Pneumocystis Pneumonia in a Non-Immunocompromised Lung Cancer Patient after Surgery: A Case Report
Abstract
1. Introduction
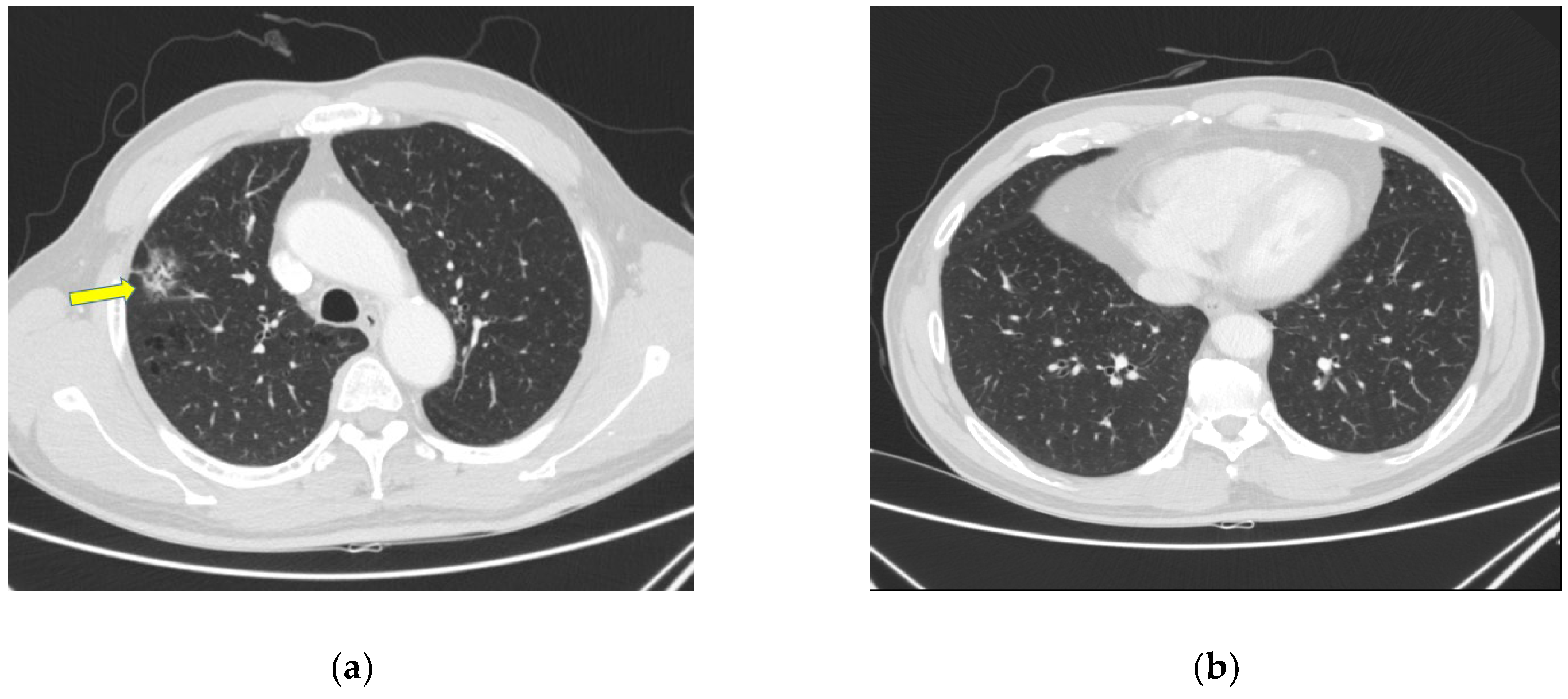
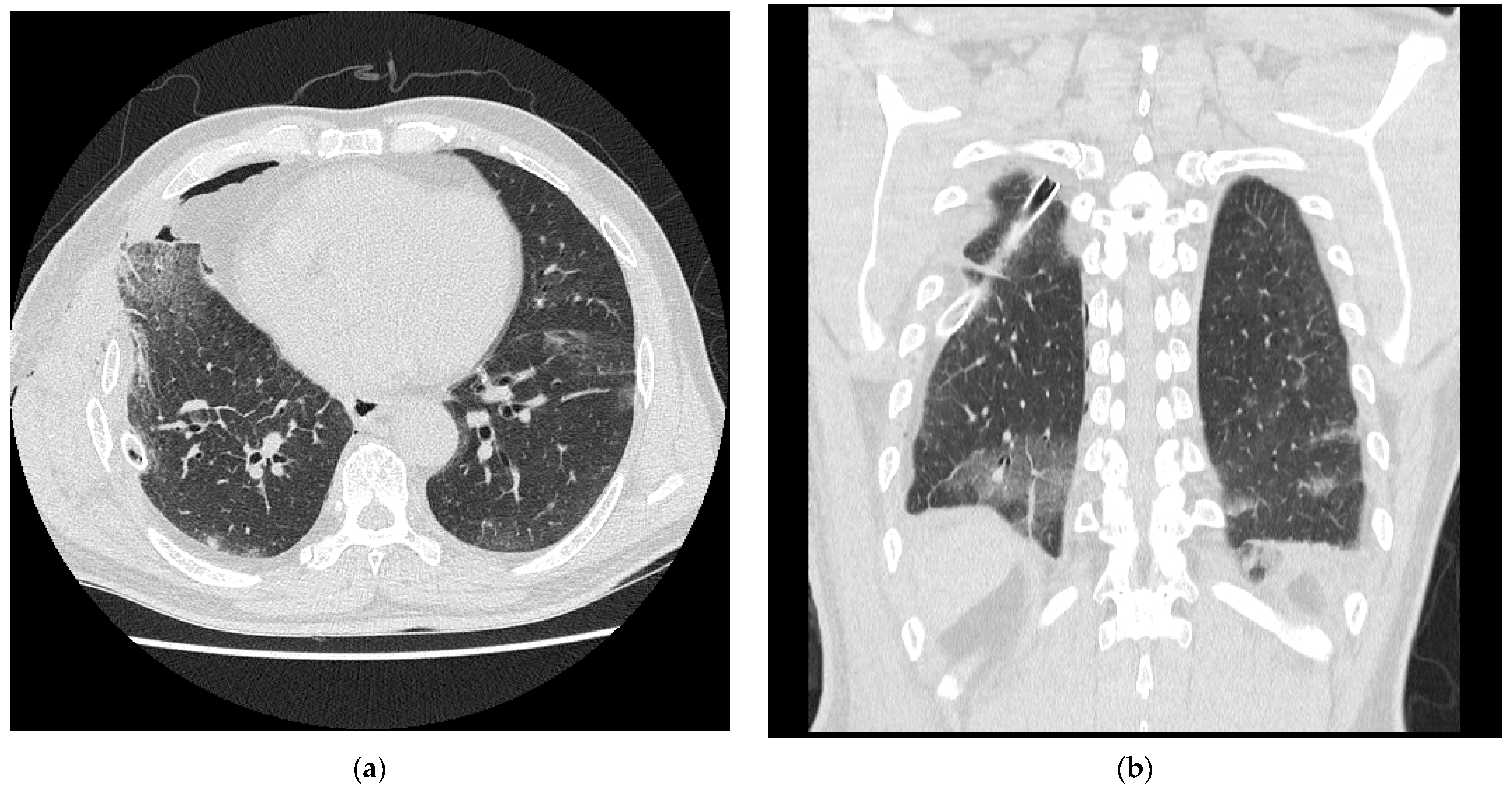
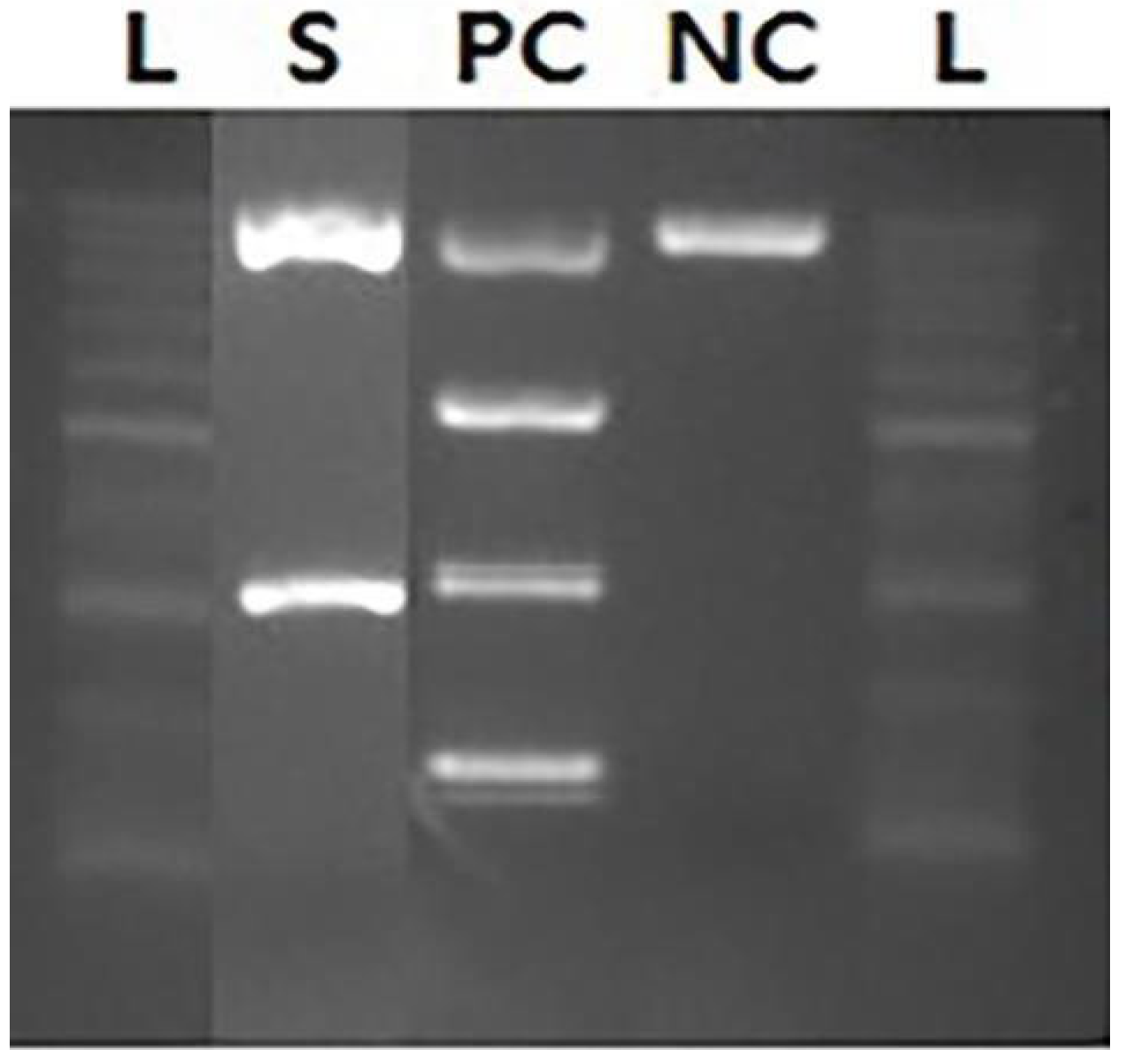
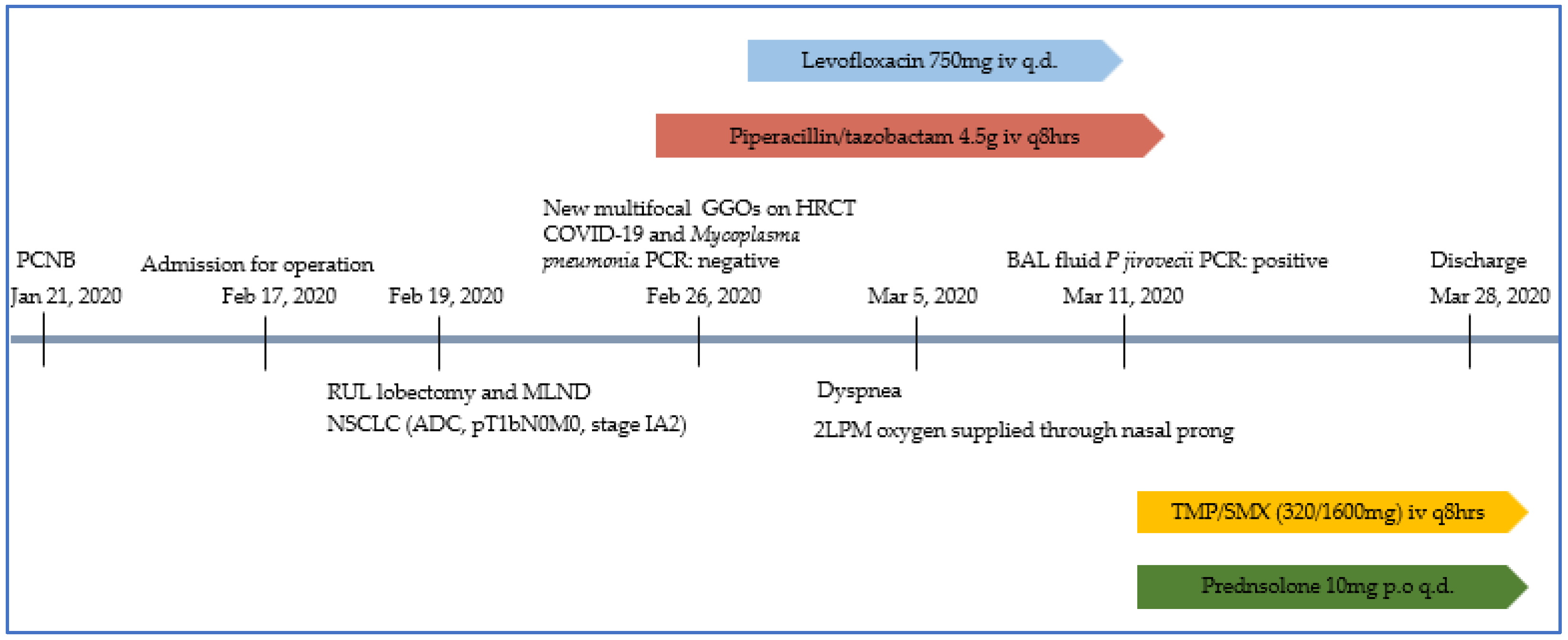
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, J.J.; Lee, C.H. Pneumocystis pneumonia. J. Formos. Med. Assoc. 2008, 107, 830–842. [Google Scholar] [CrossRef]
- Morjaria, S.; Frame, J.; Franco-Garcia, A.; Geyer, A.; Kamboj, M.; Babady, N.E. Clinical Performance of (1,3) Beta-D Glucan for the Diagnosis of Pneumocystis Pneumonia (PCP) in Cancer Patients Tested With PCP Polymerase Chain Reaction. Clin. Infect. Dis. 2019, 69, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- Luque Paz, D.; Jouneau, S.; Tattevin, P.; Ricordel, C. Pneumocystis in metastatic lung cancer, a pragmatic approach in support of prophylaxis. BMJ Case Rep. 2021, 14, e232895. [Google Scholar] [CrossRef] [PubMed]
- Fillatre, P.; Decaux, O.; Jouneau, S.; Revest, M.; Gacouin, A.; Robert-Gangneux, F.; Fresnel, A.; Guiguen, C.; Le Tulzo, Y.; Jégo, P.; et al. Incidence of Pneumocystis jiroveci pneumonia among groups at risk in HIV-negative patients. Am. J. Med. 2014, 127, 1242-e11. [Google Scholar] [CrossRef] [PubMed]
- White, P.L.; Backx, M.; Barnes, R.A. Diagnosis and management of Pneumocystis jirovecii infection. Expert Rev. Anti-Infect. Ther. 2017, 15, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Kim, E.Y.; Lee, S.H.; Roh, Y.H.; Leem, A.Y.; Song, J.H.; Kim, S.Y.; Chung, K.S.; Jung, J.Y.; Kang, Y.A.; et al. Risk factors and clinical characteristics of Pneumocystis jirovecii pneumonia in lung cancer. Sci. Rep. 2019, 9, 2094. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, A.; Schauwvlieghe, A.; Algoe, S.; van Hellemond, J.J.; Reynders, M.; Vandecasteele, S.; Boelens, J.; Depuydt, P.; Rijnders, B. Epidemiology of Pneumocystis jirovecii pneumonia and (non-)use of Prophylaxis. Front. Cell. Infect. Microbiol. 2020, 10, 224. [Google Scholar] [CrossRef] [PubMed]
- Bateman, M.; Oladele, R.; Kolls, J.K. Diagnosing Pneumocystis jirovecii pneumonia: A review of current methods and novel approaches. Med. Mycol. 2020, 58, 1015–1028. [Google Scholar] [CrossRef] [PubMed]
- Sivaraj, V.; Cliff, P.; Douthwaite, S.; Smith, M.; Kulasegaram, R. Pneumocystis jirovecii pneumonia PCR test on upper respiratory tract swab. HIV Med. 2021, 22, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Sarasombath, P.T.; Thongpiya, J.; Chulanetra, M.; Wijit, S.; Chinabut, P.; Ongrotchanakun, J.; Jitmuang, A.; Wanachiwanawin, D. Quantitative PCR to discriminate between pneumocystis pneumonia and colonization in HIV and non-HIV immunocompromised patients. Front. Microbiol. 2021, 12, 729193. [Google Scholar] [CrossRef] [PubMed]
- Le Gal, S.; Bonnet, P.; Huguenin, A.; Chapelle, C.; Boulic, P.; Tonnelier, J.M.; Moal, M.C.; Gut-Gobert, C.; Barnier, A.; Nevez, G. The shift from pulmonary colonization to Pneumocystis pneumonia. Med. Mycol. 2021, 59, 510–513. [Google Scholar] [CrossRef] [PubMed]
- Doello, K.; Amezcua, V.; Garcia, J.; Valdivia, J. Pneumocystis jirovecii Pneumonia in a Non-small Cell Lung Cancer Patient on Chemoradiotherapy: A Case Report. Saudi J. Med. Med. Sci. 2020, 8, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Harada, S.; Hayama, B.; Hoashi, K.; Enokida, T.; Sasaki, T.; Okamoto, K.; Nakano, K.; Ohkushi, D. Clinical characteristics and risk factors associated with Pneumocystis jirovecii infection in patients with solid tumors: Study of thirteen-year medical records of a large cancer center. BMC Cancer 2021, 21, 987. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, Y.; Wang, Y.; Liu, J.; Wen, Z.; Chen, H.; Zhu, Y.; Wang, J.; Wan, L.; Li, F.; et al. Lung cancer surgery in HIV-infected patients: An analysis of postoperative complications and long-term survival. Thorac Cancer 2020, 11, 2146–2154. [Google Scholar] [CrossRef] [PubMed]
- Della Rocca, G.; Vetrugno, L.; Coccia, C.; Pierconti, F.; Badagliacca, R.; Vizza, C.D.; Papale, M.; Melis, E.; Facciolo, F. Preoperative Evaluation of Patients Undergoing Lung Resection Surgery: Defining the Role of the Anesthesiologist on a Multidisciplinary Team. J. Cardiothorac. Vasc. Anesth. 2016, 30, 530–538. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.-W.; Lee, J.-H.; Lee, H.-J.; Kim, S.-W.; Choi, H.-S. Pneumocystis Pneumonia in a Non-Immunocompromised Lung Cancer Patient after Surgery: A Case Report. Healthcare 2022, 10, 2063. https://doi.org/10.3390/healthcare10102063
Kim T-W, Lee J-H, Lee H-J, Kim S-W, Choi H-S. Pneumocystis Pneumonia in a Non-Immunocompromised Lung Cancer Patient after Surgery: A Case Report. Healthcare. 2022; 10(10):2063. https://doi.org/10.3390/healthcare10102063
Chicago/Turabian StyleKim, Tae-Woo, Jun-Ho Lee, Hyo-Jin Lee, So-Woon Kim, and Hye-Sook Choi. 2022. "Pneumocystis Pneumonia in a Non-Immunocompromised Lung Cancer Patient after Surgery: A Case Report" Healthcare 10, no. 10: 2063. https://doi.org/10.3390/healthcare10102063
APA StyleKim, T.-W., Lee, J.-H., Lee, H.-J., Kim, S.-W., & Choi, H.-S. (2022). Pneumocystis Pneumonia in a Non-Immunocompromised Lung Cancer Patient after Surgery: A Case Report. Healthcare, 10(10), 2063. https://doi.org/10.3390/healthcare10102063





