Diffusion Tensor Tractography Studies on Recovery Mechanisms of Aphasia in Stroke Patients: A Narrative Mini-Review
Abstract
:1. Introduction
2. Neural Tracts Which Are Involved in Language Processing
3. The Useful Characteristics of DTT for Research on the Mechanisms of Aphasia Recovery in Stroke Patients
4. Review of DTT Studies on the Mechanisms of Aphasia Recovery in Stroke Patients
4.1. Recovery via the Neural Tracts in the Dominant Hemisphere
4.2. Recovery via Transcallosal Fibers
4.3. Recovery via the Neural Tracts in the Non-Dominant Hemisphere
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Damasio, A.R. Aphasia. N. Engl. J. Med. 1992, 326, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Wade, D.T.; Hewer, R.L.; David, R.M.; Enderby, P.M. Aphasia after stroke: Natural history and associated deficits. J. Neurol. Neurosurg. Psychiatry 1986, 49, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, P.M.; Jorgensen, H.S.; Nakayama, H.; Raaschou, H.O.; Olsen, T.S. Aphasia in acute stroke: Incidence, determinants, and recovery. Ann. Neurol. 1995, 38, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Wertz, R.T. Aphasia in acute stroke: Incidence, determinants, and recovery. Ann. Neurol. 1996, 40, 129–130. [Google Scholar] [CrossRef] [PubMed]
- Berthier, M.L. Poststroke aphasia: Epidemiology, pathophysiology and treatment. Drugs Aging 2005, 22, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Engelter, S.T.; Gostynski, M.; Papa, S.; Frei, M.; Born, C.; Ajdacic-Gross, V.; Gutzwiller, F.; Lyrer, P.A. Epidemiology of aphasia attributable to first ischemic stroke: Incidence, severity, fluency, etiology, and thrombolysis. Stroke 2006, 37, 1379–1384. [Google Scholar] [CrossRef] [PubMed]
- Breining, B.L.; Sebastian, R. Neuromodulation in post-stroke aphasia treatment. Curr. Phys. Med. Rehabil. Rep. 2020, 8, 44–56. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Y.; Hu, R.; Yang, L.; Wang, M.; Zhang, J.; Lu, H.; Wu, Y.; Du, X. rTMS treatments combined with speech training for a conduction aphasia patient: A case report with MRI study. Medicine 2017, 96, e7399. [Google Scholar] [CrossRef]
- Hartwigsen, G.; Volz, L.J. Probing rapid network reorganization of motor and language functions via neuromodulation and neuroimaging. NeuroImage 2021, 224, 117449. [Google Scholar] [CrossRef]
- Lin, B.F.; Yeh, S.C.; Kao, Y.J.; Lu, C.F.; Tsai, P.Y. Functional remodeling associated with language recovery after repetitive transcranial magnetic stimulation in chronic aphasic stroke. Front. Neurol. 2022, 13, 809843. [Google Scholar] [CrossRef]
- Muller, R.A.; Rothermel, R.D.; Behen, M.E.; Muzik, O.; Mangner, T.J.; Chugani, H.T. Differential patterns of language and motor reorganization following early left hemisphere lesion: A PET study. Arch. Neurol. 1998, 55, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Vikingstad, E.M.; George, K.P.; Johnson, A.F.; Welch, K.M. Cortical language activation in stroke patients recovering from aphasia with functional MRI. Stroke 1999, 30, 2331–2340. [Google Scholar] [CrossRef] [PubMed]
- Thulborn, K.R.; Carpenter, P.A.; Just, M.A. Plasticity of language-related brain function during recovery from stroke. Stroke 1999, 30, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Rosen, H.J.; Petersen, S.E.; Linenweber, M.R.; Snyder, A.Z.; White, D.A.; Chapman, L.; Dromerick, A.W.; Fiez, J.A.; Corbetta, M.D. Neural correlates of recovery from aphasia after damage to left inferior frontal cortex. Neurology 2000, 55, 1883–1894. [Google Scholar] [CrossRef]
- Winhuisen, L.; Thiel, A.; Schumacher, B.; Kessler, J.; Rudolf, J.; Haupt, W.F.; Heiss, W.D. Role of the contralateral inferior frontal gyrus in recovery of language function in poststroke aphasia: A combined repetitive transcranial magnetic stimulation and positron emission tomography study. Stroke 2005, 36, 1759–1763. [Google Scholar] [CrossRef]
- Saur, D.; Lange, R.; Baumgaertner, A.; Schraknepper, V.; Willmes, K.; Rijntjes, M.; Weiller, C. Dynamics of language reorganization after stroke. Brain 2006, 129, 1371–1384. [Google Scholar] [CrossRef]
- Szaflarski, J.P.; Eaton, K.; Ball, A.L.; Banks, C.; Vannest, J.; Allendorfer, J.B.; Page, S.; Holland, S.K. Poststroke aphasia recovery assessed with functional magnetic resonance imaging and a picture identification task. J. Stroke Cerebrovasc. Dis. 2011, 20, 336–345. [Google Scholar] [CrossRef]
- Schlaug, G.; Marchina, S.; Norton, A. Evidence for plasticity in white-matter tracts of patients with chronic Broca’s aphasia undergoing intense intonation-based speech therapy. Ann. N. Y. Acad. Ci. 2009, 1169, 385–394. [Google Scholar] [CrossRef]
- Breier, J.I.; Juranek, J.; Papanicolaou, A.C. Changes in maps of language function and the integrity of the arcuate fasciculus after therapy for chronic aphasia. Neurocase 2011, 17, 506–517. [Google Scholar] [CrossRef]
- Jang, S.H. Diffusion tensor imaging studies on arcuate fasciculus in stroke patients: A review. Front. Hum. Neurosci. 2013, 7, 749. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.H.; Lee, H.D. Recovery of injured arcuate fasciculus in the dominant hemisphere in a patient with an intracerebral hemorrhage. Am. J. Phys. Med. Rehabil. 2014, 93, e15–e18. [Google Scholar] [CrossRef] [PubMed]
- van Hees, S.; McMahon, K.; Angwin, A.; de Zubicaray, G.; Read, S.; Copland, D.A. Changes in white matter connectivity following therapy for anomia post stroke. Neurorehabil. Neural. Repair. 2014, 28, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Nunnari, D.; Bonanno, L.; Bramanti, P.; Marino, S. Diffusion tensor imaging and neuropsychologic assessment in aphasic stroke. J. Stroke Cerebrovasc. Dis. 2014, 23, e477–e478. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.H.; Cho, I.T.; Lim, J.W. Recovery of aphasia and change of injured arcuate fasciculus in the dominant hemisphere in stroke patients. NeuroRehabilitation 2017, 41, 759–764. [Google Scholar] [CrossRef]
- Yu, Q.; Yang, W.; Liu, Y.; Wang, H.; Chen, Z.; Yan, J. Changes in the corpus callosum during the recovery of aphasia: A case report. Medicine 2018, 97, e11155. [Google Scholar] [CrossRef]
- Blom-Smink, M.; Verly, M.; Spielmann, K.; Smits, M.; Ribbers, G.M.; van de Sandt-Koenderman, M.W.M.E. Change in right inferior longitudinal fasciculus integrity is associated with naming recovery in subacute poststroke aphasia. Neurorehabil. Neural. Repair 2020, 34, 784–794. [Google Scholar] [CrossRef]
- Choi, E.B.; Chang, C.H.; Jang, S.H. Restoration of injured arcuate fasciculus in the dominant hemisphere following cranioplasty in a stroke patient. J. Neuroradiol. 2021, 48, 468–470. [Google Scholar] [CrossRef]
- Jang, S.H.; Seo, J.P.; Kwon, Y.H. Recovery of an injured arcuate fasciculus via transcallosal fiber in a stroke patient: A case report. Medicine 2021, 100, e26840. [Google Scholar] [CrossRef]
- Kieronska, S.; Switonska, M.; Meder, G.; Piotrowska, M.; Sokal, P. Tractography alterations in the arcuate and uncinate fasciculi in post-stroke aphasia. Brain Sci. 2021, 11, 53. [Google Scholar] [CrossRef]
- Bae, C.R.; Na, Y.; Cho, M.; Hwang, Y.M.; Tae, W.S.; Pyun, S.B. Structural changes in the arcuate fasciculus and recovery of post-stroke aphasia: A 6-month follow-up study using diffusion tensor imaging. Neurorehabil. Neural. Repair 2022, 36, 633–644. [Google Scholar] [CrossRef]
- Basser, P.J.; Pierpaoli, C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J. Magn. Reson. B 1996, 111, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Assaf, Y.; Pasternak, O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: A review. J. Mol. Neurosci. 2008, 34, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.H.; Lee, H.D. Diagnostic approach to traumatic axonal injury of the spinothalamic tract in individual patients with mild traumatic brain injury. Diagnostics 2019, 9, 199. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.H.; Ha, J.W.; Kim, H.Y.; Seo, Y.S. Recovery of injured Broca’s portion of arcuate fasciculus in the dominant hemisphere in a patient with traumatic brain injury. Medicine 2017, 96, e9183. [Google Scholar] [CrossRef] [PubMed]
- Chernoff, B.L.; Teghipco, A.; Garcea, F.E.; Belkhir, R.; Sims, M.H.; Paul, D.A.; Tivarus, M.E.; Smith, S.O.; Hintz, E.; Pilcher, W.H.; et al. Reorganized language network connectivity after left arcuate fasciculus resection: A case study. Cortex 2020, 123, 173–184. [Google Scholar] [CrossRef]
- Yuan, B.; Zhang, N.; Gong, F.; Wang, X.; Yan, J.; Lu, J.; Wu, J. Longitudinal assessment of network reorganizations and language recovery in postoperative patients with glioma. Brain. Commun. 2022, 4, fcac046. [Google Scholar] [CrossRef]
- Catani, M.; Jones, D.K.; Ffytche, D.H. Perisylvian language networks of the human brain. Ann. Neurol. 2005, 57, 8–16. [Google Scholar] [CrossRef]
- Duffau, H. The anatomo-functional connectivity of language revisited. New insights provided by electrostimulation and tractography. Neuropsychologia 2008, 46, 927–934. [Google Scholar] [CrossRef]
- Saur, D.; Kreher, B.W.; Schnell, S.; Kummerer, D.; Kellmeyer, P.; Vry, M.S.; Umarova, R.; Musso, M.; Glauche, V.; Abel, S.; et al. Ventral and dorsal pathways for language. Proc. Natl. Acad. Sci. USA 2008, 105, 18035–18040. [Google Scholar] [CrossRef]
- Weiller, C.; Bormann, T.; Saur, D.; Musso, M.; Rijntjes, M. How the ventral pathway got lost—And what its recovery might mean. Brain Lang. 2011, 118, 29–39. [Google Scholar] [CrossRef]
- Axer, H.; Klingner, C.M.; Prescher, A. Fiber anatomy of dorsal and ventral language streams. Brain Lang. 2013, 127, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Kummerer, D.; Hartwigsen, G.; Kellmeyer, P.; Glauche, V.; Mader, I.; Kloppel, S.; Suchan, J.; Karnath, H.O.; Weiller, C.; Saur, D. Damage to ventral and dorsal language pathways in acute aphasia. Brain 2013, 136, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, M.V.; Isaev, D.Y.; Dragoy, O.V.; Akinina, Y.S.; Petrushevskiy, A.G.; Fedina, O.N.; Shklovsky, V.M.; Dronkers, N.F. Diffusion-tensor imaging of major white matter tracts and their role in language processing in aphasia. Cortex 2016, 85, 165–181. [Google Scholar] [CrossRef]
- Dick, A.S.; Garic, D.; Graziano, P.; Tremblay, P. The frontal aslant tract (FAT) and its role in speech, language and executive function. Cortex 2019, 111, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhong, S.C.; Zhou, L.; Yu, Y.M.; Tan, X.F.; Wu, M.; Sun, P.; Zhang, W.; Li, J.B.; Cheng, R.D.; et al. Correlations between dual-pathway white matter alterations and language impairment in patients with aphasia: A systematic review and meta-analysis. Neuropsychol. Rev. 2021, 31, 402–418. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Crain, B.J.; Chacko, V.P.; van Zijl, P.C.M. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann. Neurol. 1999, 45, 265–269. [Google Scholar] [CrossRef]
- Neil, J.J. Diffusion imaging concepts for clinicians. J. Magn. Reson. Imaging 2008, 27, 1–7. [Google Scholar] [CrossRef]
- Kim, S.H.; Jang, S.H. Prediction of aphasia outcome using diffusion tensor tractography for arcuate fasciculus in stroke. Am. J. Neuroradiol. 2013, 34, 785–790. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Wang, C.X.; Zhao, X.Q.; Chen, H.Y.; Han, Z.Z.; Wang, Y.J. Diffusion tensor imaging depicting damage to the arcuate fasciculus in patients with conduction aphasia: A study of the Wernicke-Geschwind model. Neurol. Res. 2010, 32, 775–778. [Google Scholar] [CrossRef]
- Seo, J.P.; Kwon, Y.H.; Jang, S.H. Mini-review of studies reporting the repeatability and reproducibility of diffusion tensor imaging. Investig. Magn. Reson. Imaging 2019, 23, 26–33. [Google Scholar]
- Lee, S.K.; Kim, D.I.; Kim, J. Diffusion-tensor MR imaging and fiber tractography: A new method of describing aberrant fiber connections in developmental CNS anomalies—Response. Radiographics 2005, 25, 68. [Google Scholar] [CrossRef] [PubMed]
- Parker, G.J.M.; Alexander, D.C. Probabilistic anatomical connectivity derived from the microscopic persistent angular structure of cerebral tissue. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Sakai, K.; Akazawa, K.; Yuen, S.; Nishimura, T. MR tractography: A review of its clinical applications. Magn. Reson. Med. Sci. 2009, 8, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Breier, J.I.; Juranek, J.; Maher, L.M.; Schmadeke, S.; Men, D.; Papanicolaou, A.C. Behavioral and neurophysiologic response to therapy for chronic aphasia. Arch. Phys. Med. Rehab. 2009, 90, 2026–2033. [Google Scholar] [CrossRef]
- Boyle, M.C.C. Application of semantic feature analysis as a treatment for aphasic dysnomia. Am. J. Speech. Lang. Pathol. 1995, 4, 94–98. [Google Scholar] [CrossRef]
- Leonard, C.; Rochon, E.; Laird, L. Treating naming impairments in aphasia: Findings from a phonological components analysis treatment. Aphasiology 2008, 22, 923–947. [Google Scholar]
- del Toro, C.M.; Bislick, L.P.; Comer, M.; Velozo, C.; Romero, S.; Rothi, L.J.G.; Kendall, D.L. Development of a short form of the boston naming test for individuals with aphasia. J. Speech. Lang. Hear. R. 2011, 54, 1089–1100. [Google Scholar] [CrossRef]
- Kim, H.; Na, D.L. Normative data on the korean version of the western aphasia battery. J. Clin. Exp. Neuropsyc. 2004, 26, 1011–1020. [Google Scholar] [CrossRef]
- Enderby, P.M.; Wood, V.A.; Wade, D.T.; Hewer, R.L. The frenchay aphasia screening test: A short, simple test for aphasia appropriate for non-specialists. Int. Rehabil. Med. 1987, 8, 166–170. [Google Scholar] [CrossRef]
- Yang, Q.; Tress, B.M.; Barber, P.A.; Desmond, P.M.; Darby, D.G.; Gerraty, R.P.; Li, T.; Davis, S.M. Serial study of apparent diffusion coefficient and anisotropy in patients with acute stroke. Stroke 1999, 30, 2382–2390. [Google Scholar] [CrossRef]
- Green, H.A.; Pena, A.; Price, C.J.; Warburton, E.A.; Pickard, J.D.; Carpenter, T.A.; Gillard, J.H. Increased anisotropy in acute stroke: A possible explanation. Stroke 2002, 33, 1517–1521. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zhao, J. Therapeutic effect of low-frequency repetitive transcranial magnetic stimulation on motor aphasia after acute cerebral infarction. Chin. J. Rehabil. 2016, 31, 28–30. [Google Scholar]
- Schlaug, G.; Marchina, S.; Norton, A. From singing to speaking: Why singing may lead to recovery of expressive language function in patients with Broca’s aphasia. Music Percept. 2008, 25, 315–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albert, M.L.; Sparks, R.W.; Helm, N.A. Melodic intonation therapy for aphasia. Arch. Neurol. 1973, 29, 130–131. [Google Scholar] [CrossRef] [PubMed]
- Sparks, R.; Helm, N.; Albert, M. Aphasia rehabilitation resulting from melodic intonation therapy. Cortex 1974, 10, 303–316. [Google Scholar] [CrossRef]
- Linebaugh, C.W.; Shisler, R.J.; Lehner, L. Cueing hierarchies and word retrieval: A therapy program. Aphasiology 2005, 19, 77–92. [Google Scholar] [CrossRef]
- Chang, M.C.; Jung, Y.J.; Jang, S.H. Motor recovery via transcallosal and transpontine fibers in a patient with intracerebral hemorrhage. Am. J. Phys. Med. Rehabil. 2014, 93, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.H.; Lee, J.; Seo, Y.S. Motor recovery by the aberrant pyramidal pathway in a patient with cerebral infarct. Medicine 2020, 99, e20282. [Google Scholar] [CrossRef]
- Cho, M.K.; Jang, S.H. Peri-infarct reorganization of an injured corticospinal tract in a patient with cerebral infarction. Neural. Regen. Res. 2021, 16, 1671–1672. [Google Scholar]
- Jang, S.H.; Cho, M.J. Role of the contra-lesional corticoreticular tract in motor recovery of the paretic leg in stroke: A mini-narrative review. Front. Hum. Neurosci. 2022, 16, 896367. [Google Scholar] [CrossRef]
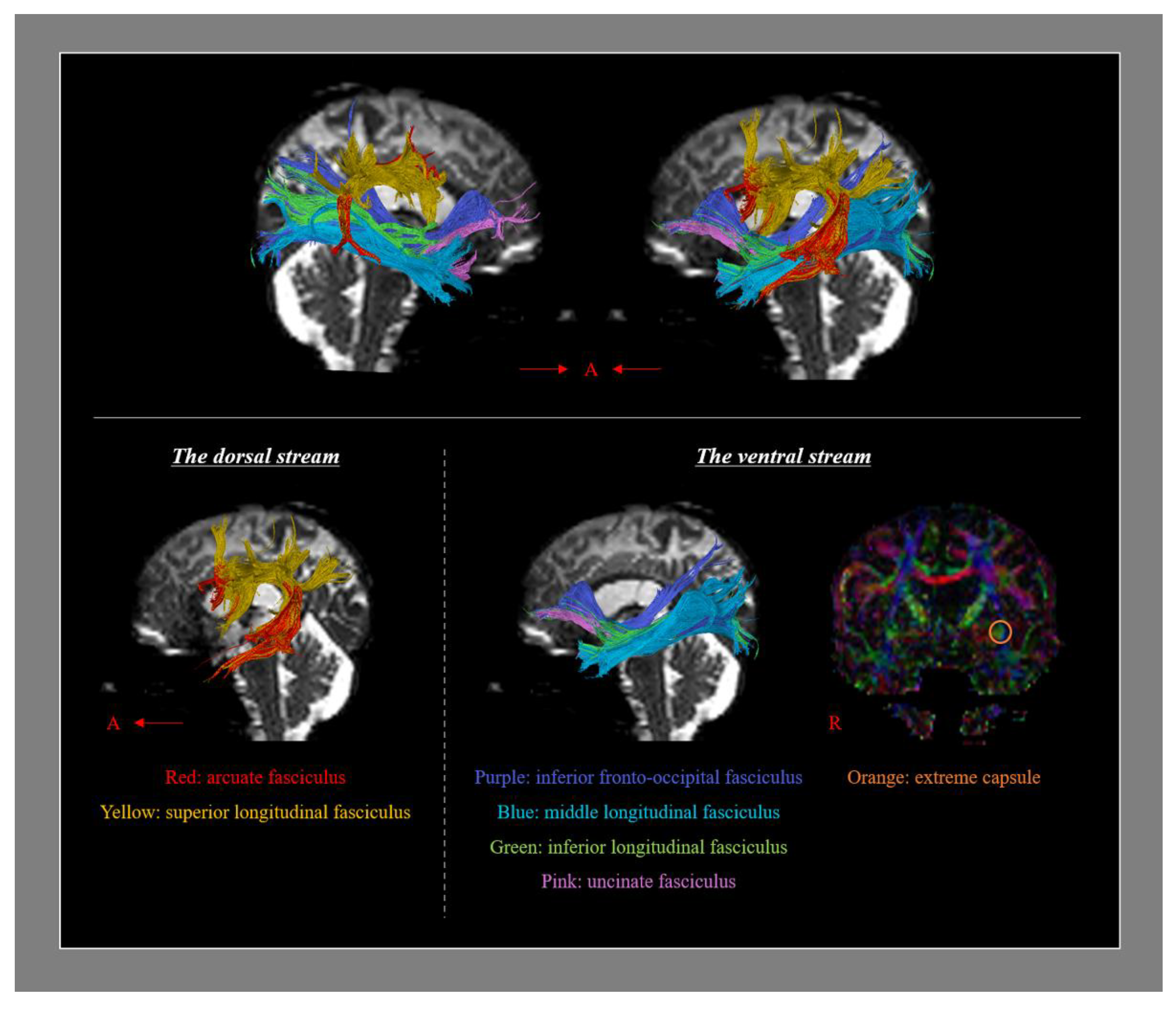


| Language Subdomains | ||||||
|---|---|---|---|---|---|---|
| Naming | Repetition | Syntactic Processing | Reading | Comprehension | ||
| Left dorsal stream | AF | - | 0.48 * | 0.61 * | - | - |
| SLF | - | - | 0.55 * | - | - | |
| Left ventral stream | IFOF | 0.56 * | - | - | 0.34 * | 0.53 * |
| UF | 0.49 * | - | - | 0.28 * | 0.38 * | |
| ILF | 0.35 * | - | - | - | 0.48 * | |
| Authors | Publication Year | Patient No. | Stroke Type | Duration to 1st DTI | Aphasia Evaluation Method | Analyzed Neural Tracts | Results |
|---|---|---|---|---|---|---|---|
| Recovery via the neural tracts in the dominant hemisphere | |||||||
| Breier et al. [19] | 2011 | 1 | Infarction | 5 years | AQ of WAB | AF | FA ↑ (Lt. AF) |
| van Hees et al. [22] | 2014 | 8 | Not described | 17~170 months | BNT | AF UF | FA ↑ |
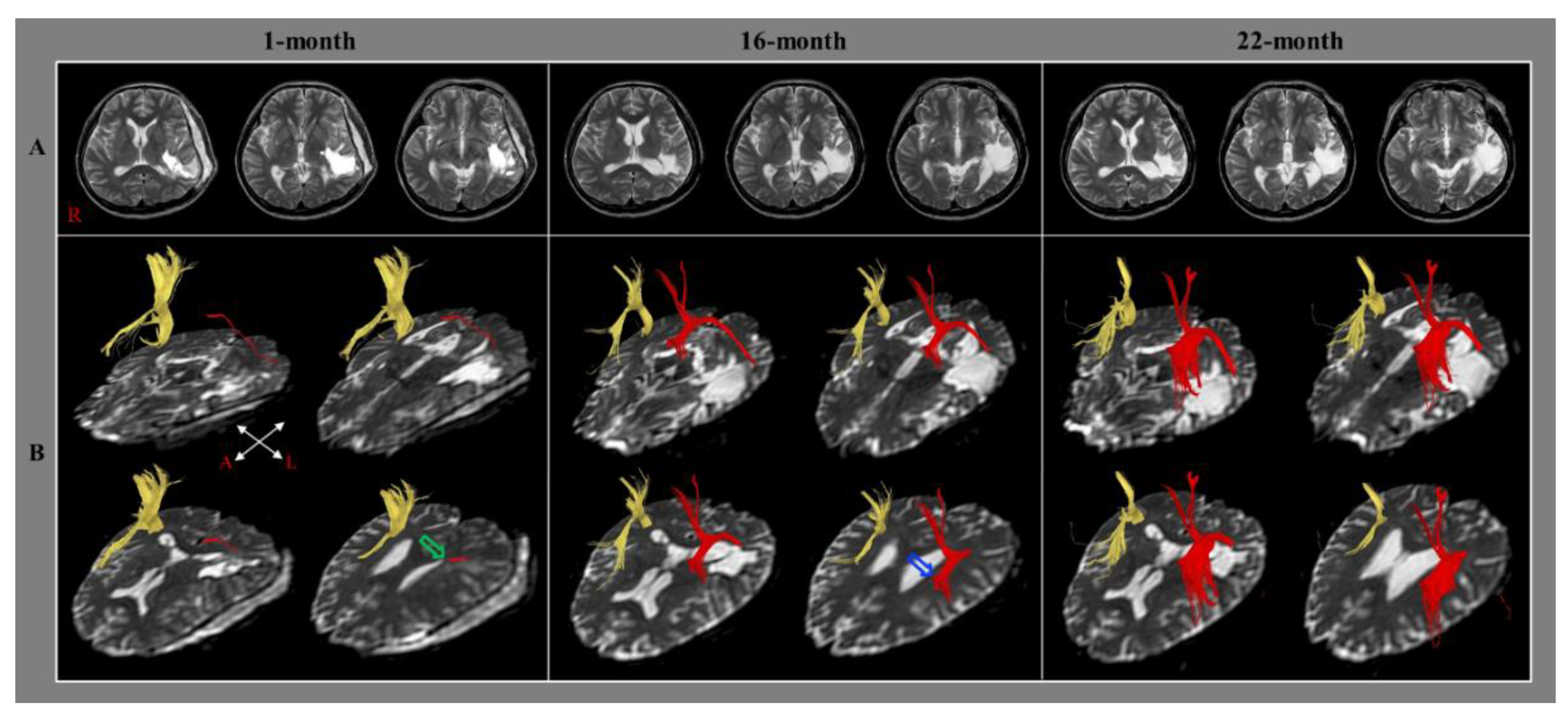
| Jang and Lee [21] | 2014 | 1 | Hemorrhage | 1 month | AQ of WAB | AF | Elongation (Lt. AF) |
| Nunnari et al. [23] | 2014 | 1 | Infarction | Not described | Neuropsychological Examination of Aphasia Battery | AF | FA ↑ Fiber number ↑ (Lt. AF) |
| Jang et al. [24] | 2017 | 16 | Infarction: 11 Hemorrhage: 5 | Within 30 days | AQ of WAB | AF | Voxel number (Lt. AF): positive correlation with AQ |
| Kieronsk et al. [29] | 2021 | 1 | Infarction | 1 day | FAST BNT | AF UF | Fiber number ↑ Tract volume ↑ (Both AFs and Lt. UF) |
| Choi et al. [27] | 2021 | 1 | Hemorrhage | 8 weeks | AQ of WAB | AF | Elongation FA ↓ Fiber number ↑ (Lt. AF) |
| Bae et al. [30] | 2022 | 35 | Stroke | Average 36.06 days | WAB | AF | FA (Lt. AF): positive correlation with AQ |
| Recovery via the transcallosal fibers | |||||||
| Yu et al. [25] | 2018 | 1 | Infarction | 14 months | Modified WAB | Corpus callosum | Transcallosal fibers ↑ (to Lt. Werniche’s and Rt. Broca’s areas) |
| Jang et al. [28] | 2021 | 1 | Infarction | 1 month | AQ of WAB | AF | Transcallosal fiber ↑ (to Rt. AF) |
| Recovery via the neural tracts in the non-dominant hemisphere | |||||||
| Schlaug et al. [18] | 2009 | 6 | Not described | 1 year | Correct information units | Rt. AF | Fiber number ↑ (Rt. AF) |
| Blom-Smink et al. [26] | 2020 | 10 | Infarction: 8 Hemorrhage: 2 | Average 39.6 days | BNT | SLF IFOF ILF MLF UF | Change of FA of Rt. ILF: positive correlation with change of BNT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, S.H.; Yeo, S.S.; Choi, E.B. Diffusion Tensor Tractography Studies on Recovery Mechanisms of Aphasia in Stroke Patients: A Narrative Mini-Review. Healthcare 2022, 10, 1927. https://doi.org/10.3390/healthcare10101927
Jang SH, Yeo SS, Choi EB. Diffusion Tensor Tractography Studies on Recovery Mechanisms of Aphasia in Stroke Patients: A Narrative Mini-Review. Healthcare. 2022; 10(10):1927. https://doi.org/10.3390/healthcare10101927
Chicago/Turabian StyleJang, Sung Ho, Sang Seok Yeo, and Eun Bi Choi. 2022. "Diffusion Tensor Tractography Studies on Recovery Mechanisms of Aphasia in Stroke Patients: A Narrative Mini-Review" Healthcare 10, no. 10: 1927. https://doi.org/10.3390/healthcare10101927
APA StyleJang, S. H., Yeo, S. S., & Choi, E. B. (2022). Diffusion Tensor Tractography Studies on Recovery Mechanisms of Aphasia in Stroke Patients: A Narrative Mini-Review. Healthcare, 10(10), 1927. https://doi.org/10.3390/healthcare10101927





