Online Questionnaire with Fibromyalgia Patients Reveals Correlations among Type of Pain, Psychological Alterations, and Effectiveness of Non-Pharmacological Therapies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participant Recruitment
2.2. Questionnaires
2.3. Statistical Analyses
3. Results
3.1. Sociodemographic Characteristics
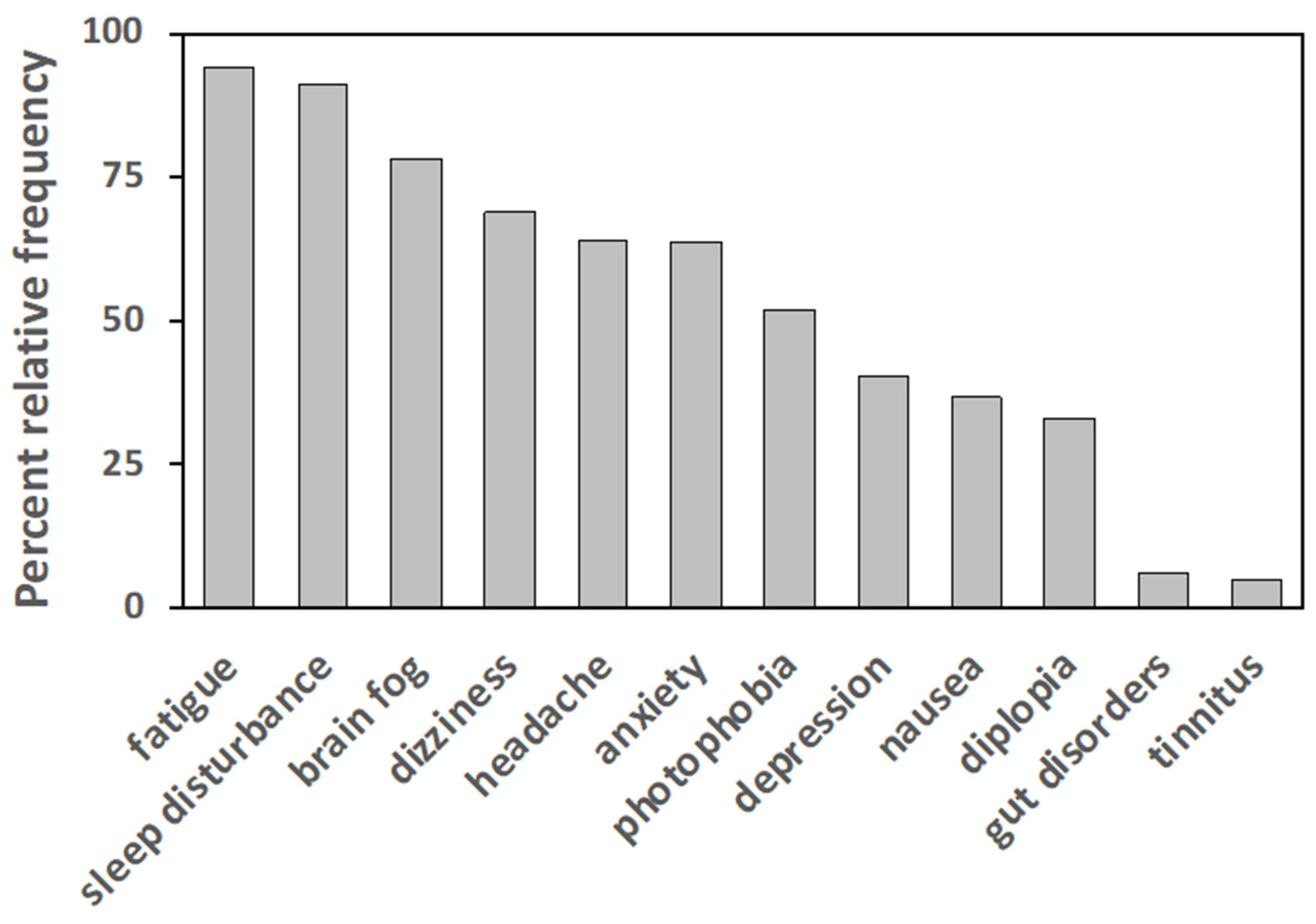
3.2. Clinical Characteristics
3.3. Psychological Profile
3.4. Evaluation of Pain
3.5. Treatment Effectiveness
4. Discussion
4.1. FM Clinical Management Is Affected by Diagnostic Drawback and Delay
4.2. FM as a Central Multisensory Disorder
4.3. Non-Pharmacological Therapies Prevail over Pharmacological Ones
4.4. Limitations of the Study
5. Conclusions
- − a prevalence of neuropathic/nociplastic pain in FM patients, correlated with anxiety, low mood, psychophysical discomfort, and an inability to relax;
- − a perceived higher effectiveness of mind-body non-pharmacological treatments with respect to pharmacological ones;
- − the role of pain types and psychological traits in determining the self-perceived effectiveness of therapies;
- − a high self-perceived effectiveness of dietary interventions, relaxation techniques, and psychotherapy.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bellato, E.; Marini, E.; Castoldi, F.; Barbasetti, N.; Mattei, L.; Bonasia, D.E.; Blonna, D. Fibromyalgia syndrome: Etiology, pathogenesis, diagnosis, and treatment. Pain Res. Treat. 2012, 2012, 426130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarzi-Puttini, P.; Giorgi, V.; Atzeni, F.; Gorla, R.; Kosek, E.; Choy, E.H.; Bazzichi, L.; Hauser, W.; Ablin, J.N.; Aloush, V.; et al. Fibromyalgia position paper. Clin. Exp. Rheumatol. 2021, 39 (Suppl. 130), 186–193. [Google Scholar] [CrossRef] [PubMed]
- Bartels, E.M.; Dreyer, L.; Jacobsen, S.; Jespersen, A.; Bliddal, H.; Danneskiold-Samsoe, B. Fibromyalgia, diagnosis and prevalence. Are gender differences explainable? Ugeskr. Laeger. 2009, 171, 3588–3592. [Google Scholar] [PubMed]
- Inanici, F.; Yunus, M.B. History of fibromyalgia: Past to present. Curr. Pain Headache Rep. 2004, 8, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Clauw, D.J.; Crofford, L.J. Chronic widespread pain and fibromyalgia: What we know, and what we need to know. Best Pract. Res. Clin. Rheumatol. 2003, 17, 685–701. [Google Scholar] [CrossRef]
- Petersel, D.L.; Dror, V.; Cheung, R. Central amplification and fibromyalgia: Disorder of pain processing. J. Neurosci. Res. 2011, 89, 29–34. [Google Scholar] [CrossRef]
- Clauw, D.J.; Arnold, L.M.; McCarberg, B.H.; FibroCollaborative. The science of fibromyalgia. Mayo Clin. Proc. 2011, 86, 907–911. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Hauser, W.; Katz, R.L.; Mease, P.J.; Russell, A.S.; Russell, I.J.; Walitt, B. 2016 revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin. Arthritis Rheum. 2016, 46, 319–329. [Google Scholar] [CrossRef]
- Bazzichi, L.; Giacomelli, C.; Consensi, A.; Giorgi, V.; Batticciotto, A.; Di Franco, M.; Sarzi-Puttini, P. One year in review 2020: Fibromyalgia. Clin. Exp. Rheumatol. 2020, 38 (Suppl. 123), 3–8. [Google Scholar]
- Chinn, S.; Caldwell, W.; Gritsenko, K. Fibromyalgia pathogenesis and treatment options update. Curr. Pain Headache Rep. 2016, 20, 25. [Google Scholar] [CrossRef]
- Bair, M.J.; Krebs, E.E. Fibromyalgia. Ann. Intern. Med. 2020, 172, ITC33–ITC48. [Google Scholar] [CrossRef]
- Solano, C.; Martinez, A.; Becerril, L.; Vargas, A.; Figueroa, J.; Navarro, C.; Ramos-Remus, C.; Martinez-Lavin, M. Autonomic dysfunction in fibromyalgia assessed by the Composite Autonomic Symptoms Scale (COMPASS). J. Clin. Rheumatol. 2009, 15, 172–176. [Google Scholar] [CrossRef]
- Boomershine, C.S. Fibromyalgia: The prototypical central sensitivity syndrome. Curr. Rheumatol. Rev. 2015, 11, 131–145. [Google Scholar] [CrossRef]
- Goldenberg, D.L. Pharmacological treatment of fibromyalgia and other chronic musculoskeletal pain. Best Pract. Res. Clin. Rheumatol. 2007, 21, 499–511. [Google Scholar] [CrossRef]
- Araujo, F.M.; DeSantana, J.M. Physical therapy modalities for treating fibromyalgia. F1000Research 2019, 8, 2030. [Google Scholar] [CrossRef]
- Maffei, M.E. Fibromyalgia: Recent advances in diagnosis, classification, pharmacotherapy and alternative remedies. Int. J. Mol. Sci. 2020, 21, 7877. [Google Scholar] [CrossRef]
- Maurer, A.J.; Lissounov, A.; Knezevic, I.; Candido, K.D.; Knezevic, N.N. Pain and sex hormones: A review of current understanding. Pain Manag. 2016, 6, 285–296. [Google Scholar] [CrossRef]
- Albert, P.R. Why is depression more prevalent in women? J. Psychiatr. Neurosci. 2015, 40, 219–221. [Google Scholar] [CrossRef]
- Todd, C.; Lagman-Bartolome, A.M.; Lay, C. Women and migraine: The role of hormones. Curr. Neurol. Neurosci. Rep. 2018, 18, 42. [Google Scholar] [CrossRef]
- Teggi, R.; Manfrin, M.; Balzanelli, C.; Gatti, O.; Mura, F.; Quaglieri, S.; Pilolli, F.; Redaelli de Zinis, L.O.; Benazzo, M.; Bussi, M. Point prevalence of vertigo and dizziness in a sample of 2672 subjects and correlation with headaches. Acta Otorhinolaryngol. Ital. 2016, 36, 215–219. [Google Scholar] [CrossRef]
- Coppens, E.; Kempke, S.; Van Wambeke, P.; Claes, S.; Morlion, B.; Luyten, P.; Van Oudenhove, L. Cortisol and subjective stress responses to acute psychosocial stress in fibromyalgia patients and control participants. Psychosom. Med. 2018, 80, 317–326. [Google Scholar] [CrossRef]
- Dhabhar, F.S. Effects of stress on immune function: The good, the bad, and the beautiful. Immunol. Res. 2014, 58, 193–210. [Google Scholar] [CrossRef]
- Ren, K.; Dubner, R. Interactions between the immune and nervous systems in pain. Nat. Med. 2010, 16, 1267–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadetoff, D.; Lampa, J.; Westman, M.; Andersson, M.; Kosek, E. Evidence of central inflammation in fibromyalgia-increased cerebrospinal fluid interleukin-8 levels. J. Neuroimmunol. 2012, 242, 33–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolcino, M.; Tinazzi, E.; Puccetti, A.; Lunardi, C. Gene expression profiling in fibromyalgia indicates an autoimmune origin of the disease and opens new avenues for targeted therapy. J. Clin. Med. 2020, 9, 1814. [Google Scholar] [CrossRef] [PubMed]
- Kundakci, B.; Kaur, J.; Goh, S.L.; Hall, M.; Doherty, M.; Zhang, W.; Abhishek, A. Efficacy of nonpharmacological interventions for individual features of fibromyalgia: A systematic review and meta-analysis of randomised controlled trials. Pain 2022, 163, 1432–1445. [Google Scholar] [CrossRef] [PubMed]
- Ader, R. On the development of psychoneuroimmunology. Eur. J. Pharmacol. 2000, 405, 167–176. [Google Scholar] [CrossRef]
- Franca, K.; Lotti, T.M. Psycho-neuro-endocrine-immunology: A psychobiological concept. Adv. Exp. Med. Biol. 2017, 996, 123–134. [Google Scholar] [CrossRef]
- Meeus, M.; Nijs, J. Central sensitization: A biopsychosocial explanation for chronic widespread pain in patients with fibromyalgia and chronic fatigue syndrome. Clin. Rheumatol. 2007, 26, 465–473. [Google Scholar] [CrossRef] [Green Version]
- Freynhagen, R.; Baron, R.; Gockel, U.; Tolle, T.R. painDETECT: A new screening questionnaire to identify neuropathic components in patients with back pain. Curr. Med. Res. Opin. 2006, 22, 1911–1920. [Google Scholar] [CrossRef]
- Zotti, A.M.; Bertolotti, G.; Michielin, P.; Sanavio, E.; Vidotto, G. Linee Guida per lo Screening di Tratti di Personalità, Cognizioni e Comportamenti Avversi alla Salute. Manuale d’Uso per il CBA Forma Hospital; Maugeri Foundation Books: Pavia, Italy, 2000; p. 126. [Google Scholar]
- Brackett, M.A.; Rivers, S.E.; Shiffman, S.; Lerner, N.; Salovey, P. Relating emotional abilities to social functioning: A comparison of self-report and performance measures of emotional intelligence. J. Pers. Soc. Psychol. 2006, 91, 780–795. [Google Scholar] [CrossRef]
- Bailly, F.; Cantagrel, A.; Bertin, P.; Perrot, S.; Thomas, T.; Lansaman, T.; Grange, L.; Wendling, D.; Dovico, C.; Trouvin, A.P. Part of pain labelled neuropathic in rheumatic disease might be rather nociplastic. RMD Open 2020, 6, e001326. [Google Scholar] [CrossRef]
- Kulekcioglu, S. Diagnostic difficulty, delayed diagnosis, and increased tendencies of surgical treatment in fibromyalgia syndrome. Clin. Rheumatol. 2021, 41, 831–837. [Google Scholar] [CrossRef]
- Martinez-Jauand, M.; Sitges, C.; Femenia, J.; Cifre, I.; Gonzalez, S.; Chialvo, D.; Montoya, P. Age-of-onset of menopause is associated with enhanced painful and non-painful sensitivity in fibromyalgia. Clin. Rheumatol. 2013, 32, 975–981. [Google Scholar] [CrossRef]
- Williams, D.A.; Clauw, D.J. Understanding fibromyalgia: Lessons from the broader pain research community. J. Pain 2009, 10, 777–791. [Google Scholar] [CrossRef] [Green Version]
- Macfarlane, G.J.; Kronisch, C.; Dean, L.E.; Atzeni, F.; Hauser, W.; Fluss, E.; Choy, E.; Kosek, E.; Amris, K.; Branco, J.; et al. EULAR revised recommendations for the management of fibromyalgia. Ann. Rheum. Dis. 2017, 76, 318–328. [Google Scholar] [CrossRef]
- Bair, M.J.; Robinson, R.L.; Katon, W.; Kroenke, K. Depression and pain comorbidity: A literature review. Arch. Intern. Med. 2003, 163, 2433–2445. [Google Scholar] [CrossRef] [Green Version]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef]
- Coaccioli, S.; Varrassi, G.; Sabatini, C.; Marinangeli, F.; Giuliani, M.; Puxeddu, A. Fibromyalgia: Nosography and therapeutic perspectives. Pain Pract. 2008, 8, 190–201. [Google Scholar] [CrossRef]
- Groh, A.; Krieger, P.; Mease, R.A.; Henderson, L. Acute and chronic pain processing in the thalamocortical system of humans and animal models. Neuroscience 2018, 387, 58–71. [Google Scholar] [CrossRef]
- Guillery, R.W.; Sherman, S.M. Thalamic relay functions and their role in corticocortical communication: Generalizations from the visual system. Neuron 2002, 33, 163–175. [Google Scholar] [CrossRef]
- Hache, G.; Coudore, F.; Gardier, A.M.; Guiard, B.P. Monoaminergic antidepressants in the relief of pain: Potential therapeutic utility of triple reuptake inhibitors (TRIs). Pharmaceuticals 2011, 4, 285–342. [Google Scholar] [CrossRef] [Green Version]
- Galvez-Sanchez, C.M.; Duschek, S.; Reyes Del Paso, G.A. Psychological impact of fibromyalgia: Current perspectives. Psychol. Res. Behav. Manag. 2019, 12, 117–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braz Ade, S.; de Paula, A.P.; Diniz Mde, F.; de Almeida, R.N. Non-pharmacological therapy and complementary and alternative medicine in fibromyalgia. Rev. Bras. Reumatol. 2011, 51, 269–282. [Google Scholar]
- Hassett, A.L.; Gevirtz, R.N. Nonpharmacologic treatment for fibromyalgia: Patient education, cognitive-behavioral therapy, relaxation techniques, and complementary and alternative medicine. Rheum. Dis. Clin. N. Am. 2009, 35, 393–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrot, S.; Russell, I.J. More ubiquitous effects from non-pharmacologic than from pharmacologic treatments for fibromyalgia syndrome: A meta-analysis examining six core symptoms. Eur. J. Pain 2014, 18, 1067–1080. [Google Scholar] [CrossRef] [PubMed]
- Coppens, E.; Van Wambeke, P.; Morlion, B.; Weltens, N.; Giao Ly, H.; Tack, J.; Luyten, P.; Van Oudenhove, L. Prevalence and impact of childhood adversities and post-traumatic stress disorder in women with fibromyalgia and chronic widespread pain. Eur. J. Pain 2017, 21, 1582–1590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagliai, G.; Giangrandi, I.; Dinu, M.; Sofi, F.; Colombini, B. Nutritional interventions in the management of fibromyalgia syndrome. Nutrients 2020, 12, 2525. [Google Scholar] [CrossRef]
- Makrani, A.H.; Afshari, M.; Ghajar, M.; Forooghi, Z.; Moosazadeh, M. Vitamin D and fibromyalgia: A meta-analysis. Korean J. Pain 2017, 30, 250–257. [Google Scholar] [CrossRef]
- Rossi, A.; Di Lollo, A.C.; Guzzo, M.P.; Giacomelli, C.; Atzeni, F.; Bazzichi, L.; Di Franco, M. Fibromyalgia and nutrition: What news? Clin. Exp. Rheumatol. 2015, 33, S117–S125. [Google Scholar]
- Erdrich, S.; Hawrelak, J.A.; Myers, S.P.; Harnett, J.E. Determining the association between fibromyalgia, the gut microbiome and its biomarkers: A systematic review. BMC Musculoskelet Disord. 2020, 21, 181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ursini, F.; Naty, S.; Grembiale, R.D. Fibromyalgia and obesity: The hidden link. Rheumatol. Int. 2011, 31, 1403–1408. [Google Scholar] [CrossRef] [PubMed]
- NCD Risk Factor Collaboration. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar] [CrossRef]
- Lovejoy, J.C.; Champagne, C.M.; de Jonge, L.; Xie, H.; Smith, S.R. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int. J. Obes. 2008, 32, 949–958. [Google Scholar] [CrossRef] [Green Version]
- Sarzi-Puttini, P.; Giorgi, V.; Marotto, D.; Atzeni, F. Fibromyalgia: An update on clinical characteristics, aetiopathogenesis and treatment. Nat. Rev. Rheumatol. 2020, 16, 645–660. [Google Scholar] [CrossRef]
- Busch, A.J.; Webber, S.C.; Brachaniec, M.; Bidonde, J.; Bello-Haas, V.D.; Danyliw, A.D.; Overend, T.J.; Richards, R.S.; Sawant, A.; Schachter, C.L. Exercise therapy for fibromyalgia. Curr. Pain Headache Rep. 2011, 15, 358–367. [Google Scholar] [CrossRef] [Green Version]
- O’Dwyer, T.; Maguire, S.; Mockler, D.; Durcan, L.; Wilson, F. Behaviour change interventions targeting physical activity in adults with fibromyalgia: A systematic review. Rheumatol. Int. 2019, 39, 805–817. [Google Scholar] [CrossRef]
- NCCI.; NIH. Relaxation Techniques: What You Need to Know. Available online: https://www.nccih.nih.gov/health/relaxation-techniques-what-you-need-to-know (accessed on 19 December 2021).
- Park, Y.J.; Park, Y.B. Clinical utility of paced breathing as a concentration meditation practice. Complement. Ther. Med. 2012, 20, 393–399. [Google Scholar] [CrossRef]
- Critchley, H.D.; Nicotra, A.; Chiesa, P.A.; Nagai, Y.; Gray, M.A.; Minati, L.; Bernardi, L. Slow breathing and hypoxic challenge: Cardiorespiratory consequences and their central neural substrates. PLoS ONE 2015, 10, e0127082. [Google Scholar] [CrossRef] [Green Version]
- Zaccaro, A.; Piarulli, A.; Laurino, M.; Garbella, E.; Menicucci, D.; Neri, B.; Gemignani, A. How breath-control can change your life: A systematic review on psycho-physiological correlates of slow breathing. Front. Hum. Neurosci. 2018, 12, 353. [Google Scholar] [CrossRef] [Green Version]
- Pavlov, V.A.; Tracey, K.J. The vagus nerve and the inflammatory reflex--linking immunity and metabolism. Nat. Rev. Endocrinol. 2012, 8, 743–754. [Google Scholar] [CrossRef]
- Buric, I.; Farias, M.; Jong, J.; Mee, C.; Brazil, I.A. What is the molecular signature of mind-body interventions? A systematic review of gene expression changes induced by meditation and related practices. Front. Immunol. 2017, 8, 670. [Google Scholar] [CrossRef] [Green Version]
- Meeus, M.; Nijs, J.; Vanderheiden, T.; Baert, I.; Descheemaeker, F.; Struyf, F. The effect of relaxation therapy on autonomic functioning, symptoms and daily functioning, in patients with chronic fatigue syndrome or fibromyalgia: A systematic review. Clin. Rehabil. 2015, 29, 221–233. [Google Scholar] [CrossRef]
- Sorrell, J.M. Diagnostic and statistical manual of mental disorders-5: Implications for older adults and their families. J. Psychosoc. Nurs. Ment. Health Serv. 2013, 51, 19–22. [Google Scholar] [CrossRef]
- Sivik, T. Psycho-neuro-endocrino-immunology (PNEI): A common language for the whole human body. In Proceedings of the 16th World Congress on Psychosomatic Medicine, Göteborg, Sweden, 24–29 August 2001. [Google Scholar]
- Bernardy, K.; Klose, P.; Welsch, P.; Hauser, W. Efficacy, acceptability and safety of cognitive behavioural therapies in fibromyalgia syndrome—A systematic review and meta-analysis of randomized controlled trials. Eur. J. Pain 2018, 22, 242–260. [Google Scholar] [CrossRef] [Green Version]
- Epstein, S.A.; Kay, G.; Clauw, D.; Heaton, R.; Klein, D.; Krupp, L.; Kuck, J.; Leslie, V.; Masur, D.; Wagner, M.; et al. Psychiatric disorders in patients with fibromyalgia. A multicenter investigation. Psychosomatics 1999, 40, 57–63. [Google Scholar] [CrossRef]
- Tan, A.C.; Jaaniste, T.; Champion, D. Chronic widespread pain and fibromyalgia syndrome: Life-course risk markers in young people. Pain Res. Manag. 2019, 2019, 6584753. [Google Scholar] [CrossRef] [Green Version]
- Alciati, A.; Nucera, V.; Masala, I.F.; Giallanza, M.; La Corte, L.; Giorgi, V.; Sarzi-Puttini, P.; Atzeni, F. One year in review 2021: Fibromyalgia. Clin. Exp. Rheumatol. 2021, 39 (Suppl. 130), 3–12. [Google Scholar] [CrossRef]








| Gender | Female | 88.1 |
|---|---|---|
| Male | 4.0 | |
| No answer | 7.9 | |
| Education | Primary | 1.4 |
| Lower secondary | 17 | |
| Upper secondary | 55.7 | |
| Academic degree | 18.5 | |
| PhD or equivalent | 7.1 | |
| No answer | 0.3 | |
| Marital status | Single | 22.2 |
| Married/cohabitant | 59.9 | |
| Separated/divorced | 15.6 | |
| Widowed | 2.3 | |
| Number of sons | 0 | 37.2 |
| 1 | 28.4 | |
| 2 | 25.9 | |
| 3 | 6.8 | |
| >3 | 1.7 | |
| Employment | Grey-collar | 39.8 |
| White-collar | 11.9 | |
| Blue-collar | 8.8 | |
| Shopkeeper | 3.4 | |
| Unemployed | 35 | |
| No answer | 1.1 |
| Min | Q1 | Median | Mean ± s.d. | Q3 | Max | |
|---|---|---|---|---|---|---|
| Height (cm) | 147 | 160 | 163 | 164 ± 6 | 168 | 193 |
| Weight (Kg) | 39 | 57 | 65 | 67.9 ± 15.4 | 76 | 125 |
| BMI | 15.6 | 21.3 | 24.2 | 25.3 ± 5.4 | 28.3 | 45.9 |
| Patient age (years) | 18 | 41 | 50 | 47.9 ± 10.8 | 56 | 86 |
| Age-of-onset (years) | 13 | 36 | 44 | 42.3 ± 10.1 | 50 | 83 |
| Disease duration (years) | <1 | 5 | 9 | 11.7 ± 9.3 | 15 | 49 |
| Diagnostic delay (years) | <1 | 1 | 3 | 6.35 ± 6 | 8 | 48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demori, I.; Molinari, E.; Rapallo, F.; Mucci, V.; Marinelli, L.; Losacco, S.; Burlando, B. Online Questionnaire with Fibromyalgia Patients Reveals Correlations among Type of Pain, Psychological Alterations, and Effectiveness of Non-Pharmacological Therapies. Healthcare 2022, 10, 1975. https://doi.org/10.3390/healthcare10101975
Demori I, Molinari E, Rapallo F, Mucci V, Marinelli L, Losacco S, Burlando B. Online Questionnaire with Fibromyalgia Patients Reveals Correlations among Type of Pain, Psychological Alterations, and Effectiveness of Non-Pharmacological Therapies. Healthcare. 2022; 10(10):1975. https://doi.org/10.3390/healthcare10101975
Chicago/Turabian StyleDemori, Ilaria, Elena Molinari, Fabio Rapallo, Viviana Mucci, Lucio Marinelli, Serena Losacco, and Bruno Burlando. 2022. "Online Questionnaire with Fibromyalgia Patients Reveals Correlations among Type of Pain, Psychological Alterations, and Effectiveness of Non-Pharmacological Therapies" Healthcare 10, no. 10: 1975. https://doi.org/10.3390/healthcare10101975
APA StyleDemori, I., Molinari, E., Rapallo, F., Mucci, V., Marinelli, L., Losacco, S., & Burlando, B. (2022). Online Questionnaire with Fibromyalgia Patients Reveals Correlations among Type of Pain, Psychological Alterations, and Effectiveness of Non-Pharmacological Therapies. Healthcare, 10(10), 1975. https://doi.org/10.3390/healthcare10101975









