Current Expertise, Opinions, and Attitude toward TNF-⍺ Antagonist Biosimilars among Physicians: A Self-Administered Online Survey in Western Switzerland
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Respondent’s Characteristics
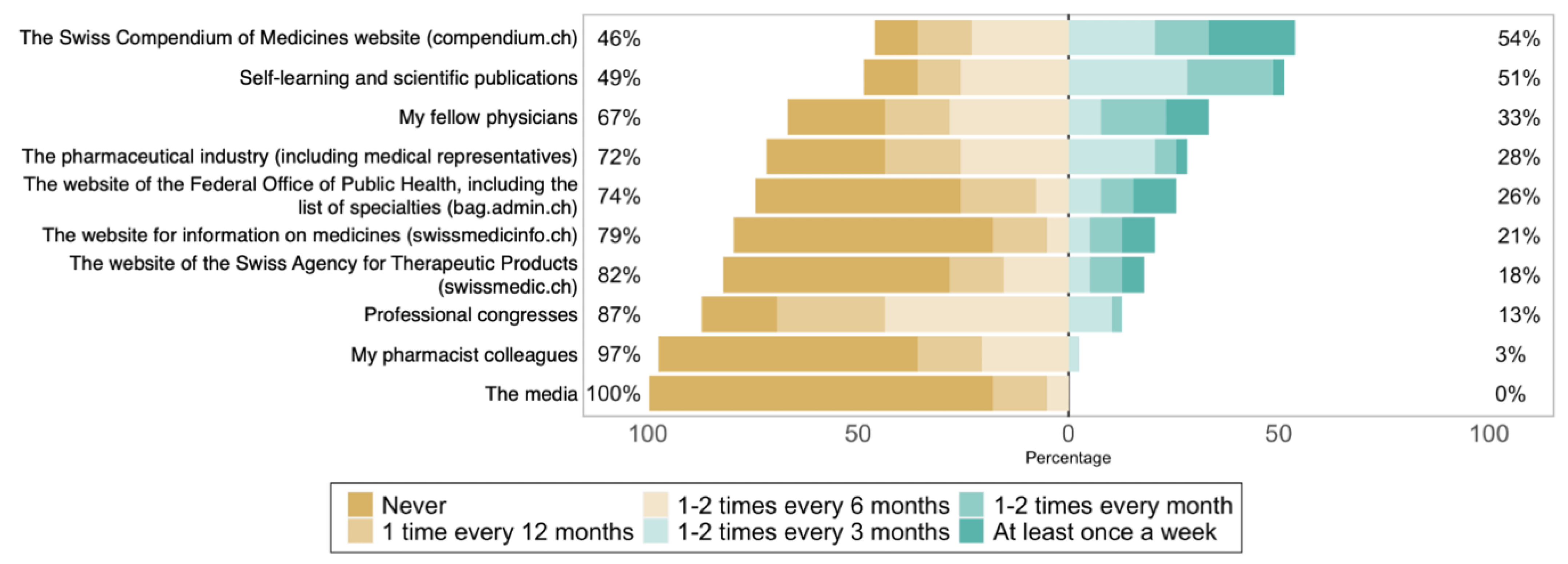
3.2. Experience and Knowledge Regarding Biosimilars
Knowledge Score (KS)
3.3. Confidence in TNF-⍺ Antagonist Biosimilars
3.4. Opinion Regarding Biosimilars of TNF-⍺ Antagonists
4. Discussion
4.1. A Slow but Positive Trend in Knowledge and Confidence in Biosimilars
4.2. Physician’s Prescription Behavior
4.3. Opinion Regarding the Prescription of TNF-⍺ Antagonists Biosimilars in Switzerland
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- BCC Research. Biologic Therapeutic Drugs: Technologies and Global Markets. Available online: https://www.bccresearch.com/market-research/biotechnology/biologic-therapeutic-drugs-technologies-markets-report.html (accessed on 14 September 2021).
- Mikulic, M. Global Pharmaceutical Industry–Statistics & Facts. Available online: https://www.statista.com/topics/1764/global-pharmaceutical-industry/ (accessed on 14 September 2021).
- Institute for Human Data Science (IQVIA). The Impact of Biosimilar Competition in Europe. Available online: https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/the-global-use-of-medicine-in-2019-and-outlook-to-2023.pdf?_=1580989922494 (accessed on 16 March 2022).
- Institute for Human Data Science (IQVIA). Advancing Biosimilar Sustainability in Europe: A Multi-Stakeholder Assessment. Available online: https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/advancing-biosimilar-sustainability-in-europe.pdf?_=1582559849932 (accessed on 24 February 2020).
- Institute fo Human Data Science (IQVIA). The Global Use of Medicine in 2019 and Outlook to 2023. Available online: https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/the-global-use-of-medicine-in-2019-and-outlook-to-2023.pdf?_=1580989922494 (accessed on 6 February 2020).
- Moorkens, E.; Vulto, A.G.; Huys, I.; Dylst, P.; Godman, B.; Keuerleber, S.; Claus, B.; Dimitrova, M.; Petrova, G.; Sović-Brkičić, L.; et al. Policies for biosimilar uptake in Europe: An overview. PLoS ONE 2017, 12, e0190147. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food & Drug Administration (FDA). Biological Product Definitions. Available online: https://www.fda.gov/media/108557/download (accessed on 6 February 2020).
- U.S. Food & Drug Administration (FDA). Biosimilars. Available online: https://www.fda.gov/drugs/therapeutic-biologics-applications-bla/biosimilars (accessed on 18 March 2022).
- European Medicine Agency (EMA). Biosimilars. Available online: https://www.ema.europa.eu/en/human-regulatory/research-development/scientific-guidelines/multidisciplinary/multidisciplinary-biosimilar (accessed on 18 March 2022).
- Murage, M.J.; Tongbram, V.; Feldman, S.R.; Malatestinic, W.N.; Larmore, C.J.; Muram, T.M.; Burge, R.T.; Bay, C.; Johnson, N.; Clifford, S.; et al. Medication adherence and persistence in patients with rheumatoid arthritis, psoriasis, and psoriatic arthritis: A systematic literature review. Patient Prefer. Adherence 2018, 12, 1483–1503. [Google Scholar] [CrossRef]
- Yasmeen, N.; Sawyer, L.M.; Malottki, K.; Levin, L.; Apol, E.D.; Jemec, G.B. Targeted therapies for patients with moderate-to-severe psoriasis: A systematic review and network meta-analysis of PASI response at 1 year. J. Dermatol. Treat. 2020, 33, 204–218. [Google Scholar] [CrossRef]
- Yu, C.L.; Yang, C.H.; Chi, C.C. Drug Survival of Biologics in Treating Ankylosing Spondylitis: A Systematic Review and Meta-analysis of Real-World Evidence. BioDrugs 2020, 34, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Peyrin-Biroulet, L.; Van Assche, G.; Gómez-Ulloa, D.; García-Álvarez, L.; Lara, N.; Black, C.; Kachroo, S. Systematic Review of Tumor Necrosis Factor Antagonists in Extraintestinal Manifestations in Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2016, 15, 25–36. [Google Scholar] [CrossRef] [PubMed]
- MSD Merck Sharp & Dohme AG. Remicade®. Available online: https://www.swissmedicinfo.ch/?Lang=EN#section4 (accessed on 18 March 2022).
- AbbVie AG. Humira®, Solution Injectable. Available online: https://www.swissmedicinfo.ch/#section4 (accessed on 18 March 2022).
- Pfizer AG. Enbrel®/Enbrel MyClic®. Available online: https://www.swissmedicinfo.ch/#section4 (accessed on 18 March 2022).
- Alliance for Safe Biologic Medicines (ASBM). ASBM Latin America Prescribers Survey. Available online: https://safebiologics.org/wp-content/uploads/2015/06/ASBM-Latin-America-2015-FINAL.pdf (accessed on 8 March 2022).
- Beck, M.; Rybarczyk-Vigouret, M.-C.; Levêque, D.; Sordet, C.; Sibilia, J.; Velten, M.; On behalf of the CRI (Club “Rhumatismes et Inflammations”). Rheumatologists’ Perceptions of Biosimilar Medicines Prescription: Findings from a French Web-Based Survey. BioDrugs 2016, 30, 585–592. [Google Scholar] [CrossRef]
- Danese, S.; Fiorino, G.; Michetti, P. Viewpoint: Knowledge and viewpoints on biosimilar monoclonal antibodies among members of the European Crohn's and Colitis Organization. J. Crohns. Colitis. 2014, 8, 1548–1550. [Google Scholar] [CrossRef]
- Tanabe, K.; Sugimoto, N.; Fujimoto, Y. A Web-Based Survey to Investigate the Extent of Awareness and Understanding for Biosimilar among Japanese Physicians and Pharmacists. Value Health 2015, 18, A658. [Google Scholar] [CrossRef][Green Version]
- Sarnola, K.; Merikoski, M.; Jyrkkä, J.; Hämeen-Anttila, K. Physicians’ perceptions of the uptake of biosimilars: A systematic review. BMJ Open 2020, 10, e034183. [Google Scholar] [CrossRef]
- Leonard, E.; Wascovich, M.; Oskouei, S.; Gurz, P.; Carpenter, D. Factors Affecting Health Care Provider Knowledge and Acceptance of Biosimilar Medicines: A Systematic Review. J. Manag. Care Spec. Pharm. 2019, 25, 102–112. [Google Scholar] [CrossRef]
- Yang, J.; Blinzler, K.; Lankin, J.; Vijayakumar, S.; Maculaitis, M.C.; Shelbaya, A. Evolving Perceptions, Utilization, and Real-World Implementation Experiences of Oncology Monoclonal Antibody Biosimilars in the USA: Perspectives from Both Payers and Physicians. BioDrugs 2021, 36, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Marín-Jiménez, I.; Carrascosa, J.M.; Guigini, M.A.; Monte-Boquet, E. Knowledge, perceptions, attitude, barriers and facilitators of biosimilars use across specialty physicians and hospital pharmacists: A national survey. Farm. Hosp. 2021, 45, 240–246. [Google Scholar] [PubMed]
- Cohen, H.P.; McCabe, D. The Importance of Countering Biosimilar Disparagement and Misinformation. BioDrugs 2020, 34, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Hadoussa, S.; Bouhlel, M.; A Soussi, M.; Drira, C.; Hadoussa, M.; Khrouf, M.R. Perception of hematologists and oncologists about the biosimilars: A prospective Tunisian study based on a survey. J. Oncol. Pharm. Pract. 2019, 26, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Alliance for Safe Biologic Medicines (ASBM). ASBM Survey of European Prescribers. Understanding and Knowledge of Biosimilar Medicines. Available online: https://safebiologics.org/wp-content/uploads/2020/06/EU-Survey-2019.pdf (accessed on 8 March 2022).
- Aoki, Y.; Sai, K.; Katsuta, Y.; Suzuki, M.; Suzuki, Y.; Ishii-Watabe, A.; Saito, Y. Questionnaire Survey on Adoption and Prescription of Biosimilars (Antibody and Its-related Products) by Medical Doctors in Japan. YAKUGAKU ZASSHI 2022, 142, 547–560. [Google Scholar] [CrossRef]
- Poon, S.Y.-K.; Hsu, J.C.; Ko, Y.; Chiang, S.-C. Assessing Knowledge and Attitude of Healthcare Professionals on Biosimilars: A National Survey for Pharmacists and Physicians in Taiwan. Healthcare 2021, 9, 1600. [Google Scholar] [CrossRef]
- Mhiri, A.; Khemakhem, M.; Kalboussi, N.; Kacem, B. Knowledge and perceptions of biosimilar medicines by health professionals in Tunisia. Ann. Pharm. Fr. 2021, 80, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Gibofsky, A.; McCabe, D. US rheumatologists’ beliefs and knowledge about biosimilars: A survey. Rheumatology 2020, 60, 896–901. [Google Scholar] [CrossRef]
- Karateev, D.; Belokoneva, N. Evaluation of Physicians’ Knowledge and Attitudes Towards Biosimilars in Russia and Issues Associated with Their Prescribing. Biomolecules 2019, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Demir-Dora, D.; Aksoyalp, Z. Medical students' knowledge and awareness levels about biologics and biosimilars: The earlier the better? Expert Opin. Biol. Ther. 2022, 22, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Krstic, M.; Devaud, J.-C.; Marti, J.; Sadeghipour, F. Exploring the Reasons Behind the Substantial Discontinuation Rate Among Patients Taking CT-P13 in a Large Tertiary Hospital in Western Switzerland: A Retrospective Cohort Study Using Routinely Collected Medical Data. Drugs Real World Outcomes 2022, 9, 425–436. [Google Scholar] [CrossRef]
- Momentive. SurveyMonkey. Available online: https://www.surveymonkey.com (accessed on 1 April 2022).
- Danese, S.; Fiorino, G.; Michetti, P. Changes in Biosimilar Knowledge among European Crohn’s Colitis Organization [ECCO] Members: An Updated Survey. J. Crohn’s Colitis 2016, 10, 1362–1365. [Google Scholar] [CrossRef]
- Cook, J.W.; McGrath, M.K.; Dixon, M.D.; Switchenko, J.M.; Harvey, R.D.; Pentz, R.D. Academic oncology clinicians’ understanding of biosimilars and information needed before prescribing. Ther. Adv. Med Oncol. 2019, 11. [Google Scholar] [CrossRef]
- The Federal Assembly of the Swiss Confederation. Federal Act on Research Involving Human Beings. Available online: https://www.fedlex.admin.ch/eli/cc/2013/617/en (accessed on 1 April 2022).
- Eysenbach, G. Improving the Quality of Web Surveys: The Checklist for Reporting Results of Internet E-Surveys (CHERRIES). J. Med Internet Res. 2004, 6, e34. [Google Scholar] [CrossRef]
- Computer Program. R: A language and environment for statistical computing. In R Foundation for Statistical Computing; Computer Program: Vienna, Austria, 2022. [Google Scholar]
- Federal Office of Public Health (FOPH). Register of Medical Professions. Available online: https://www.medregom.admin.ch/EN (accessed on 31 May 2022).
- HCI Solutions SA. Compendium.ch. Available online: https://compendium.ch (accessed on 28 July 2022).
- Cohen, H.; Beydoun, D.; Chien, D.; Lessor, T.; McCabe, D.; Muenzberg, M.; Popovian, R.; Uy, J. Awareness, Knowledge, and Perceptions of Biosimilars Among Specialty Physicians. Adv. Ther. 2016, 33, 2160–2172. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-K.; Moon, W.; Kim, E.S.; Park, S.H.; Park, D.I. Knowledge and Viewpoints on Biosimilar Monoclonal Antibodies among Asian Physicians: Comparison with European Physicians. Korean J. Gastroenterol. 2019, 74, 333–340. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). Abasaglar. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/abasaglar-previously-abasria#authorisation-details-section (accessed on 20 June 2022).
- European Medicines Agency (EMA). Binocrit. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/binocrit#authorisation-details-section (accessed on 20 June 2022).
- European Medicines Agency (EMA). Tevagrastim. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/tevagrastim#authorisation-details-section (accessed on 20 June 2022).
- European Medicines Agency (EMA). Zarzio. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/zarzio#authorisation-details-section (accessed on 20 June 2022).
- European Medicines Agency (EMA). Omnitrope. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/omnitrope#authorisation-details-section (accessed on 20 June 2022).
- European Medicines Agency (EMA). Inflectra. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/inflectra (accessed on 6 April 2021).
- European Medicine Agency (EMA). Erelzi. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/erelzi (accessed on 21 June 2022).
- European Medicine Agency (EMA). Rixathon. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/rixathon (accessed on 21 June 2022).
- European Medicine Agency (EMA). Amgevita. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/amgevita (accessed on 21 June 2022).
- European Medicine Agency (EMA). Kanjinti. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/kanjinti#authorisation-details-section (accessed on 21 June 2022).
- European Medicine Agency (EMA). Zirabev. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/zirabev (accessed on 21 June 2022).
- O'Callaghan, J.; Bermingham, M.; Leonard, M.; Hallinan, F.; Morris, J.M.; Moore, U.; Griffin, B.T. Assessing awareness and attitudes of healthcare professionals on the use of biosimilar medicines: A survey of physicians and pharmacists in Ireland. Regul. Toxicol. Pharmacol. 2017, 88, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Feldman, M.; Reilly, M.S. A white paper: US biosimilars market on pace with Europe. Generics Biosimilars Initiat. J. 2020, 9, 150–154. [Google Scholar] [CrossRef]
- Krstic, M.; Devaud, J.-C.A.; Sadeghipour, F. Pharmacists’ considerations on non-medical switching at the hospital: A systematic review of the economic outcomes of cost-saving therapeutic drug classes. Eur. J. Hosp. Pharm. 2021, 28, e2–e7. [Google Scholar] [CrossRef] [PubMed]
- Federal Office of Public Health (FOPH). Health2030. Available online: https://www.bag.admin.ch/bag/en/home/strategie-und-politik/gesundheit-2030.html (accessed on 22 July 2022).
- Curafutura bc, Intergenerika. Baromètre des biosimilaires Suisse 2022: Les Biosimilaires Encore Trop Peu Utilisés en Suisse. Available online: https://curafutura.ch/fr/barometre-des-biosimilaires-suisse-les-biosimilaires-encore-trop-peu-utilises-en-suisse/ (accessed on 23 June 2022).
- Institute fo Human Data Science (IQVIA). Homepage. Available online: https://www.iqvia.com (accessed on 23 June 2022).
- Curafutura bc, Intergenerika. Baromètre des biosimilaires Suisse 2020. Available online: https://biosimilar.ch/wp-content/uploads/simple-file-list/Biosimilar_Barometer_1_April-2021_FR.pdf (accessed on 23 June 2022).
- Curafutura bc, Intergenerika. Baromètre des biosimilaires Suisse 2021. Available online: https://biosimilar.ch/wp-content/uploads/simple-file-list/Biosimilar_Barometer_2_September-2021_FR.pdf (accessed on 23 June 2022).


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krstic, M.; Devaud, J.-C.; Sadeghipour, F.; Marti, J. Current Expertise, Opinions, and Attitude toward TNF-⍺ Antagonist Biosimilars among Physicians: A Self-Administered Online Survey in Western Switzerland. Healthcare 2022, 10, 2152. https://doi.org/10.3390/healthcare10112152
Krstic M, Devaud J-C, Sadeghipour F, Marti J. Current Expertise, Opinions, and Attitude toward TNF-⍺ Antagonist Biosimilars among Physicians: A Self-Administered Online Survey in Western Switzerland. Healthcare. 2022; 10(11):2152. https://doi.org/10.3390/healthcare10112152
Chicago/Turabian StyleKrstic, Marko, Jean-Christophe Devaud, Farshid Sadeghipour, and Joachim Marti. 2022. "Current Expertise, Opinions, and Attitude toward TNF-⍺ Antagonist Biosimilars among Physicians: A Self-Administered Online Survey in Western Switzerland" Healthcare 10, no. 11: 2152. https://doi.org/10.3390/healthcare10112152
APA StyleKrstic, M., Devaud, J.-C., Sadeghipour, F., & Marti, J. (2022). Current Expertise, Opinions, and Attitude toward TNF-⍺ Antagonist Biosimilars among Physicians: A Self-Administered Online Survey in Western Switzerland. Healthcare, 10(11), 2152. https://doi.org/10.3390/healthcare10112152





