Abstract
This study compared the anthropometric parameters of patients with fatty acid oxidation disorders (FAOD) and healthy controls, showing an increased prevalence of abnormal body weight (overweight and obesity) in the FAOD group. First, differences in BMI, BMI percentiles and z-scores, and weight and weight percentiles were compared in a cohort of 39 patients with FAOD and 156 healthy controls, as well as between patients born before and after the introduction of a populational newborn screening programme (NBS) in 2014 in Poland. We also performed a systematic literature review yielding 12 studies mentioning anthropometric parameters in 80 FAOD patients and 121 control subjects, followed by a meta-analysis of data from 8 studies and our cohort. There were significant differences in body weight percentiles (p = 0.001), BMI (p = 0.022), BMI percentiles (p = 0.003) and BMI z-scores (p = 0.001) between FAOD patients and controls in our cohort but not between pre- and post-newborn-screening patients. The meta-analysis did not show any differences in weight and BMI in all tested subgroups, i.e., all FAOD patients vs. controls, medium-chain acyl-CoA dehydrogenase (MCADD) patients vs. controls and patients with FAOD types other than MCAD vs. controls. These results, however, should be interpreted with caution due to the overall low quality of evidence as assessed by GRADE, the small sample sizes and the significant heterogeneity of the included data.
1. Introduction
Fatty acid oxidation disorders (FAOD) are rare autosomal recessive inborn errors of metabolism resulting in defective mitochondrial beta-oxidation or carnitine-mediated transport of fatty acids into the mitochondria. The prevalence of FAOD ranges from 1:20,000 for medium-chain acyl-CoA dehydrogenase deficiency (MCADD) [1] to 1:2,000,000 for multiple acyl CoA dehydrogenase deficiency (MADD). FAOD present with a broad spectrum of symptoms, such as hypotonia, recurrent rhabdomyolysis, exercise intolerance, hypoketotic hypoglycaemia, liver failure and sudden infant death syndrome [2]. Presentation depends on the type of defect, with MCADD tending to have a milder phenotype. At the same time, long-chain fatty acid oxidation disorders (LC-FAOD) and carnitine metabolism disorders present more severe symptoms. Complications include retinopathy, acute or chronic cardiomyopathy and polyneuropathy [3]. The treatment recommendations are based on experts’ consensus and include avoidance of fasting and strenuous exercise, aggressive treatment of infections, and in some cases, carnitine supplementation [2]. Most patients, especially with LC-FAOD, require dietary modifications such as diets with low LCT content, MCT oil supplementation and night feeds [4]. In the past, a high-carbohydrate diet was also recommended. Some of the research published in previous decades suggests a tendency towards overweight and obesity [5,6] among FAOD patients; however, the lack of control groups hinders conclusion.
We hypothesised that patients with FAOD, especially LC-FAOD, are at increased risk of excess weight and obesity due to the treatment modalities and lifestyle modifications mentioned above. Therefore, various anthropometric data were analysed to investigate whether FAOD patients differ from the general population regarding weight and BMI. The analysis included a cohort of FAOD patients from two metabolic centres in Poland and patients born before and after the introduction of a populational newborn screening programme (NBS) in 2014 in Poland [7], as well as a systematic review and meta-analysis of studies related to anthropometrics in patients with FAOD.
2. Materials and Methods
2.1. Case–Control Study
The study protocol was approved by the Poznań University of Medical Sciences Ethical Committee, and all procedures were performed according to the Helsinki Declaration of 1975 [8]. The study was conducted per the STROBE guidelines [9].
2.1.1. Study Population
The medical records of 39 FAOD patients from two metabolic centres in Poland (22 patients from the Department of Paediatric Gastroenterology and Metabolic Diseases at Poznan University of Medical Sciences and 17 patients from the Department of Paediatrics, Haematology and Oncology, Medical University of Gdansk) were reviewed retrospectively to retrieve age, sex and anthropometric data (weight, height, BMI). FAOD diagnosis was confirmed in all patients with blood acylcarnitine assays, urinary organic acid profiles and genetic testing. Patients in unstable clinical conditions were excluded from the study. Sex- and age- matched control subjects were recruited using the propensity score matching method. The inclusion criteria included age 0–18 years, good general health, lack of chronic disease (such as asthma, diabetes, hypertension, hypothyroidism) and documented intellectual or physical disability. Children born prematurely (before 37 weeks of gestation) were also excluded. Controls data were obtained in July 2022 by a review of the charts from a GP’s office located in a socially diverse neighbourhood in Poznań, Poland. The included data were recorded during routine health check-ups and immunisation visits. Percentiles and z-scores were calculated using the PediTools Software [10], which is based on WHO Child Growth Standards for children aged 0–2 [11] and CDC Clinical Growth Charts for children and adolescents aged 2–20 years [12].
2.1.2. Anthropometric Measurements
Measurements were recorded between May 2018 and July 2022 by treating physicians during routine clinical check-ups, in the morning, in light clothing and without shoes, using Seca 703 scales equipped with a measuring rod (Hamburg, Germany) for children > 2 years of age, and Seca 376 scales equipped with a measuring rod (Hamburg, Germany) for children < 2 years of age. The most recent set of measurements for each patient was used in the study. The weight measurements had an accuracy of 0.1 kg, while height was measured with an accuracy of 0.1 cm. BMI was calculated using the obtained values, with an accuracy of 0.1 kg/m2.
2.1.3. Statistical Analysis
Continuous data are presented as means (M) with standard deviations (SD), medians (Me) and interquartile ranges. Categorical variables are presented as absolute and relative frequencies. Normality was assessed with the Shapiro–Wilk test. Due to the non-normal distribution of the data for most studied variables, the anthropometric parameters of patients and controls and pre- and post-NBS patients were compared using the nonparametric U-Mann–Whitney test. ANOVA Kruskal–Wallis analysis and posthoc tests were used to compare different types of FAOD with the control group, and the Chi-squared statistic was used for categorical data. A p-value < 0.05 was considered statistically significant. Statistica Software version 13 (TIBCO Software Inc., Palo Alto, CA, USA) was used for the statistical calculations.
2.2. Systemic Review and Meta-Analysis
2.2.1. Protocol and Registration
The systematic review and meta-analysis were carried out and reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). Prisma Checklist is presented in Supplementary Materials (Table S3). The study protocol was registered in the international prospective register of systematic reviews (PROSPERO) database with ID number CRD42022343364.
2.2.2. Search Strategy
The following databases were searched: PubMed (Medline), Embase, Web of Science and Scopus; we used the following search terms: fatty acid oxidation disorders FAOD; long-chain 3-hydroxyacyl-coenzyme A dehydrogenase deficiency, LCHADD; medium-chain acyl-CoA dehydrogenase deficiency, MCADD; very long chain acyl-CoA dehydrogenase deficiency, VLCADD; short-chain acyl-CoA dehydrogenase deficiency, SCADD; carnitine palmitoyltransferase II deficiency, CPTIID or CPT 2D; mitochondrial trifunctional protein deficiency, TFPD. Searches were restricted to titles and abstracts, the English language and studies performed in humans. No date limits were applied. In addition, the articles were reviewed for anthropometric data (weight, BMI and percentiles and z-scores for these parameters), and the reference lists of the identified papers were checked for articles that database searches might have missed.
The search strategy was as follows:
PubMed (Medline)
#1 (disease name, i.e., LCHADD [Title/Abstract])
#2 AND (English and Human [Filter])
Embase
#1 (disease name i.e., LCHADD [Candidate Term])
#2 AND (Abstract and English and Human [Filter])
Web of Science
#1 (disease name, i.e., LCHADD [Topic])
#2 AND (English [Filter])
Scopus
#1 (disease name, i.e., LCHADD [Article title, Abstract, Keywords])
#2 AND (English and Human [Filter])
2.2.3. Eligibility Criteria
The following inclusion criteria were applied:
- Study type: observational (case–control, cohort, case series) or experimental studies (any type); a study was excluded when only an abstract was available.
- Language: English.
- Study population: children (>1 month of age) and adults with confirmed diagnosis (biochemically or genetically) of fatty acid oxidation disorder (LCHADD, MCADD, VLCADD, SCADD, CPT IID or TFPD).
- Outcomes: anthropometric parameters (weight, BMI and percentiles and z-scores for these parameters).
Exclusion criteria:
- Study type: letters, case studies, conference abstracts, non-human studies.
- Language: papers published in a language other than English.
- Population: newborns (age < 1 month), pregnant and breastfeeding women, patients in unstable or critical clinical condition.
2.2.4. Study Selection Process
Three researchers (MWG, JG and KB) searched each database independently. First, the reviewers checked titles, abstracts and then full texts based on the eligibility criteria. Studies deemed appropriate by at least one of the reviewers were incorporated in the next step, with any disagreements resolved by consensus within the review team.
2.2.5. Data Extraction
Two reviewers (MWG and JG) independently performed data extraction, and two others (MJ and JW) checked it. The corresponding authors were contacted for missing information. The following information was extracted from each paper:
- General information: full title of the article, list of authors, country, journal name, year of publication.
- Study characteristics: study design (experimental or observational).
- Population characteristics: number of participants in patient groups and control groups (if applicable), types of FAOD diagnosed in patients, age of the participants and sex of the participants.
- Outcomes recorded: anthropometric parameters of patients and controls (if present)—weight, BMI and percentiles and z-scores for these parameters.
2.2.6. Certainty of Evidence Assessment
Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) was used to estimate the quality of the included data [13]. Two independent researchers (MWG and MJ) conducted the assessment, and any disagreements were resolved by discussion.
2.2.7. Statistical Analysis
The meta-analysis was performed using Comprehensive Meta-Analysis version 3.0 software (Biostat, Inc. Englewood, CO, USA). A p-value < 0.05 was considered as statistically significant. In some studies, M and SD were calculated based on individual patients’ and control subjects’ data. The most recent data set was used if several data points were available for the patient. For the meta-analysis, two scenarios for patient groups were considered. In the first one, all patients with diagnosed FAOD were put in one group and analysed vs. controls. In the second one, the patients were divided into an MCAD group and an other-types-of-FAOD group and analysed vs. controls. The meta-analysis was performed if a given outcome was reported in at least two studies. There was no need to unify the data, as they were presented in SI units in all included studies. The analyses were undertaken to compare weight and BMI between patients with FAOD vs. control subjects. Data analysis was performed, including calculating the effect of sizes with 95% CI, using either random-effect models (to analyse studies with moderate to high heterogeneity) or fixed-effect models (to analyze outcomes without heterogeneity). Mean differences (MD) were used as a summary statistic to facilitate comparing outcomes between groups. Forest plots were generated to visualise the study-specific effect sizes, with 95% CI. Funnel plots were generated, and Begg’s and Egger’s tests were performed to assess publication bias. Heterogeneity between studies was evaluated using Cochran’s Q test, with p < 0.1 indicating significant heterogeneity. The I2 test was used to assess consistency between studies, with an I2 value of <30% indicating a low risk of heterogeneity, 30–75% indicating a moderate risk of heterogeneity, and >75% indicating a high risk of heterogeneity. Sensitivity analysis and cumulative analysis were also performed.
3. Results
3.1. Case–Control Study Results
The population characteristics are summarised in Table 1, showing no differences in age and sex structure between the groups. The FAOD cohort included 19 patients with MCADD (48.7%), 14 patients with LCHADD (35.9%), 5 patients with VLCADD (12.8%) and one patient with CDSP (2.6%). In total, 17 patients (43.6%) were born before the introduction of NBS in Poland, while 22 patients (56.4%) were born after it. We observed statistically significant differences in body weight percentiles (p = 0.001), BMI (p = 0.022), BMI percentiles (p = 0.003) and BMI z-scores (p = 0.001), with FAOD patients having larger body weight and BMI than healthy controls. There were no statistically significant differences in weight percentiles (p = 0.702), BMI percentiles (p = 0.590) and BMI z-scores (p = 0.365) observed for pre- and post-NBS patients. The Kruskal–Wallis test was conducted to determine differences in anthropometric parameters between different types of FAOD patients and controls, showing statistically significant differences in weight centiles (p = 0.009), BMI centiles (p = 0.044) and BMI z-scores (p = 0.021). There were no differences in weight (p = 0.164) and BMI (p = 0.260), but the posthoc analysis confirmed differences in weight centiles between MCAD and the control group (p = 0.036).

Table 1.
Population characteristics.
3.2. Results from the Systematic Review and Meta-Analysis
3.2.1. Search Results
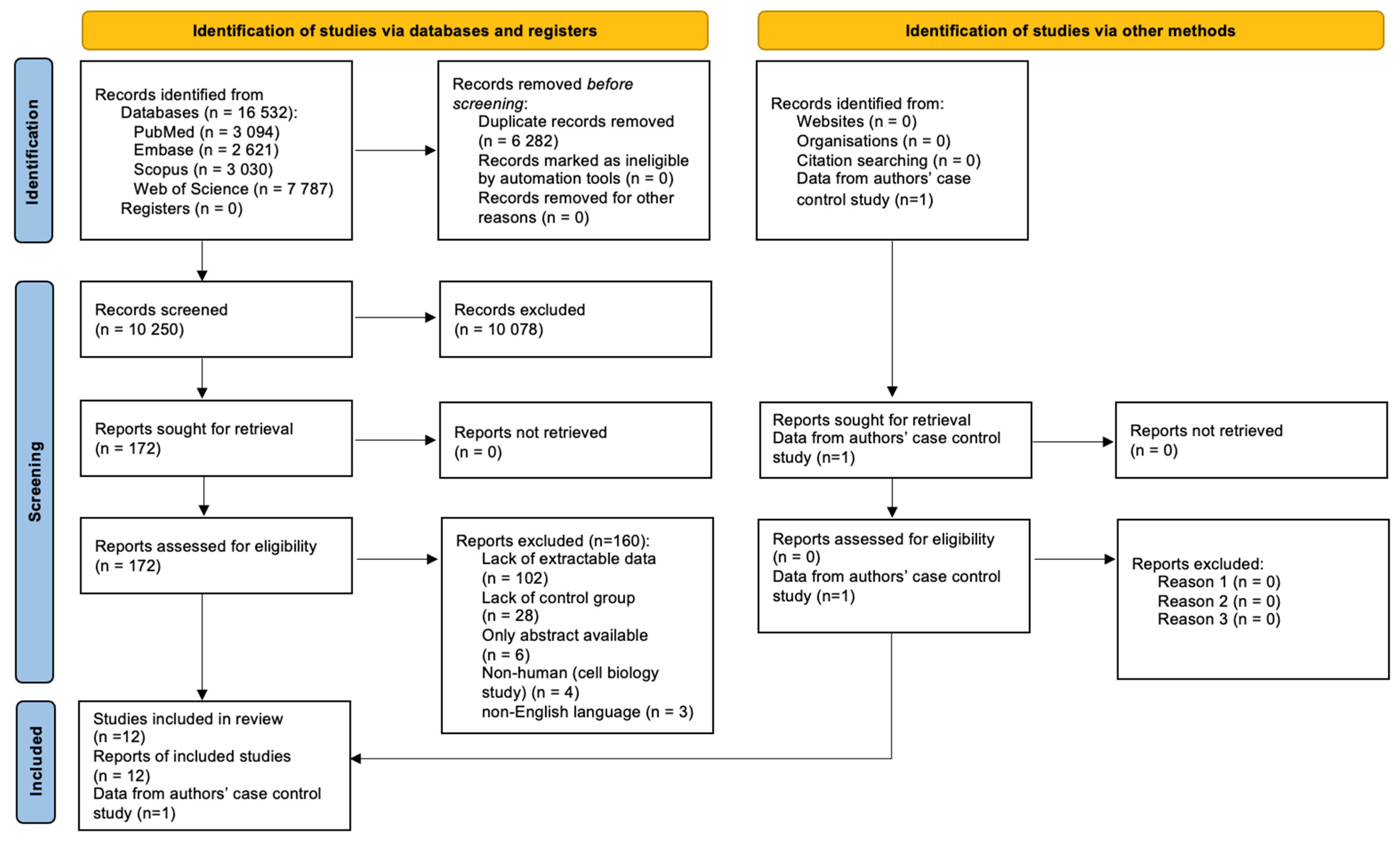
Figure 1 shows the results of the search identifying a total of 16,532 records, of which, 6282 were duplicates. The titles and abstracts of the following 10,250 records were screened, and 172 full texts were retrieved, of which, 12 articles were included in the study.
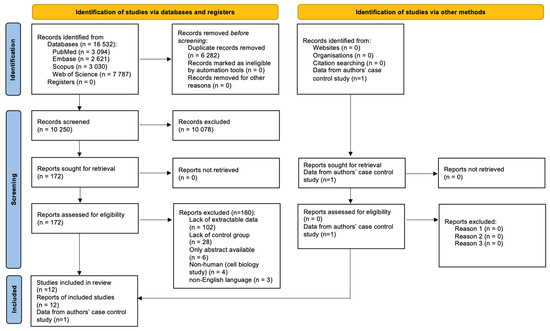
Figure 1.
PRISMA 2020 Flow Diagram.
However, the studies by McCoin et al., 2019, McCoin et al., 2016 and Gillingham et al., 2013 [14,15,16] were conducted in the same centre on overlapping populations; therefore, we decided to include a study by McCoin et al., 2016 in the meta-analysis, since it had the largest study and control populations. Of the remaining papers, seven had extractable data on the study and control groups [17,18,19,20,21,22]; therefore, eight papers were incorporated into the meta-analysis along with our case–control study.
3.2.2. Reported Anthropometric Parameters
Weight was reported in seven studies [14,16,18,19,21,22,23]. The data on weight were presented in the form of individual patient data, M, SD, Me and Z-score Me and Z-score SD. BMI was reported in 10 papers [14,15,16,17,18,19,20,23,24,25]. The data were presented as individual patient data, M, SD, Me, Z-score Me and Z-score SD.
3.2.3. Characteristics of the Included Studies
The characteristics of the included studies are presented in Table 2, and the data from the included studies are presented in Table 3. Characteristics and data from studies without control groups that contained data on anthropometric parameters, but were not included into the meta-analysis, are presented in Tables S1 and S2 in Supplementary Materials.

Table 2.
Characteristics of the studies with control groups.

Table 3.
Data extracted form studies with control groups.
All the included 12 papers were observational studies. Seven studies were performed in Europe, four in the Netherlands [18,19,22,24], two in Denmark [17,25], one in Spain [23], three in North America (USA) [14,15,16], one in Asia (Japan) [20] and one in Australia [21].
3.2.4. Characteristics of the Study Participants
A total of 201 participants were included in the studies, of which, 80 were FAOD patients, while 121 were control subjects. The study size varied from 6 to 24 participants. In nine studies, children were incorporated into the study groups. Among the included FAOD patients, 16 had MCADD, 14 had VLCADD, 33 had LCHADD, 9 had CPT2D, 1 had CPT1D, 3 had MADD, and four had SCADD. FAOD diagnosis was confirmed via biochemical or genetic testing. In one study, the patients were (mostly) diagnosed by the NBS [23], one study stated that the patients were diagnosed before the NBS programme [24], and ten papers included no information on the NBS status. As mentioned above, some of the study groups were overlapping.
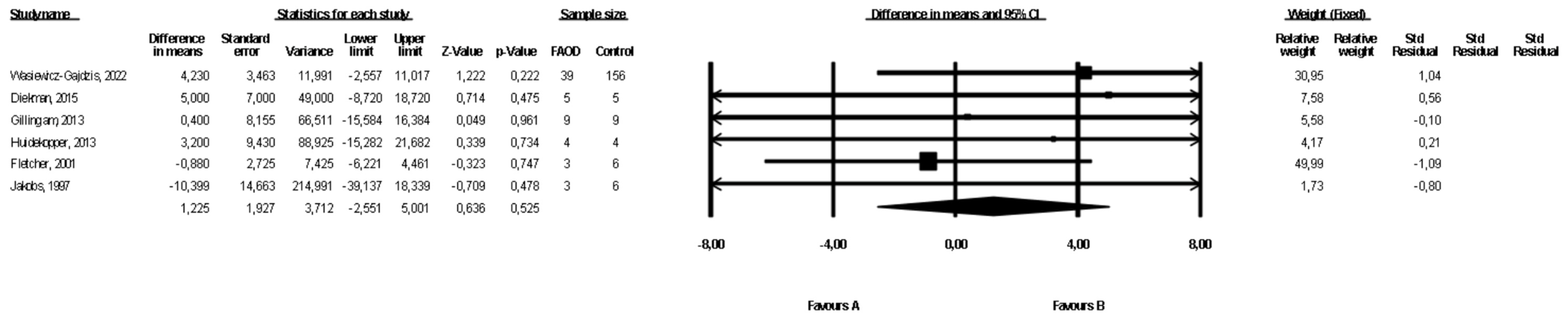
3.3. Comparison of Weight between FAOD Patients and Controls
Six studies presented data on weight for the FAOD and the control groups ([16,18,19,21,22], Wasiewicz-Gajdzis et al., 2022). No study reported statistically significant differences between the groups. The meta-analysis showed no difference in weight between the FAOD and the control groups (fixed-effects model, SE: 1.93, 95% CI: −2.55, 5.00, p = 0.53, Figure 2). The risk of heterogeneity was assessed as small (Q value = 0.232, p = 0.80, I2 = 0). The funnel plot of standard error by a difference in means of weight is presented in the Supplementary Materials (Figure S1). The Begg and Mazumdar’s rank correlation test (p = 0.70) and the Egger’s regression test (p = 0.96) were performed.

Figure 2.
Forest plot—comparison of body weight in FAOD patients vs. controls [16,18,19,21,22].
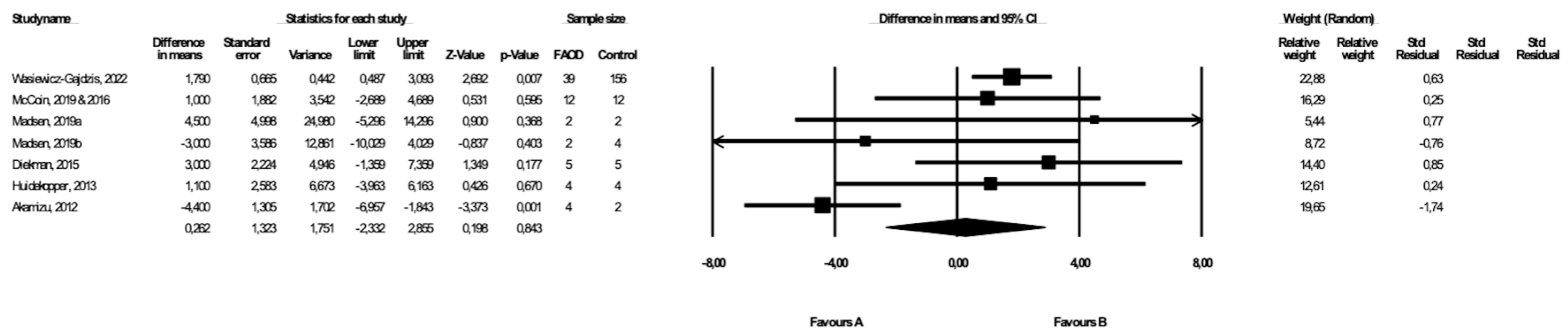
3.4. Comparison of BMI between FAOD Patients and Controls
BMI was compared between FAOD patients and control subjects in seven studies ([14,17,18,19,20,25], Wasiewicz-Gajdzis et al., 2022), with a statistically significant difference in BMI in two studies ([20], Wasiewicz-Gajdzis et al., 2022). The meta-analysis showed no difference in BMI between FAOD and control groups (random-effects model, SE: 1.32, 95% CI: −2.33, 2.86, p = 0.843, Figure 3), but there was a substantial risk for heterogeneity (Q value = 20.73, p = 0.00, I2 = 71.05). The funnel plot of standard error by a difference in means of BMI is presented in the Supplementary Materials (Figure S2); the Begg and Mazumdar’s rank correlation test (p = 0.76) and the Egger’s regression test (p = 0.73) were also performed.

Figure 3.
Forest plot—comparison of BMI in FAOD patients vs. controls [14,15,17,18,19,20,25].
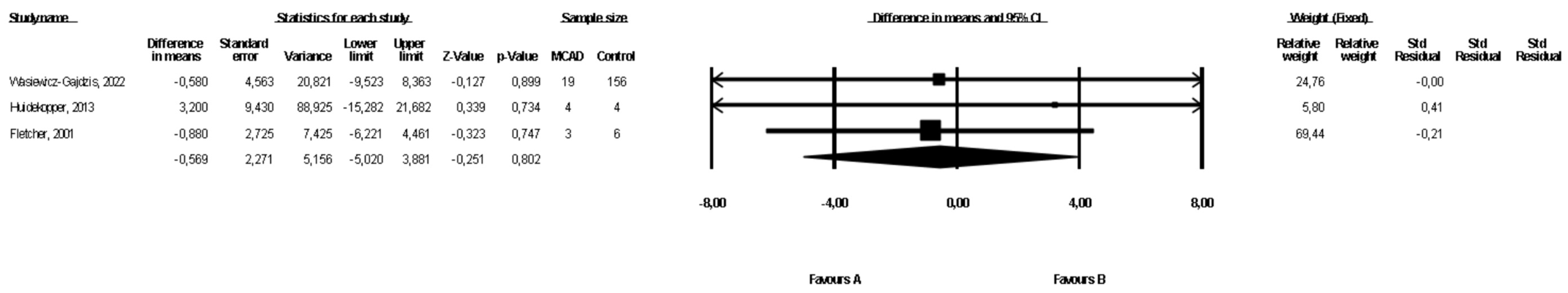
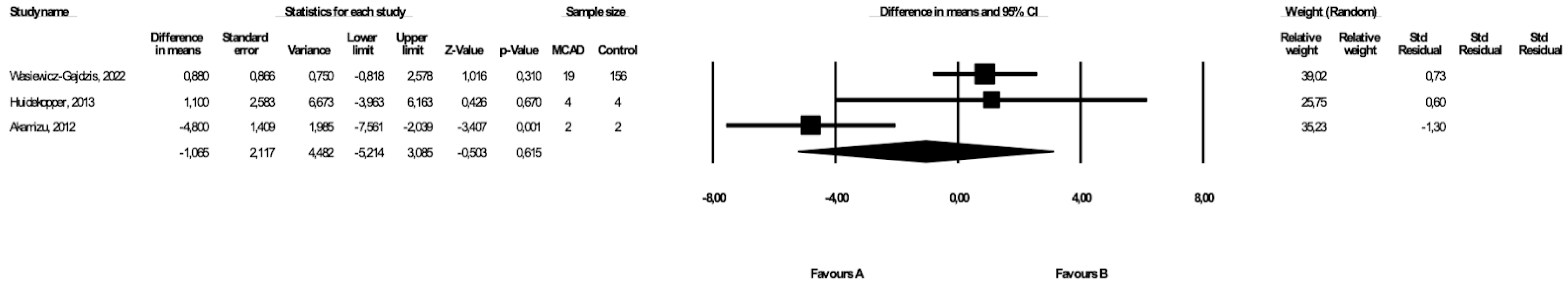
3.5. Comparison of Weight between MCAD Patients and Controls
The weight of the MCAD patients and controls was compared in three studies ([19,21], Wasiewicz-Gajdzis et al., 2022) with no study reporting a statistically significant difference in weight. The meta-analysis showed no difference in weight between MCAD patients and the control group (fixed-effects model, SE: 2.27, 95% CI: −5.02, 3.88, p = 0.80, Figure 4), and there was a small risk of heterogeneity (Q value = 0.17, p = 0.91, I2 = 0). The funnel plot of standard error by a difference in means of BMI is presented in the Supplementary Materials (Figure S3); the Begg and Mazumdar’s rank correlation test (p = 0.30) and the Egger’s regression test (p = 0.23) were also performed.

Figure 4.
Forest plot—comparison of bodyweight in MCAD patients vs. controls [19,20].
3.6. Comparison of BMI between MCAD Patients and Controls
Three studies presented data on BMI in MCAD patients and controls ([19,20], Wasiewicz-Gajdzis et al., 2022), with no study reporting a statistically significant difference in BMI. The meta-analysis showed no difference in BMI between the MCAD patients and the control group (random-effects model, SE: 2.12, 95% CI: −5.21, 3.09, p = 0.62, Figure 5). The risk of heterogeneity was considered small (Q value = 12.23, p = 0.002, I2 = 83.65). A funnel plot of standard error by a difference in means of BMI is presented in the Supplementary Materials (Figure S4). The Begg and Mazumdar’s rank correlation test (p = 1.00) and the Egger’s regression test (p = 0.76) were also performed.

Figure 5.
Forest plot—comparison of BMI in MCAD patients vs. controls [19,20].
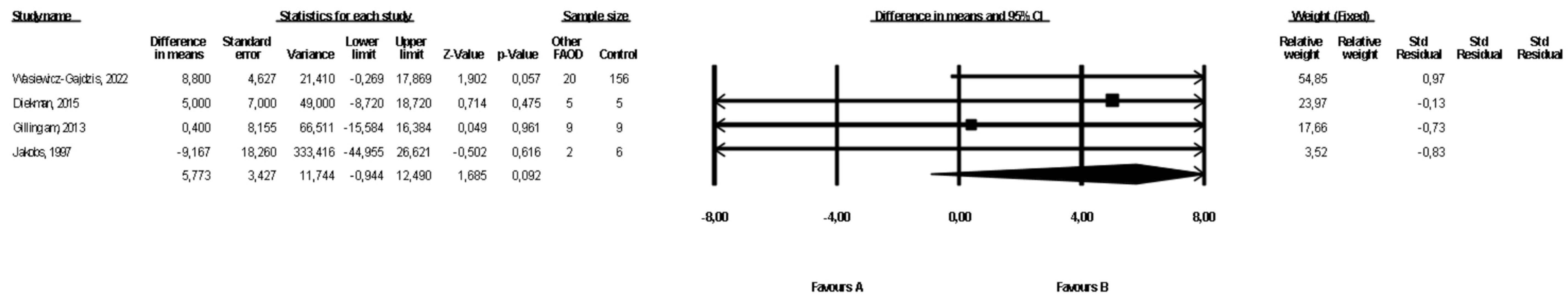
3.7. Comparison of Weight between Patients with Types of FAOD Other Than MCAD and Controls
Data on the weight of patients with FAOD types other than MCAD were available in four studies ([16,18,22], Wasiewicz-Gajdzis et al., 2022), with no study reporting a statistically significant difference in weight. The meta-analysis showed no difference in weight between patients and the control group (fixed-effects model, SE: 3.43, 95% CI: −0.94, 12.49, p = 0.09, Figure 6), and the risk of heterogeneity was assessed as small (Q value = 1.54, p = 0.68, I2 = 0). A funnel plot of the standard error by a difference in means of weight is presented in the Supplementary Materials (Figure S5). We performed the Begg and Mazumdar’s rank correlation test (p = 0.09) and the Egger’s regression test (p = 0.05).

Figure 6.
Forest plot—comparison of body weight in patients with FAOD other than MCAD vs. controls [16,18,22].
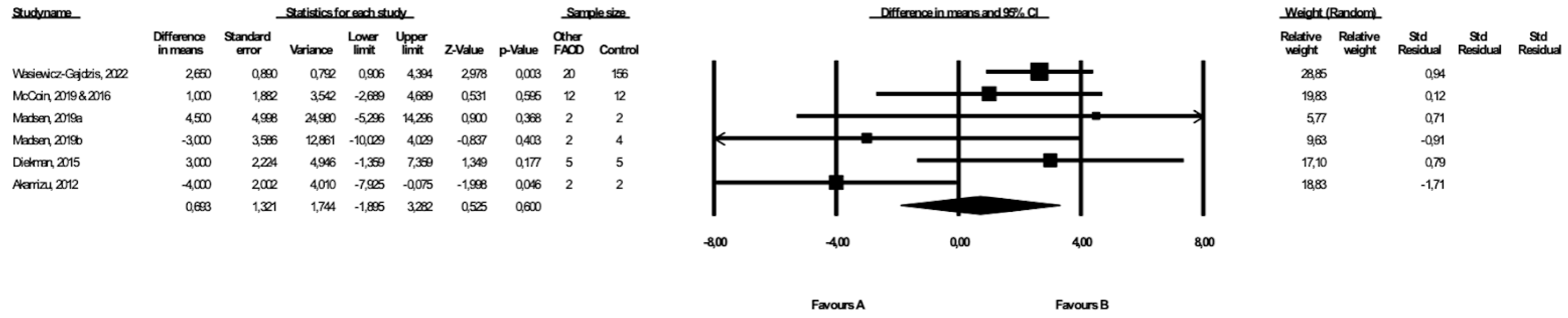
3.8. Comparison of BMI between Patients with Types of FAOD Other Than MCAD and Controls
Six studies included data on the BMI of patients with types of FAOD other than MCAD ([14,17,18,20,25,26], Wasiewicz-Gajdzis et al., 2022), with no study reporting a statistically significant difference in BMI. The meta-analysis showed no difference in BMI between patients and the control group (random-effects model, SE: 1.32, 95% CI: −1.90, 3.26, p = 0.60, Figure 7), and the risk of heterogeneity was substantial (Q value = 11.67, p = 0.04, I2 = 57.17). A funnel plot of the standard error by a difference in means of weight is presented in the Supplementary Materials (Figure S6). We performed the Begg and Mazumdar’s rank correlation test (p = 0.71) and the Egger’s regression test (p = 0.42).

Figure 7.
Forest plot—comparison of BMI in patients with FAOD other than MCAD vs. controls [14,15,17,18,20,25].
3.9. Sensitivity and Cumulative Meta-Analyses
The sensitivity and cumulative meta-analyses revealed no statistically significant differences, and the relevant figures are provided in the Supplementary Materials (Figures S6–S18).
3.10. Certainty of Evidence Assessment
The certainty in the evidence for each outcome is presented in Table 4.

Table 4.
Certainty of evidence evaluated using GRADE framework.
4. Discussion
A case–control study and a systematic review and meta-analysis were conducted to compare anthropometric parameters between FAOD patients and healthy people. The case–control study revealed a statistically significant difference in weight percentiles, BMI, BMI percentiles and BMI z-scores between our FAOD cohort and healthy control subjects, with FAOD patients being heavier and having a higher BMI. However, there were no significant differences in anthropometric parameters of patients born pre- and post-NBS introduction in Poland. There were no differences in anthropometric parameters between patients with different FAOD diagnoses, either.
Our systematic review and meta-analysis revealed no differences in the anthropometric parameters of patients with all types of FAOD and controls. There was also no statistically significant difference in weight and BMI when comparing a group of people with MCAD and a group of patients with types of FAOD other than MCAD to healthy controls.
Some of the studies which were not included in the meta-analysis due to lack of extractable data yielded similar conclusions as our meta-analysis: Rücklová et al. observed good growth and weight control among patients [27], Rovelli et al. and Anderson et al. found that the anthropometric parameters of FAOD patients were not different from those of the general population [28,29]. Some of the older works described a failure to thrive [30,31] and an increased incidence of overweight and obesity [5,6,32]. Two papers reported increased adiposity in patients with FAOD [16,33].
Differences in treatment guidelines implementation, such as the extent of dietary restrictions, the maximal recommended duration of fasting, the implementation of night feeds, or enteral nutrition in metabolic centres, might have caused the differences in results in our case–control study and meta-analysis. There were also differences in healthcare services provided to patients that might affect the nutritional status; some studies described a close supervision of patients by dieticians [28], while such services were not widely available to patients in other centres. Furthermore, a fear of metabolic decompensation and complications could be differentiated depending on the care possibilities available to patients and personal experience, which might drive overfeeding and excessive avoidance of physical activity.
This is the first meta-analysis comparing the anthropometric parameters of patients with FAOD to those of healthy controls; the cohort from Polish centres was significant compared to other studies. The strength of this work is that it was based on a comprehensive search of four large databases: PubMed, Embase, Scopus and Web of Science, using broad search terms. Furthermore, the double-counting of patients from publications prepared in the same centres or by the same authors was prevented.
The major limitation of this study is the overall low level of evidence, as assessed by GRADE. Other limitations of our study comprise the small sample sizes in the included groups, which were due to the low prevalence of inborn errors of metabolism in the population. Furthermore, many studies incorporated children and adults, hindering the analysis. Another limitation was the lack of extractable data in publications for the systemic review, and the authors did not provide data when contacted. In addition, some publications had overlapping patient groups, and only a few publications considered wth control groups, which limited the number of papers that could be included in the meta-analysis. Due to a lack of research, we could not perform a meta-analysis to compare the effect of NBS on the anthropometric parameters. Therefore, the results from this study should be interpreted with caution. More research is needed to clearly establish the impact of FAOD on the nutritional state of patients.
5. Conclusions
There were statistically significant differences in anthropometric parameters between FAOD patients and healthy controls in our case–control study, with patients having higher body weight and BMI, but no differences were observed for pre- and post-NBS patients and patients with different types of FAOD. However, the meta-analysis showed no differences in anthropometric parameters between patients and controls, and the results must be interpreted cautiously due to the small sample sizes and heterogeneous data.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/healthcare10122405/s1, Table S1. Characteristics of the studies without control groups, Table S2. Data extracted form studies without control groups, Table S3: PRISMA 2020 Checklist; Figure S1: Funnel plot of Standard Error by a difference in the means of weight (FAOD vs. Controls); Figure S2: Funnel plot of Standard Error by a difference in the means of BMI (FAOD vs. Controls); Figure S3: Funnel plot of Standard Error by a difference in the means of weight (MCADD vs. Controls); Figure S4: Funnel plot of Standard Error by a difference in the means of BMI (MCADD vs. Controls) Figure S5: Funnel plot of Standard Error by a difference in the means of weight (other FAOD vs. Controls); Figure S6: Funnel plot of Standard Error by a difference in the means (other FAOD vs. Controls); Figure S7: Cumulative meta-analysis: Forest plot: comparison of body weight in FAOD patients vs. controls; Figure S8: Sensitivity meta-analysis: Forest plot: comparison of weight in FAOD patients vs. controls; Figure S9: Cumulative meta-analysis: Forest plot: comparison of BMI in FAOD patients vs. controls; Figure S10: Sensitivity meta-analysis: Forest plot: comparison of BMI in FAOD patients vs. controls; Figure S11: Cumulative meta-analysis: Forest plot: comparison of weight in MCADD patients vs. controls; Figure S12: Sensitivity meta-analysis: Forest plot: comparison of weight in MCADD patients vs. controls; Figure S13: Cumulative meta-analysis: Forest plot: comparison of BMI in MCADD patients vs. controls; Figure S14: Sensitivity meta-analysis: Forest plot: comparison of BMI in MCADD patients vs. controls; Figure S15: Cumulative meta-analysis: Forest plot: comparison of weight in patients with types of FAOD other than MCADD vs. controls; Figure S16: Sensitivity meta-analysis: Forest plot: comparison of weight in patients with types of FAOD other than MCADD vs. controls; Figure S17: Cumulative meta-analysis: Forest plot: comparison of BMI in patients with types of FAOD other than MCADD vs. controls; Figure S18: Sensitivity meta-analysis: Forest plot: comparison of BMI in patients with types of FAOD other than MCADD vs. controls.
Author Contributions
Conceptualization, M.J. and J.W.; methodology, M.W.-G., M.J. and J.W.; formal analysis, M.W.-G., M.J. and J.G.; investigation, M.W.-G., J.G. and K.B.; resources, Ł.K. and J.J.; data curation, M.J. and M.W.-G.; writing—original draft preparation, M.W.-G.; writing—review and editing, M.W.-G., M.J., J.W. and Ł.K.; visualization, M.W.-G. and K.B.; supervision, J.W.; project administration, J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this article are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Merritt, J.L.; Chang, I.J. Medium-Chain Acyl-Coenzyme A Dehydrogenase Deficiency. In GeneReviews®; Adam, M.P., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Merritt II, J.L.; Norris, M.; Kanungo, S. Fatty Acid Oxidation Disorders. Ann. Transl. Med. 2018, 6, 473. [Google Scholar] [CrossRef] [PubMed]
- Spiekerkoetter, U.; Lindner, M.; Santer, R.; Grotzke, M.; Baumgartner, M.R.; Boehles, H.; Das, A.; Haase, C.; Hennermann, J.B.; Karall, D.; et al. Management and Outcome in 75 Individuals with Long-Chain Fatty Acid Oxidation Defects: Results from a Workshop. J. Inherit. Metab. Dis. 2009, 32, 488–497. [Google Scholar] [CrossRef]
- Saudubray, J.M.; Martin, D.; de Lonlay, P.; Touati, G.; Poggi-Travert, F.; Bonnet, D.; Jouvet, P.; Boutron, M.; Slama, A.; Vianey-Saban, C.; et al. Recognition and Management of Fatty Acid Oxidation Defects: A Series of 107 Patients. J. Inherit. Metab. Dis. 1999, 22, 488–502. [Google Scholar] [CrossRef]
- Derks, T.G.J.; Reijngoud, D.-J.; Waterham, H.R.; Gerver, W.-J.M.; van den Berg, M.P.; Sauer, P.J.J.; Smit, G.P.A. The Natural History of Medium-Chain Acyl CoA Dehydrogenase Deficiency in the Netherlands: Clinical Presentation and Outcome. J. Pediatr. 2006, 148, 665–670.e3. [Google Scholar] [CrossRef] [PubMed]
- Gillingham, M.B.; Purnell, J.Q.; Jordan, J.; Stadler, D.; Haqq, A.M.; Harding, C.O. Effects of Higher Dietary Protein Intake on Energy Balance and Metabolic Control in Children with Long-Chain 3-Hydroxy Acyl-CoA Dehydrogenase (LCHAD) or Trifunctional Protein (TFP) Deficiency. Mol. Genet. Metab. 2007, 90, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Wszystkie Noworodki w Polsce Zostały Objęte Badaniem Przesiewowym w Kierunku Rzadkich Wad Metabolizmu—Institute of Mother and Child. Available online: https://imid.med.pl/en/409/wszystkie-noworodki-w-polsce-zostaly-objete-badaniem-przesiewowym-w-kierunku-rzadkich-wad-metabolizmu (accessed on 28 August 2022).
- Sawicka-Gutaj, N.; Gruszczyński, D.; Guzik, P.; Mostowska, A.; Walkowiak, J. Publication Ethics of Human Studies in the Light of the Declaration of Helsinki—A Mini-Review. J. Med. Sci. 2022, 91, e700. [Google Scholar] [CrossRef]
- Field, N.; Cohen, T.; Struelens, M.J.; Palm, D.; Cookson, B.; Glynn, J.R.; Gallo, V.; Ramsay, M.; Sonnenberg, P.; MacCannell, D.; et al. Strengthening the Reporting of Molecular Epidemiology for Infectious Diseases (STROME-ID): An Extension of the STROBE Statement. Lancet Infect. Dis. 2014, 14, 341–352. [Google Scholar] [CrossRef]
- Chou, J.H.; Roumiantsev, S.; Singh, R. PediTools Electronic Growth Chart Calculators: Applications in Clinical Care, Research, and Quality Improvement. J. Med. Internet Res. 2020, 22, e16204. [Google Scholar] [CrossRef]
- The WHO Child Growth Standards. Available online: https://www.who.int/tools/child-growth-standards/standards (accessed on 15 August 2022).
- Growth Charts—Clinical Growth Charts. Available online: https://www.cdc.gov/growthcharts/clinical_charts.htm (accessed on 15 August 2022).
- Puhan, M.A.; Schunemann, H.J.; Murad, M.H.; Li, T.; Brignardello-Petersen, R.; Singh, J.A.; Kessels, A.G.; Guyatt, G.H.; for the GRADE Working Group. A GRADE Working Group Approach for Rating the Quality of Treatment Effect Estimates from Network Meta-Analysis. BMJ 2014, 349, g5630. [Google Scholar] [CrossRef]
- McCoin, C.S.; Piccolo, B.D.; Knotts, T.A.; Matern, D.; Vockley, J.; Gillingham, M.B.; Adams, S.H. Unique Plasma Metabolomic Signatures of Individuals with Inherited Disorders of Long-Chain Fatty Acid Oxidation. J. Inherit. Metab. Dis. 2016, 39, 399–408. [Google Scholar] [CrossRef]
- McCoin, C.S.; Gillingham, M.B.; Knotts, T.A.; Vockley, J.; Ono-Moore, K.D.; Blackburn, M.L.; Norman, J.E.; Adams, S.H. Blood Cytokine Patterns Suggest a Modest Inflammation Phenotype in Subjects with Long-chain Fatty Acid Oxidation Disorders. Physiol. Rep. 2019, 7, e14037. [Google Scholar] [CrossRef] [PubMed]
- Gillingham, M.B.; Harding, C.O.; Schoeller, D.A.; Matern, D.; Purnell, J.Q. Altered Body Composition and Energy Expenditure but Normal Glucose Tolerance among Humans with a Long-Chain Fatty Acid Oxidation Disorder. Am. J. Physiol.-Endocrinol. Metab. 2013, 305, E1299–E1308. [Google Scholar] [CrossRef] [PubMed]
- Madsen, K.L.; Stemmerik, M.G.; Buch, A.E.; Poulsen, N.S.; Lund, A.M.; Vissing, J. Impaired Fat Oxidation During Exercise in Long-Chain Acyl-CoA Dehydrogenase Deficiency Patients and Effect of IV-Glucose. J. Clin. Endocrinol. Metab. 2019, 104, 3610–3613. [Google Scholar] [CrossRef] [PubMed]
- Diekman, E.F.; Visser, G.; Schmitz, J.P.J.; Nievelstein, R.A.J.; de Sain-van der Velden, M.; Wardrop, M.; Van der Pol, W.L.; Houten, S.M.; van Riel, N.A.W.; Takken, T.; et al. Altered Energetics of Exercise Explain Risk of Rhabdomyolysis in Very Long-Chain Acyl-CoA Dehydrogenase Deficiency. PLoS ONE 2016, 11, e0147818. [Google Scholar] [CrossRef]
- Huidekoper, H.H.; Ackermans, M.T.; Koopman, R.; van Loon, L.J.C.; Sauerwein, H.P.; Wijburg, F.A. Normal Rates of Whole-Body Fat Oxidation and Gluconeogenesis after Overnight Fasting and Moderate-Intensity Exercise in Patients with Medium-Chain Acyl-CoA Dehydrogenase Deficiency. J. Inherit. Metab. Dis. 2013, 36, 831–840. [Google Scholar] [CrossRef]
- Akamizu, T.; Sakura, N.; Shigematsu, Y.; Tajima, G.; Ohtake, A.; Hosoda, H.; Iwakura, H.; Ariyasu, H.; Kangawa, K. Analysis of Plasma Ghrelin in Patients with Medium-Chain Acyl-CoA Dehydrogenase Deficiency and Glutaric Aciduria Type II. Eur. J. Endocrinol. 2012, 166, 235–240. [Google Scholar] [CrossRef]
- Fletcher, J.M.; Pitt, J.J. Fasting Medium Chain Acyl-Coenzyme a Dehydrogenase-Deficient Children Can Make Ketones. Metabolism 2001, 50, 161–165. [Google Scholar] [CrossRef]
- Jakobs, C.; Kneer, J.; Martin, D.; Boulloche, J.; Brivet, M.; Poll-The, B.T.; Saudubray, J.M. In Vivo Stable Isotope Studies in Three Patients Affected with Mitochondrial Fatty Acid Oxidation Disorders: Limited Diagnostic Use of 1-13C Fatty Acid Breath Test Using Bolus Technique. Eur. J. Pediatr. 1997, 156, S78–S82. [Google Scholar] [CrossRef]
- de Castro, M.-J.; Sánchez-Pintos, P.; Abdelaziz-Salem, N.; Leis, R.; Couce, M.L. Evaluation of Body Composition, Physical Activity, and Food Intake in Patients with Inborn Errors of Intermediary Metabolism. Nutrients 2021, 13, 2111. [Google Scholar] [CrossRef]
- Knottnerus, S.J.G.; Bleeker, J.C.; Ferdinandusse, S.; Houtkooper, R.H.; Langeveld, M.; Nederveen, A.J.; Strijkers, G.J.; Visser, G.; Wanders, R.J.A.; Wijburg, F.A.; et al. Subclinical Effects of Long-chain Fatty Acid Β-oxidation Deficiency on the Adult Heart: A Case-control Magnetic Resonance Study. J. Inherit. Metab. Dis. 2020, 43, 969–980. [Google Scholar] [CrossRef]
- Madsen, K.L.; Preisler, N.; Buch, A.E.; Stemmerik, M.G.; Laforêt, P.; Vissing, J. Impaired Fat Oxidation during Exercise in Multiple Acyl-CoA Dehydrogenase Deficiency. JIMD Rep. 2019, 46, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Orngreen, M.C.; Madsen, K.L.; Preisler, N.; Andersen, G.; Vissing, J.; Laforet, P. Bezafibrate in Skeletal Muscle Fatty Acid Oxidation Disorders: A Randomized Clinical Trial. Neurology 2014, 82, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Rücklová, K.; Hrubá, E.; Pavlíková, M.; Hanák, P.; Farolfi, M.; Chrastina, P.; Vlášková, H.; Kousal, B.; Smolka, V.; Foltenová, H.; et al. Impact of Newborn Screening and Early Dietary Management on Clinical Outcome of Patients with Long Chain 3-Hydroxyacyl-CoA Dehydrogenase Deficiency and Medium Chain Acyl-CoA Dehydrogenase Deficiency—A Retrospective Nationwide Study. Nutrients 2021, 13, 2925. [Google Scholar] [CrossRef] [PubMed]
- Rovelli, V.; Manzoni, F.; Viau, K.; Pasquali, M.; Longo, N. Clinical and Biochemical Outcome of Patients with Very Long-Chain Acyl-CoA Dehydrogenase Deficiency. Mol. Genet. Metab. 2019, 127, 64–73. [Google Scholar] [CrossRef]
- Anderson, D.R.; Viau, K.; Botto, L.D.; Pasquali, M.; Longo, N. Clinical and Biochemical Outcomes of Patients with Medium-Chain Acyl-CoA Dehydrogenase Deficiency. Mol. Genet. Metab. 2020, 129, 13–19. [Google Scholar] [CrossRef] [PubMed]
- De Biase, I.; Viau, K.S.; Liu, A.; Yuzyuk, T.; Botto, L.D.; Pasquali, M.; Longo, N. Diagnosis, Treatment, and Clinical Outcome of Patients with Mitochondrial Trifunctional Protein/Long-Chain 3-Hydroxy Acyl-CoA Dehydrogenase Deficiency. JIMD Rep. 2016, 31, 63–71. [Google Scholar] [CrossRef]
- Iafolla, A.K.; Thompson, R.J.; Roe, C.R. Medium-Chain Acyl-Coenzyme A Dehydrogenase Deficiency: Clinical Course in 120 Affected Children. J. Pediatr. 1994, 124, 409–415. [Google Scholar] [CrossRef]
- Haglind, C.B.; Stenlid, M.H.; Ask, S.; Alm, J.; Nemeth, A.; Döbeln, U.; Nordenström, A. Growth in Long-Chain 3-Hydroxyacyl-CoA Dehydrogenase Deficiency. JIMD Rep.—Case Res. Rep. 2012/5 2012, 8, 81–90. [Google Scholar] [CrossRef]
- Evans, M.; Andresen, B.S.; Nation, J.; Boneh, A. VLCAD Deficiency: Follow-up and Outcome of Patients Diagnosed through Newborn Screening in Victoria. Mol. Genet. Metab. 2016, 118, 282–287. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).