients With Coronary Artery Disease. Front. Physiol. 2022, 13, 837482. [Google Scholar] [CrossRef] [PubMed]
| Cardiac Rehabilitation Participants (N = 26) | Cardiac Rehabilitation Non-Participants (N = 16) | p-Value | ||
|---|---|---|---|---|
| Age (years) | 61.3 ± 6.52 | 64.3 ± 8.70 | 0.213 | |
| Sex ratio (men/women) | 24/2 | 13/3 | 0.283 | |
| LVEF (%) | 39.3 ± 8.68 | 35.3 ± 4.96 | 0.103 | |
| CHD event type | STEMI (%) | 15 (57.7) | 9 (56.3) | 0.927 |
| NSTEMI (%) | 11 (42.3) | 7 (43.7) | ||
| Hypertension | 7 (26.9) | 9 (56.3) | 0.057 | |
| Diabetes mellitus | 11 (42.3) | 3 (18.8) | 0.116 | |
| Dyslipidemia | 19 (73.1) | 8 (50.0) | 0.130 | |
| Smoking status | Current | 13 (50.0) | 5 (31.3) | 0.488 |
| Former | 5 (19.2) | 4 (25.0) | ||
| Never | 8 (30.8) | 7 (43.8) | ||
| Family history | 3 (11.5) | 3 (2.3) | 0.517 | |
| BMI (kg/m2) | 25.3 ± 3.10 | 24.8 ± 2.22 | 0.576 | |
| Initial ETT | Cardiac Rehabilitation Participants (N = 26) | Cardiac Rehabilitation Non-Participants (N = 16) | p-Value |
| VO2 peak (mL/kg/min) | 23.07 ± 6.30 | 19.74 ± 4.94 | 0.079 |
| VE peak (L/min) | 59.71 ± 18.85 | 52.53 ± 19.12 | 0.240 |
| RER | 1.06 ± 0.07 | 1.02 ± 0.09 | 0.146 |
| Stage | 4.81 ± 1.06 | 4.19 ± 1.11 | 0.078 |
| METs | 6.60 ± 1.80 | 5.64 ± 1.40 | 0.078 |
| Exercise time (s) | 753.38 ± 239.27 | 685.50 ± 165.71 | 0.326 |
| SBP max (mmHg) | 167.88 ± 26.96 | 161.88 ± 27.54 | 0.491 |
| DBP max (mmHg) | 76.96 ± 15.40 | 76.63 ± 13.78 | 0.943 |
| HR max (beat/min) | 142.46 ± 18.92 | 136.13 ± 18.75 | 0.297 |
| ETT that Occurred After 3 Months | Cardiac Rehabilitation Participants (N = 26) | Cardiac Rehabilitation Non-Participants (N = 16) | p-Value |
| VO2 peak (mL/kg/min) | 26.24 ± 7.11 | 21.45 ± 6.40 | 0.033 * |
| VE peak (L/min) | 70.02 ± 23.02 | 56.15 ± 20.37 | 0.055 |
| RER | 1.09 ± 0.07 | 1.05 ± 0.08 | 0.197 |
| Stage | 4.88 ± 1.37 | 3.38 ± 1.45 | 0.002 * |
| METs | 7.49 ± 2.06 | 6.13 ± 1.83 | 0.037 * |
| Exercise time (s) | 768.08 ± 249.29 | 500.13 ± 235.49 | <0.001 * |
| SBP max (mmHg) | 194.46 ± 123.80 | 167.19 ± 34.38 | 0.396 |
| DBP max (mmHg) | 78.31 ± 10.91 | 80.69 ± 12.57 | 0.521 |
| HR max (beat/min) | 150.15 ± 21.98 | 144.56 ± 17.85 | 0.396 |
| Cardiac Rehabilitation Participants (N = 26) | ||||
| Intial | 3 months after | Δ | p-Value | |
| VO2 peak (mL/kg/min) | 23.07 ± 6.30 | 26.24 ± 7.11 | 3.17 ± 4.01 | <0.001 * |
| VE peak (L/min) | 59.71 ± 18.85 | 70.02 ± 23.02 | 10.31 ± 12.40 | <0.001 * |
| RER | 1.06 ± 0.07 | 1.09 ± 0.07 | 0.03 ± 0.07 | 0.064 |
| Stage | 4.81 ± 1.06 | 4.88 ± 1.37 | 0.08 ± 1.16 | 0.739 |
| METs | 6.60 ± 1.80 | 7.49 ± 2.06 | 0.89 ± 1.17 | 0.001 * |
| Exercise time (s) | 753.38 ± 239.27 | 768.08 ± 249.29 | 14.69 ± 218.96 | 0.735 |
| SBP max (mmHg) | 167.88 ± 26.96 | 194.46 ± 123.80 | 26.58 ± 119.59 | 0.268 |
| DBP max (mmHg) | 76.96 ± 15.40 | 78.31 ± 10.91 | 1.35 ± 12.83 | 0.597 |
| HR max (beat/min) | 142.46 ± 18.92 | 150.15 ± 21.98 | 7.69 ± 13.33 | 0.007 * |
| Cardiac Rehabilitation Non-Participants (N = 16) | ||||
| Intial | 3 months after | Δ | p-Value | |
| VO2 peak (mL/kg/min) | 19.74 ± 4.94 | 21.45 ± 6.40 | 1.71 ± 3.70 | 0.084 |
| VE peak (L/min) | 52.53 ± 19.12 | 56.15 ± 20.37 | 3.62 ± 10.20 | 0.176 |
| RER | 1.02 ± 0.09 | 1.05 ± 0.08 | 0.03 ± 0.08 | 0.150 |
| Stage | 4.19 ± 1.11 | 3.38 ± 1.45 | −0.81 ± 1.05 | 0.007 * |
| METs | 5.64 ± 1.40 | 6.13 ± 1.83 | 0.49 ± 1.06 | 0.087 |
| Exercise time (s) | 685.50 ± 165.71 | 500.13 ± 235.49 | −185.38 ± 186.12 | 0.001 * |
| SBP max (mmHg) | 161.88 ± 27.54 | 167.19 ± 34.38 | 5.31 ± 23.12 | 0.373 |
| DBP max (mmHg) | 19.74 ± 4.94 | 21.45 ± 6.40 | 1.71 ± 3.70 | 0.084 |
| HR max (beat/min) | 52.53 ± 19.12 | 56.15 ± 20.37 | 3.62 ± 10.20 | 0.176 |
| Parameters | Cardiac Rehabilitation Participants (N = 26) | Cardiac Rehabilitation Non-Participants (N = 16) | ||||||
|---|---|---|---|---|---|---|---|---|
| Initial | 3 months | Δ | p value | Initial | 3 months | Δ | p value | |
| EF (%) | 37.76 ± 8.68 | 46.77 ± 11.82 | 9.01 ± 11.28 | 0.002 * | 36.24 ± 4.03 | 43.64 ± 8.74 | 7.41 ± 8.21 | 0.005 * |
| LVESD (cm) | 4.32 ± 0.88 | 4.04 ± 0.86 | −0.28 ± 0.99 | 0.221 | 4.49 ± 0.81 | 4.22 ± 0.73 | −0.27 ± 0.69 | 0.163 |
| LVEDD (cm) | 5.42 ± 0.62 | 5.45 ± 0.77 | −0.03 ± 0.84 | 0.875 | 5.59 ± 0.66 | 5.54 ± 0.54 | −0.05 ± 0.46 | 0.718 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.Y.; Kim, J.H. Effects of Cardiac Rehabilitation in Cardiopulmonary Fitness with High-Risk Myocardial Infarction. Healthcare 2022, 10, 1849. https://doi.org/10.3390/healthcare10101849
Choi SY, Kim JH. Effects of Cardiac Rehabilitation in Cardiopulmonary Fitness with High-Risk Myocardial Infarction. Healthcare. 2022; 10(10):1849. https://doi.org/10.3390/healthcare10101849
Chicago/Turabian StyleChoi, Seok Yeon, and Ji Hee Kim. 2022. "Effects of Cardiac Rehabilitation in Cardiopulmonary Fitness with High-Risk Myocardial Infarction" Healthcare 10, no. 10: 1849. https://doi.org/10.3390/healthcare10101849






