Effect of Pirfenidone on Risk of Pulmonary Fibrosis in COVID-19 Patients Experiencing Cytokine Storm
Abstract
:1. Introduction
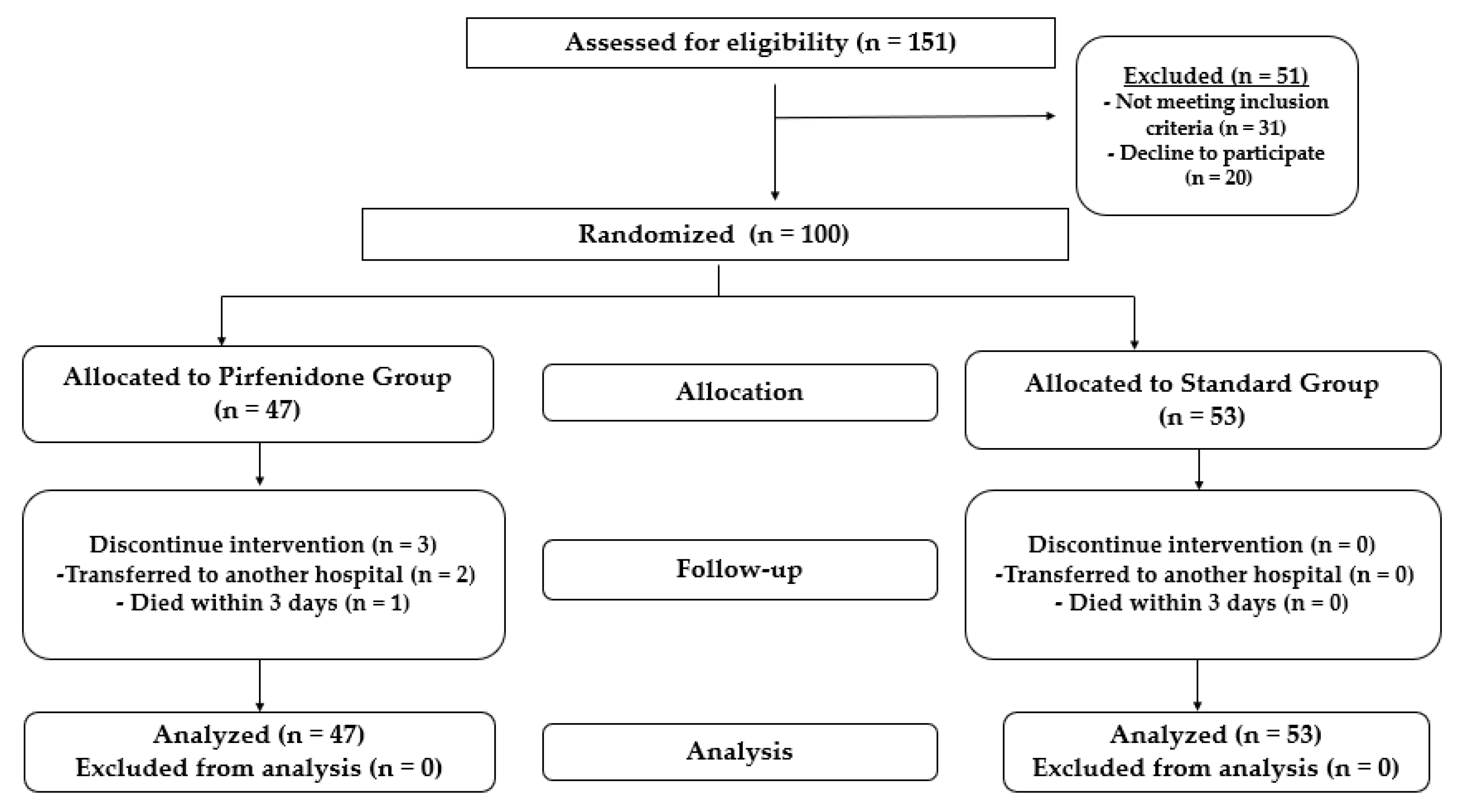
2. Patients and Methods
2.1. Study Design
2.2. Protocol and Study Population
2.3. Patient Follow-Up and Clinical Outcomes
2.4. Statistical Analysis
2.4.1. Sample Size Calculation
2.4.2. Descriptive and Inferential Statistics
2.5. Ethics Statement
3. Results
3.1. Patient Demographics
3.2. Patient Clinical Features, Baseline Pulmonary Functions, and Inflammatory Markers
3.3. Clinical Outcomes and Follow-Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Schaalan, M.; Warda, A.E.A.; Osman, S.M.; Fathy, S.; Sarhan, R.M.; Boshra, M.S.; Sarhan, N.; Gaber, S.; Ali, A.M.A. The Impact of Sociodemographic, Nutritional, and Health Factors on the Incidence and Complications of COVID-19 in Egypt: A Cross-Sectional Study. Viruses 2022, 14, 448. [Google Scholar] [CrossRef]
- Seifirad, S. Pirfenidone: A novel hypothetical treatment for COVID-19. Med. Hypotheses 2020, 144, 110005. [Google Scholar] [CrossRef] [PubMed]
- Ciotti, M.; Ciccozzi, M.; Terrinoni, A.; Jiang, W.; Wang, C.; Bernardini, S. The COVID-19 pandemic. Crit. Rev. Clin. Lab. Sci. 2020, 57, 365–388. [Google Scholar] [CrossRef]
- Tzotzos, S.J.; Fischer, B.; Fischer, H.; Zeitlinger, M. Incidence of ARDS and outcomes in hospitalized patients with COVID-19: A global literature survey. Crit. Care 2020, 24, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Du, R.-H.; Wang, R.; Cao, T.-Z.; Guan, L.-L.; Yang, C.-Q.; Zhu, Q.; Hu, M.; Li, X.-Y.; Li, Y.; et al. Comparison of Hospitalized Patients With ARDS Caused by COVID-19 and H1N1. Chest 2020, 158, 195–205. [Google Scholar] [CrossRef] [PubMed]
- George, P.M.; Wells, A.U.; Jenkins, R.G. Pulmonary fibrosis and COVID-19: The potential role for antifibrotic therapy. Lancet Respir. Med. 2020, 8, 807–815. [Google Scholar] [CrossRef]
- Velazquez-Salinas, L.; Verdugo-Rodriguez, A.; Rodriguez, L.L.; Borca, M.V. The Role of Interleukin 6 during Viral Infections. Front. Microbiol. 2019, 10, 1057. [Google Scholar] [CrossRef] [Green Version]
- Hasan, D.; Shono, A.; van Kalken, C.K.; van der Spek, P.J.; Krenning, E.P.; Kotani, T. A novel definition and treatment of hyperinflammation in COVID-19 based on purinergic signalling. Purinergic Signal. 2022, 18, 13–59. [Google Scholar]
- Mahmudpour, M.; Roozbeh, J.; Keshavarz, M.; Farrokhi, S.; Nabipour, I. COVID-19 cytokine storm: The anger of inflammation. Cytokine 2020, 133, 155151. [Google Scholar] [CrossRef]
- Cron, R.Q.; Schulert, G.S.; Tattersall, R.S. Defining the scourge of COVID-19 hyperinflammatory syndrome. Lancet Rheumatol. 2020, 2, e727–e729. [Google Scholar] [CrossRef]
- Hu, B.; Huang, S.; Yin, L. The cytokine storm and COVID-19. J. Med. Virol. 2021, 93, 250–256. [Google Scholar] [CrossRef]
- Spagnolo, P.; Balestro, E.; Aliberti, S.; Cocconcelli, E.; Biondini, D.; della Casa, G.; Sverzellati, N.; Maher, T.M. Pulmonary fibrosis secondary to COVID-19: A call to arms? Lancet Respir. Med. 2020, 8, 750–752. [Google Scholar] [CrossRef]
- Choudhary, S.; Sharma, K.; Silakari, O. The interplay between inflammatory pathways and COVID-19: A critical review on pathogenesis and therapeutic options. Microb. Pathog. 2021, 150, 104673. [Google Scholar] [CrossRef]
- Reyfman, P.A.; Walter, J.M.; Joshi, N.; Anekalla, K.R.; McQuattie-Pimentel, A.C.; Chiu, S.; Fernandez, R.; Akbarpour, M.; Chen, C.-I.; Ren, Z.; et al. Single-Cell Transcriptomic Analysis of Human Lung Provides Insights into the Pathobiology of Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2019, 199, 1517–1536. [Google Scholar] [CrossRef]
- Liao, M.; Liu, Y.; Yuan, J.; Wen, Y.; Xu, G.; Zhao, J.; Chen, L.; Li, J.; Wang, X.; Wang, F.; et al. The landscape of lung bronchoalveolar immune cells in COVID-19 revealed by single-cell RNA sequencing. medRxiv, 2020; ahead of print. [Google Scholar]
- Garcia-Revilla, J.; Deierborg, T.; Venero, J.L.; Boza-Serrano, A. Hyperinflammation and Fibrosis in Severe COVID-19 Patients: Galectin-3, a Target Molecule to Consider. Front. Immunol. 2020, 11, 2069. [Google Scholar] [CrossRef]
- Chung, M.P.; Park, M.S.; Oh, I.-J.; Lee, H.B.; Kim, Y.W.; Park, J.S.; Uh, S.T.; Kim, Y.S.; Jegal, Y.; Song, J.W. Safety and Efficacy of Pirfenidone in Advanced Idiopathic Pulmonary Fibrosis: A Nationwide Post-Marketing Surveillance Study in Korean Patients. Adv. Ther. 2020, 37, 2303–2316. [Google Scholar] [CrossRef] [Green Version]
- George, P.M.; Wells, A.U. Pirfenidone for the treatment of idiopathic pulmonary fibrosis. Expert Rev. Clin. Pharmacol. 2017, 10, 483–491. [Google Scholar] [CrossRef]
- Raghu, G.; Selman, M. Nintedanib and Pirfenidone. New Antifibrotic Treatments Indicated for Idiopathic Pulmonary Fibrosis Offer Hopes and Raises Questions. Am. J. Respir. Crit. Care Med. 2015, 191, 252–254. [Google Scholar] [CrossRef]
- Antar, S.A.; Saleh, M.A.; Al-Karmalawy, A.A. Investigating the possible mechanisms of pirfenidone to be targeted as a promising anti-inflammatory, anti-fibrotic, anti-oxidant, anti-apoptotic, anti-tumor, and/or anti-SARS-CoV-2. Life Sci. 2022, 309, 121048. [Google Scholar] [CrossRef]
- Takeda, Y.; Tsujino, K.; Kijima, T.; Kumanogoh, A. Efficacy and safety of pirfenidone for idiopathic pulmonary fibrosis. Patient Prefer. Adherence 2014, 8, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, S.; Natt, B.; Bime, C.; Knox, K.S.; Glassberg, M.K. Antifibrotics in COVID-19 lung disease: Let us stay focused. Front. Med. 2020, 7, 539. [Google Scholar] [CrossRef]
- Hochhegger, B.; Marchiori, E.; Zanon, M.; Rubin, A.S.; Fragomeni, R.; Altmayer, S.; Carvalho, C.R.R.; Baldi, B.G. Imaging in idiopathic pulmonary fibrosis: Diagnosis and mimics. Clinics 2019, 74, e225. [Google Scholar] [CrossRef]
- Edey, A.J.; Devaraj, A.A.; Barker, R.P.; Nicholson, A.G.; Wells, A.U.; Hansell, D.M. Fibrotic idiopathic interstitial pneumonias: HRCT findings that predict mortality. Eur. Radiol. 2011, 21, 1586–1593. [Google Scholar] [CrossRef]
- Akira, M.; Inoue, Y.; Arai, T.; Okuma, T.; Kawata, Y. Long-term follow-up high-resolution CT findings in non-specific interstitial pneumonia. Thorax 2011, 66, 61–65. [Google Scholar] [CrossRef] [Green Version]
- Walsh, S.L.F.; Sverzellati, N.; Devaraj, A.; Keir, G.J.; Wells, A.U.; Hansell, D.M. Connective tissue disease related fibrotic lung disease: High resolution computed tomographic and pulmonary function indices as prognostic determinants. Thorax 2014, 69, 216–222. [Google Scholar] [CrossRef] [Green Version]
- Sumikawa, H.; Johkoh, T.; Fujimoto, K.; Arakawa, H.; Colby, T.V.; Fukuoka, J.; Taniguchi, H.; Kondoh, Y.; Kataoka, K.; Ogura, T.; et al. Pathologically Proved Nonspecific Interstitial Pneumonia: CT Pattern Analysis as Compared with Usual Interstitial Pneumonia CT Pattern. Radiology 2014, 272, 549–556. [Google Scholar] [CrossRef] [Green Version]
- Francone, M.; Iafrate, F.; Masci, G.M.; Coco, S.; Cilia, F.; Manganaro, L.; Panebianco, V.; Andreoli, C.; Colaiacomo, M.C.; Zingaropoli, M.A.; et al. Chest CT score in COVID-19 patients: Correlation with disease severity and short-term prognosis. Eur. Radiol. 2020, 4, 1–10. [Google Scholar]
- Pan, F.; Ye, T.; Sun, P.; Gui, S.; Liang, B.; Li, L.; Zheng, D.; Wang, J.; Hesketh, R.L.; Yang, L.; et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19). Radiology 2020, 295, 715–721. [Google Scholar] [CrossRef] [Green Version]
- Nisar, S.; Tanvir, M.; Wagay, I.; Ahmed, R.N.; Maqbool, M.; Kareem, O.; Muzaffer, U. Early intervention with anti-fibrotic pirfenidone is effective than corticosteroids in preventing pulmonary fibrosis in severe COVID pneumonia patients. Curr. Med. Res. Pr. 2022, 12, 53. [Google Scholar] [CrossRef]
- Noble, P.W.; Albera, C.; Bradford, W.Z.; Costabel, U.; Glassberg, M.K.; Kardatzke, D., Jr.; Lancaster, L.; Sahn, S.A.; Szwarcberg, J.; Valeyre, D.; et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): Two randomised trials. Lancet 2011, 377, 1760–1769. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, A.; Pelliccia, C.; Ferrara, F. COVID-19 Patients with Pulmonary Fibrotic Tissue: Clinical Pharmacological Rational of Antifibrotic Therapy. SN Compr. Clin. Med. 2020, 2, 1709–1712. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Huang, Q.; Wu, X.; Zheng, X.; Luo, S.; Xu, S.; Weng, J. Targeting inflammation and cytokine storm in COVID-19. Pharmacol. Res. 2020, 159, 105051. [Google Scholar] [CrossRef]
- Sun, X.; Wang, T.; Cai, D.; Hu, Z.; Chen, J.; Liao, H.; Zhi, L.; Wei, H.; Zhang, Z.; Qiu, Y.; et al. Cytokine storm intervention in the early stages of COVID-19 pneumonia. Cytokine Growth Factor Rev. 2020, 53, 38–42. [Google Scholar] [CrossRef]
- Acat, M.; Gulhan, P.Y.; Oner, S.; Turan, M.K. Comparison of pirfenidone and corticosteroid treatments at the COVID-19 pneumonia with the guide of artificial intelligence supported thoracic computed tomography. Int. J. Clin. Pract. 2021, 75, e14961. [Google Scholar] [CrossRef] [PubMed]
- King, T.E.; Bradford, W.Z.; Castro-Bernardini, S.; Fagan, E.A.; Glaspole, I.; Glassberg, M.K.; Gorina, E.; Hopkins, P.M.; Kardatzke, D.; Lancaster, L.; et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014, 370, 2083–2092. [Google Scholar] [CrossRef] [Green Version]
- Kreuter, M.; Ehlers-Tenenbaum, S.; Palmowski, K.; Bruhwyler, J.; Oltmanns, U.; Muley, T.; Heussel, C.P.; Warth, A.; Kolb, M.; Herth, F.J.F. Impact of Comorbidities on Mortality in Patients with Idiopathic Pulmonary Fibrosis. PLoS ONE 2016, 11, e0151425. [Google Scholar] [CrossRef] [Green Version]
- Raghu, G.; Amatto, V.C.; Behr, J.; Stowasser, S. Comorbidities in idiopathic pulmonary fibrosis patients: A systematic literature review. Eur. Respir. J. 2015, 46, 1113–1130. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Wei, Y.; He, L.; Zhang, H.; Hu, Q.; Yue, H.; He, J.; Dai, H. A trial of pirfenidone in hospitalized adult patients with severe coronavirus disease 2019. Chin. Med. J. 2021, 135, 368–370. [Google Scholar] [CrossRef]
- Zhou, S.; Li, W.; Tian, M.; Zhang, N.; Yang, X.; Li, W.; Peng, Y.; Zheng, J. Metabolic Activation of Pirfenidone Mediated by Cytochrome P450s and Sulfotransferases. J. Med. Chem. 2020, 63, 8059–8068. [Google Scholar] [CrossRef] [PubMed]
- Valeyre, D.; Albera, C.; Bradford, W.Z.; Costabel, U.; King, T.E.; Leff, J.A.; Noble, P.W.; Sahn, S.A.; Du Bois, R.M. Comprehensive assessment of the long-term safety of pirfenidone in patients with idiopathic pulmonary fibrosis. Respirology 2014, 19, 740–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamidi, S.H.; Veethil, S.K.; Hamidi, S.H. Role of pirfenidone in TGF-β pathways and other inflammatory pathways in acute respiratory syndrome coronavirus 2 (SARS-Cov-2) infection: A theoretical perspective. Pharmacol. Rep. 2021, 73, 712–727. [Google Scholar] [CrossRef]
- Lancaster, L.H.; de Andrade, J.A.; Zibrak, J.D.; Padilla, M.L.; Albera, C.; Nathan, S.D.; Wijsenbeek, M.S.; Stauffer, J.L.; Kirchgaessler, K.-U.; Costabel, U. Pirfenidone safety and adverse event management in idiopathic pulmonary fibrosis. Eur. Respir. Rev. 2017, 26, 170057. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Pirfenidone Group (n = 47) | Standard Group (n = 53) | p |
|---|---|---|---|
| Age (years), Mean ± SD | 64.68 ± 10.60 | 62.42 ± 11.77 | 0.317 |
| Gender (male), n% | 32 (68.1%) | 36 (67.9%) | 0.986 |
| BMI (kg/m2), Mean ± SD | 29.07 ± 3.77 | 29.06 ± 4.24 | 0.892 |
| Temperature °C, Mean ± SD | 38.0 ± 0.85 | 38.1 ± 0.93 | 0.794 |
| Patients need low-flow oxygen, n% | 12 (25.5%) | 19 (35.8%) | 0.287 |
| Patients need high-flow oxygen, n% | 31 (66.0%) | 34 (64.2%) | 1.000 |
| Pulmonary functions | |||
| Oxygen saturation, Mean ± SD | 81.09 ± 12.44 | 81.45 ± 12.59 | 0.561 |
| FVC %, Mean ± SD | 75.96 ± 12.18 | 75.87 ± 12.23 | 0.758 |
| FEV1 %, Mean ± SD | 71.77 ± 11.42 | 71.85 ± 11.77 | 0.742 |
| Po2/Fio2 ratio | 141.6 ± 73.89 | 152.5 ± 71.35 | 0.499 |
| Comorbidities | |||
| Hypertension, n% | 21 (44.7%) | 31 (58.5%) | 0.229 |
| Diabetes, n% | 14 (29.8%) | 21 (39.6%) | 0.401 |
| Ischemic heart disease, n% | 5 (10.6%) | 15 (28.3%) | 0.044 * |
| AF, n% | 4 (8.5%) | 5 (9.4%) | 1.000 |
| Asthma, n% | 3 (6.4%) | 3 (5.7%) | 1.000 |
| Others, n% | 12 (25.5%) | 13 (24.5%) | 1.000 |
| Current smoking habits, n% | 9 (19.1%) | 17 (32.1%) | 0.174 |
| Medications | |||
| Remdesivir, n% | 46 (97.9%) | 46 (86.8%) | 0.063 |
| Lopinavir/Ritonavir, n% | 16 (34.0%) | 11 (20.8%) | 0.177 |
| Ivermectin, n% | 5 (10.6%) | 13 (24.5%) | 0.116 |
| Infliximab, n% | 18 (38.3%) | 21 (39.6%) | 1.000 |
| Tocilizumab, n% | 40 (85.1%) | 43 (81.1%) | 0.790 |
| Parameters | Pirfenidone Group (n = 47) | Standard Group (n = 53) | p |
|---|---|---|---|
| D-Dimer, Mean ± SD | 2.75 ± 10.86 | 0.857 ± 1.32 | 0.013 |
| LDH, Mean ± SD | 447.1 ± 242.2 | 483.1 ± 291.4 | 0.824 |
| Ferritin, Mean ± SD | 1158.1 ± 962.2 | 866.16 ± 763.9 | 0.096 |
| CRP, Mean ± SD | 133.59 ± 90.3 | 127.4 ± 90.45 | 0.671 |
| WBCs, Median (IQR) | 8.7 (7.0–12.4) | 7.3 (4.8–11.2) | 0.058 |
| Neutrophil/lymphocytes ratio, Mean ± SD | 10.85 ± 7.47 | 9.21 ± 7.09 | 0.268 |
| ALT, Mean ± SD | 48.93 ± 34.68 | 43.56 ± 25.4 | 0.842 |
| AST, Mean ± SD | 48.65 ± 29.95 | 50.78 ± 35.4 | 0.968 |
| Parameters | Pirfenidone Group (n = 47) | Standard Group (n = 53) | p |
|---|---|---|---|
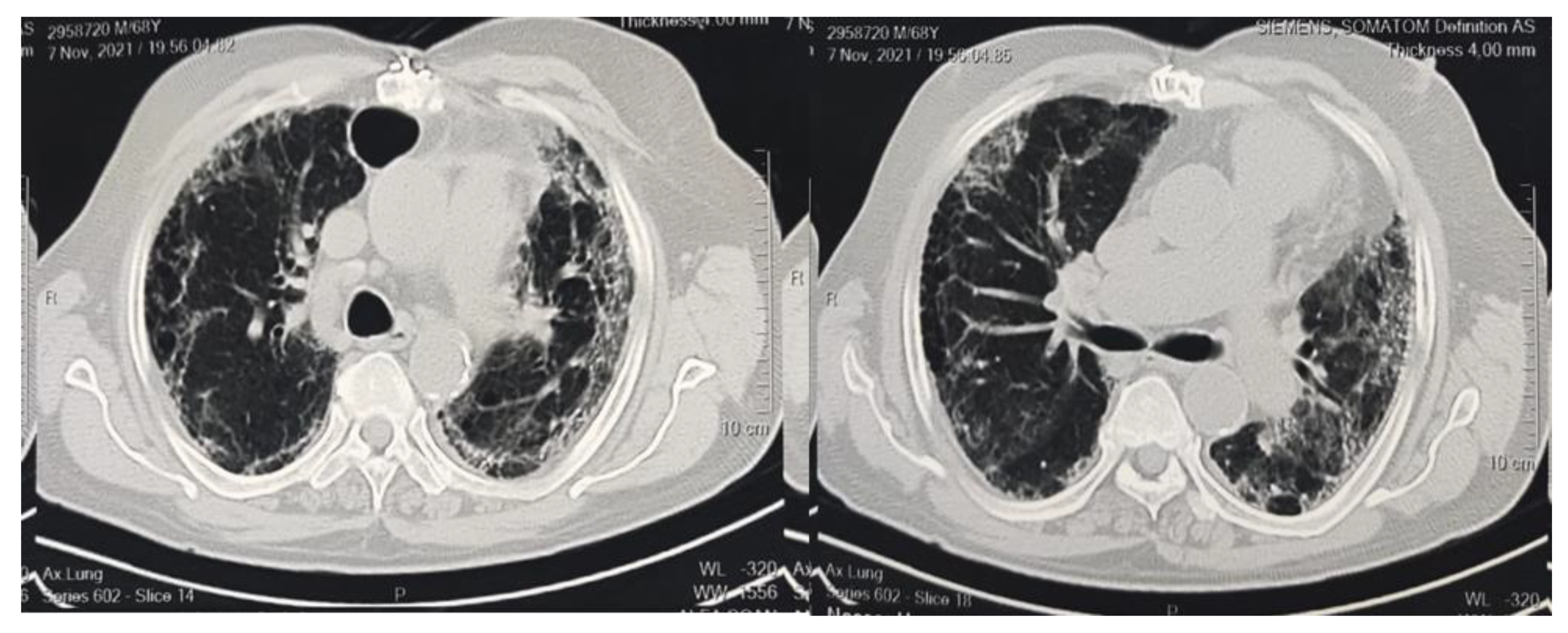
| Broncho vascular bundle distortion, n% | 25 (53.1%) | 19 (35.8%) | 0.749 |
| Traction bronchiectasis, n% | 37 (78.7%) | 31 (58.4%) | 0.451 |
| Fibrotic strips, n% | 7 (14.8%) | 4 (7.5%) | 0.529 |
| Architectural distortion, n% | 29 (61.7%) | 22 (41.5%) | 0.532 |
| Interlobar septal thickening, n% | 10 (21.2%) | 12 (22.6%) | 0.422 |
| Honeycombing appearance, n% | 8 (17.02%) | 15 (28.3%) | 0.167 |
| Scale | Initial Lung Involvement | Lung Involvement at Time of Discharge | ||||
|---|---|---|---|---|---|---|
| Pirfenidone Group (n = 47) | Standard Group (n = 53) | p | Pirfenidone Group (n = 47) | Standard Group (n = 53) | p | |
| Scale 1, n% Less than 5% involvement | 11 (23.4%) | 12 (22.6%) | 0.558 | 7 (14.9%) | 9 (17.0%) | 0.140 |
| Scale 2, n% 5–25% involvement | 24 (51.1%) | 26 (49.1%) | 0.500 | 15 (31.9%) | 17 (32.1%) | 0.138 |
| Scale 3, n% 26–49% involvement | 7 (14.9%) | 7 (13.2%) | 0.517 | 9 (19.1%) | 7 (13.2%) | 0.296 |
| Scale 4, n% 50–75% involvement | 5 (10.6%) | 8 (15.1%) | 0.360 | 11 (23.4%) | 3 (5.7%) | 0.011 * |
| Scale 5, n% More than 75% involvement | - | - | – | 5 (10.6%) | 17(32.1%) | 0.009 * |
| Parameters | Pirfenidone Group (n = 47) | Standard Group (n = 53) | p |
|---|---|---|---|
| Development of pulmonary fibrosis, n% | 14 (29.8%) | 19 (35.8%) | 0.532 |
| Mortality within isolation unit, n% | 10 (21.3%) | 16 (30.2%) | 0.365 |
| Discharge without progression in lung fibrosis, n% | 10 (21.3%) | 3 (5.7%) | 0.034 * |
| Time to recovery without lung fibrosis (days), Mean ± SD | 4.60 ± 2.93 | 4.66 ± 2.56 | 0.734 |
| Duration of cytokine storm (days), Median (IQR) | 3 (3–6) | 4 (3–6) | 0.596 |
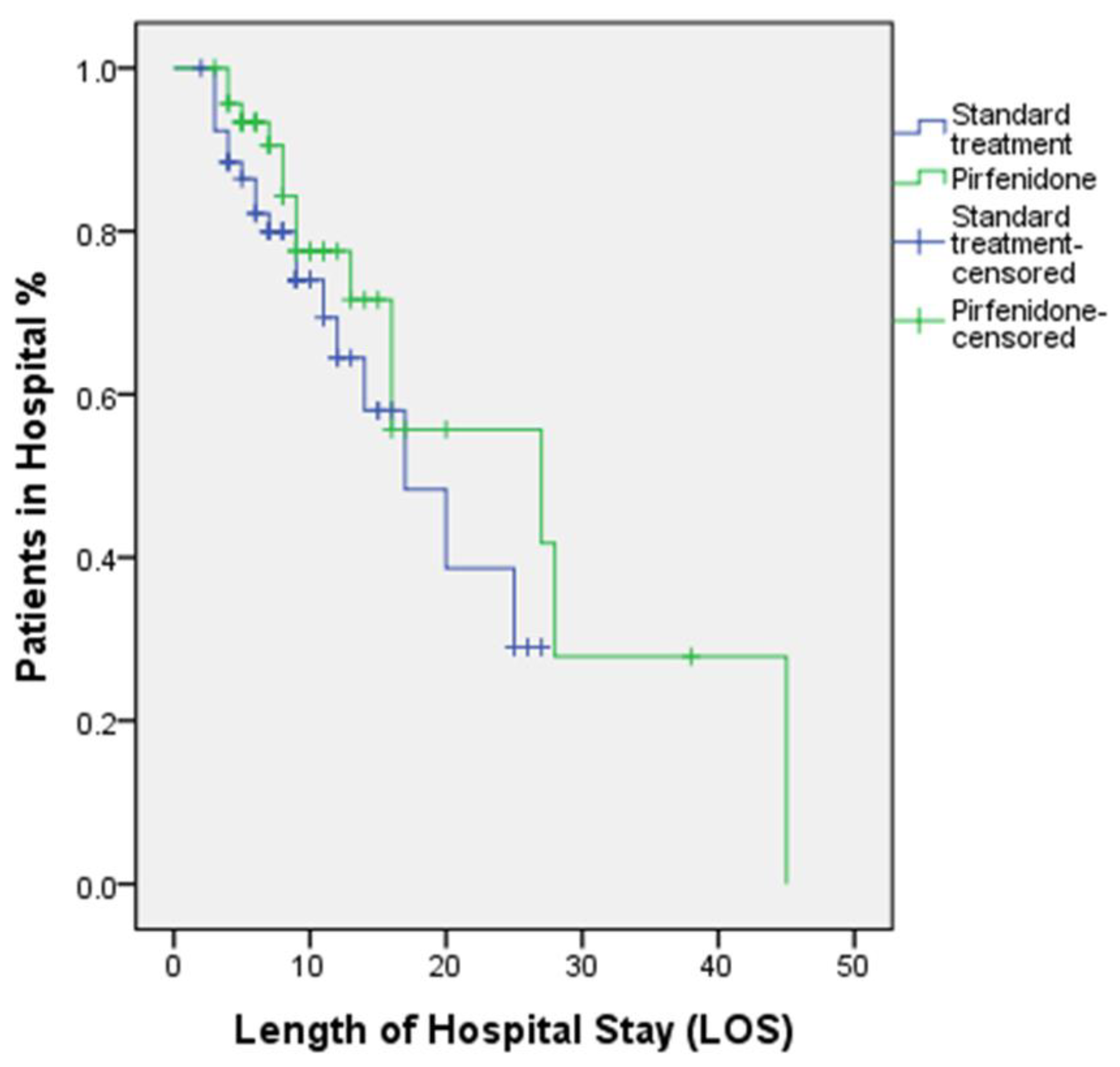
| Isolation unit stay (days), Mean ± SD | 9.34 ± 5.21 | 9.11 ± 4.99 | 0.909 |
| Length of hospital stay (LOS) (days), Mean ± SD | 11.23 ± 8.48 | 9.72 ± 6.03 | 0.448 |
| 30-day mortality, n% | 14 (29.8%) | 18 (34.0%) | 0.674 |
| Incidence of hepatotoxicity, n% | 19 (40.4%) | 8 (15.1%) | 0.006 * |
| ALT, Median (IQR) | 80 (52–182) | 53 (38–79) | 0.030 * |
| AST, Median (IQR) | 60 (33.75–82) | 41 (29–65) | 0.102 |
| Incidence of GIT disturbances, n% | 17 (36.2%) | 7 (13.2%) | 0.01 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boshra, M.S.; Abou Warda, A.E.; Sayed, M.A.; Elkomy, M.H.; Alotaibi, N.H.; Mohsen, M.; Sarhan, R.M. Effect of Pirfenidone on Risk of Pulmonary Fibrosis in COVID-19 Patients Experiencing Cytokine Storm. Healthcare 2022, 10, 2387. https://doi.org/10.3390/healthcare10122387
Boshra MS, Abou Warda AE, Sayed MA, Elkomy MH, Alotaibi NH, Mohsen M, Sarhan RM. Effect of Pirfenidone on Risk of Pulmonary Fibrosis in COVID-19 Patients Experiencing Cytokine Storm. Healthcare. 2022; 10(12):2387. https://doi.org/10.3390/healthcare10122387
Chicago/Turabian StyleBoshra, Marian S., Ahmed E. Abou Warda, Mahmoud Abdulbasser Sayed, Mohammed H. Elkomy, Nasser H. Alotaibi, Marwa Mohsen, and Rania M. Sarhan. 2022. "Effect of Pirfenidone on Risk of Pulmonary Fibrosis in COVID-19 Patients Experiencing Cytokine Storm" Healthcare 10, no. 12: 2387. https://doi.org/10.3390/healthcare10122387
APA StyleBoshra, M. S., Abou Warda, A. E., Sayed, M. A., Elkomy, M. H., Alotaibi, N. H., Mohsen, M., & Sarhan, R. M. (2022). Effect of Pirfenidone on Risk of Pulmonary Fibrosis in COVID-19 Patients Experiencing Cytokine Storm. Healthcare, 10(12), 2387. https://doi.org/10.3390/healthcare10122387






