Dietary Antioxidants and Lung Cancer Risk in Smokers and Non-Smokers
Abstract
:1. Introduction
2. Methods
3. Associations between Dietary Antioxidants Intake and LC Risk in Smokers and Non-Smokers
3.1. Smokers
3.2. Non-Smokers
4. Cigarette Smoke-Induced Oxidative Stress in Lung Cancer
5. Potential Role of Dietary Antioxidants against Cigarette Smoke-Induced Oxidative Stress in Lung Cancer
5.1. Antioxidant Vitamins
5.1.1. Retinol
5.1.2. β-Cryptoxanthin and Lycopene
5.1.3. β-Carotene, Vitamins C and E
5.1.4. Vitamin D
5.2. Antioxidant Minerals
5.2.1. Iron
5.2.2. Copper, Zinc and Selenium
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABCG1 | ATP-binding cassette transporter G1 |
| ADC | Adenocarcinoma |
| AKT | Serine-threonine kinase |
| AOC | Antioxidant capacity |
| AP-1 | Activator protein-1 |
| BCX | β-cryptoxanthin |
| BRAF | v-raf murine sarcoma viral oncogene homolog B1 |
| CS | Cigarette smoke |
| CuZnSOD | Copper-zinc superoxide dismutase |
| Cx43 | Connexin-43 |
| DDR2 | Discoidin Domain Receptor Tyrosine Kinase 2 |
| EGFR | Epidermal growth factor receptor |
| EGFR-TKI | EGFR tyrosine kinase inhibitor |
| EML4-ALK | Echinoderm microtubule–associate protein like 4/anaplastic lymphoma kinase |
| ERK | Extracellular signal-regulated kinase |
| FFQ | Food frequency questionnaire |
| FGFR | Fibroblast growth factor receptor |
| GPx | Glutathione peroxidase |
| GSH | Glutathione |
| HO-1 | Heme oxygenase-1 |
| α7nAChR | homomeric α7 nicotinic acetylcholine receptor |
| 8OHdG | 8-hydroxy-20-deoxyguanosine |
| H2O2 | Hydrogen peroxide |
| IFN-γ | Interferons |
| IL- | Interleukin |
| 8-iso-PGF2α | Isoprostane-8-iso prostaglandin F2α |
| JNK | c-JUN NH2-terminal kinase |
| KRAS | Kirsten rat sarcoma viral oncogene homolog |
| LC | Lung cancer |
| LP | Lipid peroxidation |
| LSH | Lymphoid-specific helicase |
| LXRα | Liver X receptor-α |
| MDA | Malondialdehyde |
| MMP | Metalloproteinase |
| MPO | Myeloperoxidase |
| mTOR | mammalian target of rapamycin |
| NEIL | Nei like DNA glycosylases |
| NF-κB | Transcription factor-kappaB |
| NNAL | Nicotine equivalents and total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol |
| NNK | N-nitrosamines |
| NO | Nitric oxide |
| NO2 | Nitrogen oxides |
| NOS | Nitric oxide synthase |
| NSCLC | Non-small-cell lung cancer |
| OGG1 | 8-oxoguanine DNA glycosylase |
| OS | Oxidative stress |
| PARP | Poly ADP ribose polymerase |
| P21 | Protein 21 |
| P53 | Protein 53 |
| PD-1 | Anti-programmed death receptor 1 |
| PI3K | Phosphatidylinositol-3 kinase |
| PON1 | Paraoxonase 1 |
| PPARα | Peroxisome proliferator-activated receptor α |
| PTEN | Phosphatase and tensin homolog |
| RA | Retinoid acid |
| RARβ | RA receptor beta |
| SIRT1 | Sirtuin 1 |
| ROS | Reactive oxygen species |
| ROS1 | ROS Proto-Oncogene 1, Receptor Tyrosine Kinase 1 |
| RCTs | Randomised controlled trials |
| SCC | Squamous cell carcinoma |
| SCD-1 | Stearoyl-coa desaturase-1 |
| SCLC | Small-cell lung cancer |
| SHS | Secondhand smoke |
| SICAM-1 | Soluble intercellular adhesion molecule-1 |
| Smad | deca-pentaplegic homolog |
| SOD | Superoxide dismutase |
| SOX2 | SRY-Box Transcription Factor 2 |
| TNFα | Tumor necrosis factor-α |
| VEGF | Vascular endothelial growth factor |
References
- Institute for Health Metrics and Evaluation. Global Burden of Disease 2017. 2017. Available online: http://vizhub.healthdata.org/gbd-compare/# (accessed on 1 September 2022).
- Lee, P.N.; Forey, B.A.; Coombs, K.J. Systematic review with meta-analysis of the epidemiological evidence in the 1900s relating smoking to lung cancer. BMC Cancer 2012, 12, 385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayes, L.; Haslam, P.L.; Gratziou, C.G.; Powell, P.; Britton, J.; Vardavas, C.; Jimenez-Ruiz, C.; Leonardi-Bee, J.; Tobacco Control Committee of the European Respiratory Society. SmokeHaz: Systematic reviews and meta-analyses of the effects of smoking on respiratory health. Chest 2016, 150, 164–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubin, S. Lung cancer in non-smokers. Mo. Med. 2020, 117, 375–379. [Google Scholar] [PubMed]
- Cheng, E.S.; Weber, M.; Steinberg, J.; Yu, X.Q. Lung cancer risk in never-smokers: An overview of environmental and genetic factors. Chin. J. Cancer Res. 2021, 33, 548–562. [Google Scholar] [CrossRef]
- Hecht, S.S. Lung carcinogenesis by tobacco smoke. Int. J. Cancer 2012, 131, 2724–2732. [Google Scholar] [CrossRef] [Green Version]
- Price, L.R.; Martinez, J. Cardiovascular, carcinogenic and reproductive effects of nicotine exposure: A narrative review of the scientific literature. F1000Research 2019, 8, 1586. [Google Scholar] [CrossRef]
- Alsharairi, N.A. Scutellaria baicalensis and their natural flavone compounds as potential medicinal drugs for the treatment of nicotine-induced non-small-cell lung cancer and asthma. Int. J. Environ. Res. Public Health 2021, 18, 5243. [Google Scholar] [CrossRef]
- Alsharairi, N.A. Insights into the mechanisms of action of proanthocyanidins and anthocyanins in the treatment of nicotine-induced non-small cell lung cancer. Int. J. Mol. Sci. 2022, 23, 7905. [Google Scholar] [CrossRef]
- Inamura, K. Lung cancer: Understanding its molecular pathology and the 2015 WHO Classification. Front. Oncol. 2017, 7, 193. [Google Scholar] [CrossRef] [Green Version]
- Polański, J.; Chabowski, M.; Jankowska-Polańska, B.; Janczak, D.; Rosińczuk, J. Histological subtype of lung cancer affects acceptance of illness, severity of pain, and quality of life. J. Pain Res. 2018, 11, 727–733. [Google Scholar] [CrossRef]
- Torok, S.; Hegedus, B.; Laszlo, V.; Hoda, M.A.; Ghanim, B.; Berger, W.; Klepetko, W.; Dome, B.; Ostoros, G. Lung cancer in never smokers. Future Oncol. 2011, 7, 1195–1211. [Google Scholar] [CrossRef]
- Pesch, B.; Kendzia, B.; Gustavsson, P.; Jöckel, K.-H.; Johnen, G.; Pohlabeln, H.; Olsson, A.; Ahrens, W.; Gross, I.M.; Brüske, I.; et al. Cigarette smoking and lung cancer-relative risk estimates for the major histological types from a pooled analysis of case-control studies. Int. J. Cancer 2012, 131, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Saji, H. Worldwide trend of increasing primary adenocarcinoma of the lung. Surg. Today 2014, 44, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Vogtmann, E.; Jia, M.-M.; Parascandola, M.; Li, J.-B.; Wu, Y.-L.; Feng, Q.-F.; Zou, X.-N. Tobacco smoking and trends in histological subtypes of female lung cancer at the cancer hospital of the Chinese Academy of Medical Sciences over 13 years. Thorac. Cancer 2019, 10, 1717–1724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gou, l-Y.; Niu, F.-Y.; Wu, Y.-L.; Zhong, W.-Z. Differences in driver genes between smoking-related and non-smoking-related lung cancer in the Chinese population. Cancer 2015, 121, 3069–3079. [Google Scholar] [CrossRef]
- Chapman, A.M.; Sun, K.Y.; Ruestow, P.; Cowan, D.M.; Madl, A.K. Lung cancer mutation profile of EGFR, ALK, and KRAS: Meta-analysis and comparison of never and ever smokers. Lung Cancer 2016, 102, 122–134. [Google Scholar] [CrossRef]
- Cho, J.; Choi, S.M.; Lee, J.; Lee, C.-H.; Lee, S.-M.; Kim, D.-W.; Yim, J.-J.; Kim, Y.T.; Yoo, C.-G.; Kim, Y.W.; et al. Proportion and clinical features of never-smokers with non-small cell lung cancer. Chin. J. Cancer 2017, 36, 20. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-J.; Roumeliotis, T.I.; Chang, Y.-H.; Chen, C.-T.; Han, C.-L.; Lin, M.-H.; Chen, H.-W.; Chang, G.-C.; Chang, Y.-L.; Wu, C.-T.; et al. Proteogenomics of non-smoking lung cancer in East Asia delineates molecular signatures of pathogenesis and progression. Cell 2020, 182, 226–244.e17. [Google Scholar] [CrossRef]
- Liu, X.; Lin, X.J.; Wang, C.P.; Yan, K.K.; Zhao, L.Y.; An, W.X.; Liu, X.D. Association between smoking and p53 mutation in lung cancer: A meta-analysis. Clin. Oncol. R. Coll. Radiol. 2014, 26, 18–24. [Google Scholar] [CrossRef]
- Filipits, M. New developments in the treatment of squamous cell lung cancer. Curr. Opin. Oncol. 2014, 26, 152–158. [Google Scholar] [CrossRef]
- Dafni, U.; Tsourti, Z.; Vervita, K.; Peters, S. Immune checkpoint inhibitors, alone or in combination with chemotherapy, as first-line treatment for advanced non-small cell lung cancer. A systematic review and network meta-analysis. Lung Cancer 2019, 134, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Nasser, N.J.; Gorenberg, M.; Agbarya, A. First line immunotherapy for non-small cell lung cancer. Pharmaceuticals 2020, 13, 373. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Serrano, A.; Gella, P.; Jiménez, E.; Zugazagoitia, J.; Rodríguez, L.P. Targeting EGFR in lung cancer: Current standards and developments. Drugs 2018, 78, 893–911. [Google Scholar] [CrossRef] [PubMed]
- Sanchala, D.; Bhatt, L.K.; Prabhavalkar, K.S. Therapeutic approaches for the treatment of epidermal growth factor receptor mutated lung cancer. Curr. Cancer Drug Targets 2018, 18, 773–791. [Google Scholar] [CrossRef] [PubMed]
- Tfayli, A.; Mohty, R. EGFR tyrosine kinase inhibitors in non-small cell lung cancer: Treatment paradigm, current evidence, and challenges. Tumori 2021, 107, 376–384. [Google Scholar] [CrossRef]
- Si, J.-F.; Xiang, J.; Wei, J.-W.; Hao, Y.; Song, Z.B. Clinical outcomes of EGFR-TKIs in advanced squamous cell lung cancer. Neoplasma 2022, 69, 976–982. [Google Scholar] [CrossRef]
- Alsharairi, N.A. The effects of dietary supplements on asthma and lung cancer risk in smokers and non-smokers: A review of the literature. Nutrients 2019, 11, 725. [Google Scholar] [CrossRef] [Green Version]
- Alsharairi, N.A. Supplements for smoking-related lung diseases. Encyclopedia 2021, 1, 76–86. [Google Scholar] [CrossRef]
- Landete, J.M. Dietary intake of natural antioxidants: Vitamins and polyphenols. Crit. Rev. Food Sci. Nutr. 2013, 53, 706–721. [Google Scholar] [CrossRef]
- Olza, J.; Aranceta-Bartrina, J.; González-Gross, M.; Ortega, R.M.; Serra-Majem, L.; Varela-Moreiras, G.; Gil, A. Reported dietary intake and food sources of zinc, selenium, and vitamins A, E and C in the Spanish population: Findings from the ANIBES Study. Nutrients 2017, 9, 697. [Google Scholar] [CrossRef]
- Dyer, A.R.; Elliott, P.; Stamler, J.; Chan, Q.; Ueshima, H.; Zhou, B.F.; INTERMAP Research Group. Dietary intake in male and female smokers, ex-smokers, and never smokers: The INTERMAP study. J. Hum. Hypertens. 2003, 17, 641–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raatz, S.K.; Jahns, L.; Johnson, L.K.; Scheett, A.; Carriquiry, A.; Lemieux, A.; Nakajima, M.; al’Absi, M. Smokers report lower intake of key nutrients than nonsmokers yet both fall short of meeting recommended intakes. Nutr. Res. 2017, 45, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Alberg, A. The influence of cigarette smoking on circulating concentrations of antioxidant micronutrients. Toxicology 2002, 180, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Männistö, S.; Smith-Warner, S.A.; Spiegelman, D.; Albanes, D.; Erson, K.; van den Brandt, P.A.; Cerhan, J.R.; Colditz, G.; Feskanich, D.; Freudenheim, J.L.; et al. Dietary carotenoids and risk of lung cancer in a pooled analysis of seven cohort studies. Cancer Epidemiol. Biomark. Prev. 2004, 13, 40–48. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.-J.; Bo, Y.-C.; Liu, X.-X.; Qiu, C.-G. Association of dietary vitamin E intake with risk of lung cancer: A dose-response meta-analysis. Asia Pac. J. Clin. Nutr. 2017, 26, 271–277. [Google Scholar]
- Muka, T.; Kraja, B.; Ruiter, R.; Lahousse, L.; de Keyser, C.E.; Hofman, A.; Franco, O.H.; Brusselle, G.; Stricker, B.H.; Kiefte-de Jong, J.C. Dietary mineral intake and lung cancer risk: The Rotterdam Study. Eur. J. Nutr. 2017, 56, 1637–1646. [Google Scholar] [CrossRef] [Green Version]
- You, D.; Zhang, M.; He, W.; Wang, D.; Yu, Y.; Yu, Z.; Lange, T.; Yang, S.; Wei, Y.; Ma, H.; et al. Association between dietary sodium, potassium intake and lung cancer risk: Evidence from the prostate, lung, colorectal and ovarian cancer screening trial and the Women’s Health Initiative. Transl. Lung Cancer Res. 2021, 10, 45–56. [Google Scholar] [CrossRef]
- Dana, N.; Karimi, R.; Mansourian, M.; Javanmard, S.H.; Laher, I.; Vasegh, G. Magnesium intake and lung cancer risk: A systematic review and meta-analysis. Int. J. Vitam. Nutr. Res. 2021, 91, 539–546. [Google Scholar] [CrossRef]
- Luo, J.; Shen, L.; Zheng, D. Association between vitamin C intake and lung cancer: A dose-response meta-analysis. Sci. Rep. 2014, 4, 6161. [Google Scholar] [CrossRef] [Green Version]
- Mahabir, S.; Schendel, K.; Dong, Y.Q.; Barrera, S.L.; Spitz, M.R.; Forman, M.R. Dietary α-, β-, γ- and δ-tocopherols in lung cancer risk. Int. J. Cancer 2008, 123, 1173–1180. [Google Scholar] [CrossRef] [Green Version]
- Yong, L.C.; Brown, C.C.; Schatzkin, A.; Dresser, C.M.; Slesinski, M.J.; Cox, C.S.; Taylor, P.R. Intake of vitamins E, C, and A and risk of lung cancer. The NHANES I epidemiologic followup study. First National Health and Nutrition Examination Survey. Am. J. Epidemiol. 1997, 146, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Narita, S.; Saito, E.; Sawada, N.; Shimazu, T.; Yamaji, T.; Iwasaki, M.; Ishihara, J.; Takachi, R.; Shibuya, K.; Inoue, M.; et al. Dietary consumption of antioxidant vitamins and subsequent lung cancer risk: The Japan Public Health Center-based prospective study. Int. J. Cancer 2018, 142, 2441–2460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shareck, M.; Rousseau, M.C.; Koushik, A.; Siemiatycki, J.; Parent, M.E. Inverse association between dietary intake of selected carotenoids and vitamin C and risk of lung cancer. Front. Oncol. 2017, 7, 23. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Park, S.; Liu, G.; Miller, D.P.; Wang, L.I.; Pothier, L.; Wain, J.C.; Lynch, T.J.; Giovannucci, E.; Christiani, D.C. Dietary iron, zinc, and calcium and the risk of lung cancer. Epidemiology 2005, 16, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Mahabir, S.; Spitz, M.R.; Barrera, S.L.; Beaver, S.H.; Etzel, C.; Forman, M.R. Dietary zinc, copper and selenium, and risk of lung cancer. Int. J. Cancer 2007, 120, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Ward, H.A.; Whitman, J.; Muller, D.C.; Johansson, M.; Jakszyn, P.; Weiderpass, E.; Palli, D.; Fanidi, A.; Vermeulen, R.; Tjønneland, A.; et al. Haem iron intake and risk of lung cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Eur. J. Clin. Nutr. 2019, 73, 1122–1132. [Google Scholar] [CrossRef] [Green Version]
- Mayne, S.T.; Janerich, D.T.; Greenwald, P.; Chorost, S.; Tucci, C.; Zaman, M.B.; Melamed, M.R.; Kiely, M.; McKneally, M.F. Dietary beta carotene and lung cancer risk in U.S. nonsmokers. J. Natl. Cancer Inst. 1994, 86, 33–38. [Google Scholar] [CrossRef]
- Wu, Q.J.; Xiang, Y.B.; Yang, G.; Li, H.L.; Lan, Q.; Gao, Y.T.; Zheng, W.; Shu, X.O.; Fowke, J.H. Vitamin E intake and the lung cancer risk among female nonsmokers: A report from the Shanghai Women’s Health Study. Int. J. Cancer 2015, 136, 610–617. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Dong, Y.; Lu, C.; Wang, Y.; Peng, L.; Jiang, M.; Tang, Y.; Zhao, Q. Meta-analysis of the correlation between vitamin D and lung cancer risk and outcomes. Oncotarget 2017, 8, 81040–81051. [Google Scholar] [CrossRef] [Green Version]
- Astori, E.; Garavaglia, M.L.; Colombo, G.; Landoni, L.; Portinaro, N.M.; Milzani, A.; Dalle-Donne, I. Antioxidants in smokers. Nutr. Res. Rev. 2022, 35, 70–97. [Google Scholar] [CrossRef]
- Hakim, I.A.; Harris, R.; Garland, L.; Cordova, C.A.; Mikhael, D.M.; Chow, H.-H.S. Gender difference in systemic oxidative stress and antioxidant capacity in current and former heavy smokers. Cancer Epidemiol. Biomark. Prev. 2012, 21, 2193–2200. [Google Scholar] [CrossRef] [PubMed]
- Orhan, H.; Evelo, C.T.A.; Sahin, G. Erythrocyte antioxidant defense response against cigarette smoking in humans--the glutathione S-transferase vulnerability. J. Biochem. Mol. Toxicol. 2005, 19, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Giuca, M.R.; Giuggioli, E.; Metelli, M.R.; Pasini, M.; Iezzi, G.; D’Ercole, S.; Tripodi, D. Effects of cigarette smoke on salivary superoxide dismutase and glutathione peroxidase activity. J. Biol. Regul. Homeost. Agents 2010, 24, 359–366. [Google Scholar] [PubMed]
- Padmavathi, P.; Raghu, P.S.; Reddy, V.D.; Bulle, S.; Marthadu, S.B.; Maturu, P.; Varadacharyulu, N.C. Chronic cigarette smoking-induced oxidative/nitrosative stress in human erythrocytes and platelets. Mol. Cell Toxicol. 2018, 14, 27–34. [Google Scholar] [CrossRef]
- Ho, J.C.; Chan-Yeung, M.; Ho, S.P.; Mak, J.C.; Ip, M.C.; Ooi, G.C.; Wong, M.P.; Tsang, K.W.; Lam, W.K. Disturbance of systemic antioxidant profile in nonsmall cell lung carcinoma. Eur. Respir. J. 2007, 29, 273–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulikowska-Karpińska, E.; Czerw, K. Estimation of 8-hydroxy-2’-deoxyguanosine (8-OHdG) concentration in the urine of cigarette smokers. Wiad. Lek. 2015, 68, 32–38. [Google Scholar]
- Ito, K.; Yano, T.; Morodomi, Y.; Yoshida, T.; Kohno, M.; Haro, A.; Shikada, Y.; Okamoto, T.; Maruyama, R.; Maehara, Y. Serum antioxidant capacity and oxidative injury to pulmonary DNA in never-smokers with primary lung cancer. Anticancer Res. 2012, 32, 1063–1067. [Google Scholar]
- An, A.R.; Kim, K.M.; Park, H.S.; Jang, K.Y.; Moon, W.S.; Kang, M.J.; Lee, Y.C.; Kim, J.H.; Chae, H.J.; Chung, M.J. Association between expression of 8-OHdG and cigarette smoking in non-small cell lung cancer. J. Pathol. Transl. Med. 2019, 53, 217–224. [Google Scholar] [CrossRef]
- Lu, C.-Y.; Ma, Y.-C.; Lin, J.-M.; Chuang, C.-Y.; Sung, F.-C. Oxidative DNA damage estimated by urinary 8-hydroxydeoxyguanosine and indoor air pollution among non-smoking office employees. Environ. Res. 2007, 103, 331–337. [Google Scholar] [CrossRef]
- Isik, B.; Ceylan, A.; Isik, R. Oxidative stress in smokers and non-smokers. Inhal. Toxicol. 2007, 19, 767–769. [Google Scholar] [CrossRef]
- Van der Plas, A.; Pouly, S.; de La Bourdonnaye, G.; Baker, G.; Lüdicke, F. Influence of smoking on levels of urinary 8-iso Prostaglandin F2α. Toxicol. Rep. 2019, 6, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.-M.; Carmella, S.G.; Wang, R.; Tan, Y.-T.; Adams-Haduch, J.; Gao, Y.-T.; Hecht, S.S. Relationship of the oxidative damage biomarker 8-epi-prostaglandin F2α to risk of lung cancer development in the Shanghai Cohort Study. Carcinogenesis 2018, 39, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Chávez, J.; Cano, C.; Souki, A.; Bermúdez, V.; Medina, M.; Ciszek, A.; Amell, A.; Vargas, M.E.; Reyna, N.; Toledo, A.; et al. Effect of cigarette smoking on the oxidant/antioxidant balance in healthy subjects. Am. J. Ther. 2007, 14, 189–193. [Google Scholar] [CrossRef]
- Haswell, L.E.; Papadopoulou, E.; Newland, N.; Shepperd, C.J.; Lowe, F.J. A cross-sectional analysis of candidate biomarkers of biological effect in smokers, never-smokers and ex-smokers. Biomarkers 2014, 19, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.T.; Vivar, J.C.; Tam, J.; Hammad, H.T.; Christensen, C.H.; van Bemmel, D.M.; Das, B.; Danilenko, U.; Chang, C.M. Biomarkers of potential harm among adult cigarette and smokeless tobacco users in the PATH Study wave 1 (2013-2014): A cross-sectional analysis. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1320–1327. [Google Scholar] [CrossRef] [PubMed]
- Song, M.-A.; Freudenheim, J.L.; Brasky, T.M.; Mathe, E.A.; McElroy, J.P.; Nickerson, Q.A.; Reisinger, S.A.; Smiraglia, D.J.; Weng, D.Y.; Ying, K.L.; et al. Biomarkers of exposure and effect in the lungs of smokers, nonsmokers, and electronic cigarette users. Cancer Epidemiol. Biomark. Prev. 2020, 29, 443–451. [Google Scholar] [CrossRef] [Green Version]
- Gackowski, D.; Kowalewski, J.; Siomek, A.; Olinski, R. Oxidative DNA damage and antioxidant vitamin level: Comparison among lung cancer patients, healthy smokers and nonsmokers. Int. J. Cancer 2005, 114, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Van Helden, Y.G.J.; Keijer, J.; Knaapen, A.M.; Heil, S.G.; Briedé, J.J.; van Schooten, F.J.; Godschalk, R.W.L. Beta-carotene metabolites enhance inflammation-induced oxidative DNA damage in lung epithelial cells. Free Radic. Biol. Med. 2009, 46, 299–304. [Google Scholar] [CrossRef]
- Chen, G.-Q.; Lin, B.; Dawson, M.I.; Zhang, X.-K. Nicotine modulates the effects of retinoids on growth inhibition and RAR beta expression in lung cancer cells. Int. J. Cancer 2002, 99, 171–178. [Google Scholar] [CrossRef]
- Tripathi, S.K.; Pandey, K.; Panda, M.; Spinella, M.J.; Rengasamy, K.R.; Biswal, B.K. The potential of retinoids for combination therapy of lung cancer: Updates and future directions. Pharm. Res. 2019, 147, 104331. [Google Scholar] [CrossRef]
- Lian, F.; Hu, F.-Q.; Russell, R.M.; Wang, X.-D. Beta-cryptoxanthin suppresses the growth of immortalized human bronchial epithelial cells and non-small-cell lung cancer cells and up-regulates retinoic acid receptor beta expression. Int. J. Cancer 2006, 119, 2084–2089. [Google Scholar] [CrossRef] [PubMed]
- Iskandar, A.R.; Miao, B.; Li, X.; Hu, K.-Q.; Liu, C.; Wang, X.-D. β-Cryptoxanthin reduced lung tumor multiplicity and inhibited lung cancer cell motility by downregulating nicotinic ccetylcholine receptor α7 signaling. Cancer Prev. Res. 2016, 9, 875–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Bronson, R.T.; Russell, R.M.; Wang, X.-D. β-Cryptoxanthin supplementation prevents cigarette smoke-induced lung inflammation, oxidative damage, and squamous metaplasia in ferrets. Cancer Prev. Res. 2011, 4, 1255–1266. [Google Scholar] [CrossRef] [PubMed]
- Iskandar, A.R.; Liu, C.; Smith, D.E.; Hu, K.-Q.; Choi, S.-W.; Ausman, L.M.; Wang, X.-D. β-Cryptoxanthin restores nicotine-reduced lung SIRT1 to normal levels and inhibits nicotine-promoted lung tumorigenesis and emphysema in A/J mice. Cancer Prev. Res. 2013, 6, 309–320. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Miller, B.; Balbuena, E.; Eroglu, A. Lycopene protects against smoking-induced lung cancer by inducing base excision repair. Antioxidants 2020, 9, 643. [Google Scholar] [CrossRef]
- Aizawa, K.; Liu, C.; Tang, S.; Veeramachaneni, S.; Hu, K.-Q.; Smith, D.E.; Wang, X.-D. Tobacco carcinogen induces both lung cancer and non-alcoholic steatohepatitis and hepatocellular carcinomas in ferrets which can be attenuated by lycopene supplementation. Int. J. Cancer 2016, 139, 1171–1181. [Google Scholar] [CrossRef] [Green Version]
- Campos, K.K.D.; de Oliveira Ramos, C.; Martins, T.L.; de Paula Costa, G.; Talvani, A.; Garcia, C.C.M.; Oliveira, L.A.M.; Cangussú, S.D.; Costa, D.C.; Bezerra, F.S. Lycopene mitigates pulmonary emphysema induced by cigarette smoke in a murine model. J. Nutr. Biochem. 2019, 65, 93–100. [Google Scholar] [CrossRef]
- Rakic, J.M.; Liu, C.; Veeramachaneni, S.; Wu, D.; Paul, L.; Chen, C.-Y.O.; Ausman, L.M.; Wang, X.-D. Lycopene inhibits smoke-induced chronic Obstructive pulmonary disease and lung carcinogenesis by modulating reverse cholesterol transport in ferrets. Cancer Prev. Res. 2019, 12, 421–432. [Google Scholar] [CrossRef]
- Palozza, P.; Simone, R.; Mele, M.C. Interplay of carotenoids with cigarette smoking: Implications in lung cancer. Curr. Med. Chem. 2008, 15, 844–854. [Google Scholar] [CrossRef]
- Hurst, J.S.; Contreras, J.E.; Siems, W.G.; van Kuijk, F.J.G.M. Oxidation of carotenoids by heat and tobacco smoke. Biofactors 2004, 20, 23–35. [Google Scholar] [CrossRef]
- Baker, D.L.; Krol, E.S.; Jacobsen, N.; Liebler, D.C. Reactions of beta-carotene with cigarette smoke oxidants. Identification of carotenoid oxidation products and evaluation of the prooxidant/antioxidant effect. Chem. Res. Toxicol. 1999, 12, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Willhite, C.A.; Liebler, D.C. Interactions of beta-carotene and cigarette smoke in human bronchial epithelial cells. Carcinogenesis 2001, 22, 1173–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haider, C.; Ferk, F.; Bojaxhi, E.; Martano, G.; Stutz, H.; Bresgen, N.; Knasmüller, S.; Alija, A.; Eckl, P.M. Effects of β-Carotene and its cleavage products in primary pneumocyte type II cells. Antioxidants 2017, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- Eiserich, J.P.; van der Vliet, A.; Handelman, G.J.; Halliwell, B.; Cross, C.E. Dietary antioxidants and cigarette smoke-induced biomolecular damage: A complex interaction. Am. J. Clin. Nutr. 1995, 62, 1490S–1500S. [Google Scholar] [CrossRef]
- Guénégou, A.; Leynaert, B.; Pin, I.; Le Moël, G.; Zureik, M.; Neukirch, F. Serum carotenoids, vitamins A and E, and 8 year lung function decline in a general population. Thorax 2006, 61, 320–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruno, R.S.; Ramakrishnan, R.; Montine, T.J.; Bray, T.M.; Traber, M.G. {alpha}-Tocopherol disappearance is faster in cigarette smokers and is inversely related to their ascorbic acid status. Am. J. Clin. Nutr. 2005, 81, 95–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dey, N.; Chattopadhyay, D.J.; Chatterjee, I.B. Molecular mechanisms of cigarette smoke-induced proliferation of lung cells and prevention by vitamin C. J. Oncol. 2011, 2011, 561862. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.M.; Lee, S.K.; Kim, H.S. Inhibition of oxidative DNA damage, 8-OHdG, and carbonyl contents in smokers treated with antioxidants (vitamin E, vitamin C, beta-carotene and red ginseng). Cancer Lett. 1998, 132, 219–227. [Google Scholar] [CrossRef]
- Dietrich, M.; Block, G.; Hudes, M.; Morrow, J.D.; Norkus, E.P.; Traber, M.G.; Cross, C.E.; Packer, L. Antioxidant supplementation decreases lipid peroxidation biomarker F(2)-isoprostanes in plasma of smokers. Cancer Epidemiol. Biomark. Prev. 2002, 11, 7–13. [Google Scholar]
- Guertin, K.A.; Grant, R.K.; Arnold, K.B.; Burwell, L.; Hartline, J.; Goodman, P.J.; Minasian, L.M.; Lippman, S.M.; Klein, E.; Cassano, P.A. Effect of long-term vitamin E and selenium supplementation on urine F2-isoprostanes, a biomarker of oxidative stress. Free Radic. Biol. Med. 2016, 95, 349–356. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Lee, B.M. Protective effects of antioxidant supplementation on plasma lipid peroxidation in smokers. J. Toxicol. Env. Health A 2001, 63, 583–598. [Google Scholar] [CrossRef]
- Ismail, N.M.; Harun, A.; Yusof, A.A.; Zaiton, Z.; Marzuki, A. Role of vitamin e on oxidative stress in smokers. Malays J. Med. Sci. 2002, 9, 34–42. [Google Scholar]
- Howard, D.J.; Ota, R.B.; Briggs, L.A.; Hampton, M.; Pritsos, C.A. Oxidative stress induced by environmental tobacco smoke in the workplace is mitigated by antioxidant supplementation. Cancer Epidemiol. Biomark. Prev. 1998, 7, 981–988. [Google Scholar]
- Woodson, K.; Tangrea, J.A.; Barrett, M.J.; Virtamo, J.; Taylor, P.R.; Albanes, D. Serum alpha-tocopherol and subsequent risk of lung cancer among male smokers. J. Natl. Cancer Inst. 1999, 91, 1738–1743. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Weinstein, S.J.; Yu, K.; Männistö, S.; Albanes, D. A prospective study of serum vitamin E and 28-year risk of lung cancer. J. Natl. Cancer Inst. 2020, 112, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Traber, M.G.; Winklhofer-Roob, B.M.; Roob, J.M.; Khoschsorur, G.; Aigner, R.; Cross, C.; Ramakrishnan, R.; Brigelius-Flohé, R. Vitamin E kinetics in smokers and nonsmokers. Free Radic. Biol. Med. 2001, 31, 1368–1374. [Google Scholar] [CrossRef] [PubMed]
- Bruno, R.S.; Leonard, S.W.; Atkinson, J.; Montine, T.J.; Ramakrishnan, R.; Bray, T.M.; Traber, M.G. Faster plasma vitamin E disappearance in smokers is normalized by vitamin C supplementation. Free Radic. Biol. Med. 2006, 40, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Møller, P.; Viscovich, M.; Lykkesfeldt, J.; Loft, S.; Jensen, A.; Poulsen, H.E. Vitamin C supplementation decreases oxidative DNA damage in mononuclear blood cells of smokers. Eur. J. Nutr. 2004, 43, 267–274. [Google Scholar] [CrossRef]
- Lykkesfeldt, J.; Christen, S.; Wallock, L.M.; Chang, H.H.; Jacob, R.A.; Ames, B.N. Ascorbate is depleted by smoking and repleted by moderate supplementation: A study in male smokers and nonsmokers with matched dietary antioxidant intakes. Am. J. Clin. Nutr. 2000, 71, 530–536. [Google Scholar] [CrossRef] [Green Version]
- Pathak, A.K.; Bhutani, M.; Guleria, R.; Bal, S.; Mohan, A.; Mohanti, B.K.; Sharma, A.; Pathak, R.; Bhardwaj, N.K.; Prasad, K.N.; et al. Chemotherapy alone vs. chemotherapy plus high dose multiple antioxidants in patients with advanced non-small cell lung cancer. J. Am. Coll. Nutr. 2005, 24, 16–21. [Google Scholar] [CrossRef]
- Lim, S.-J.; Choi, M.K.; Kim, M.J.; Kim, J.K. Alpha-tocopheryl succinate potentiates the paclitaxel-induced apoptosis through enforced caspase 8 activation in human H460 lung cancer cells. Exp. Mol. Med. 2009, 41, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhao, H.; Liu, K.; Wang, Y.; Liu, Q.; Sun, T.; Chen, S.; Ren, L. Smoking behavior and circulating vitamin D levels in adults: A meta-analysis. Food Sci. Nutr. 2021, 9, 5820–5832. [Google Scholar] [CrossRef] [PubMed]
- Lange, N.E.; Sparrow, D.; Vokonas, P.; Litonjua, A.A. Vitamin D deficiency, smoking, and lung function in the Normative Aging Study. Am. J. Respir. Crit. Care Med. 2012, 186, 616–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, X.; Qu, N.; Wang, L.; Wang, G.; Jiao, R.; Deng, H.; Li, S.; Qin, Y. Effect of vitamin D3 on lung damage induced by cigarette smoke in mice. Open Med. 2019, 14, 827–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akiba, T.; Morikawa, T.; Odaka, M.; Nakada, T.; Kamiya, N.; Yamashita, M.; Yabe, M.; Inagaki, T.; Asano, H.; Mori, S.; et al. Vitamin D supplementation and survival of patients with non-small cell lung cancer: A Randomized, double-blind, placebo-controlled trial. Clin. Cancer Res. 2018, 24, 4089–4097. [Google Scholar] [CrossRef] [Green Version]
- Songyang, Y.; Song, T.; Shi, Z.; Li, W.; Yang, S.; Li, D. Effect of vitamin D on malignant behavior of non-small cell lung cancer cells. Gene 2021, 768, 145309. [Google Scholar] [CrossRef]
- Ma, K.; Xu, W.; Wang, C.; Li, B.; Su, K.; Li, W. Vitamin D deficiency is associated with a poor prognosis in advanced non-small cell lung cancer patients treated with platinum-based first-line chemotherapy. Cancer Biomark. 2017, 18, 297–303. [Google Scholar] [CrossRef]
- Wang, Q.; Cui, Q.; Gao, J.-P.; Xing, R. Role of iron biomarkers and iron intakes in lung cancer risk: A systematic review and meta-analysis. J. Trace Elem. Med. Biol. 2022, 74, 127060. [Google Scholar] [CrossRef]
- Park, E.; Ha, E.; Leem, J.; Lee, K.; Chung, J.; Hong, Y. Dietary iron uptake increases lipid peroxidation by exposure to smoking. Epidemiology 2007, 18, pS180. [Google Scholar] [CrossRef]
- Oba, S.; Inaba, Y.; Shibuya, T.; Oshima, J.; Seyama, K.; Kobayashi, T.; Kunugita, N.; Ino, T. Changes in oxidative stress levels during two weeks of smoking cessation treatment and their association with nutritional characteristics in Japanese smokers. Exp. Med. 2019, 17, 2757–2764. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Zhu, C.; Tang, D.; Dou, Q.P.; Shen, J.; Chen, X. The role of ferroptosis in lung cancer. Biomark. Res. 2021, 9, 82. [Google Scholar] [CrossRef] [PubMed]
- Fukano, Y.; Yoshimura, H.; Yoshida, T. Heme oxygenase-1 gene expression in human alveolar epithelial cells (A549) following exposure to whole cigarette smoke on a direct in vitro exposure system. Exp. Toxicol. Pathol. 2006, 57, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Baglole, C.J.; Sime, P.J.; Phipps, R.P. Cigarette smoke-induced expression of heme oxygenase-1 in human lung fibroblasts is regulated by intracellular glutathione. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 295, L624–L636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, G.-Z.; Xu, T.R.; Chen, C. Tobacco carcinogen NNK-induced lung cancer animal models and associated carcinogenic mechanisms. Acta Biochim. Biophys. Sin. 2015, 47, 477–487. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.; Liu, N.; Chen, L.; Jiang, Y.; Shi, Y.; Mao, C.; Liu, Y.; Wang, M.; Lai, W.; Tang, H.; et al. LSH interacts with and stabilizes GINS4 transcript that promotes tumourigenesis in non-small cell lung cancer. J. Exp. Clin. Cancer Res. 2019, 38, 280. [Google Scholar] [CrossRef] [Green Version]
- Ma, T.-L.; Zhou, Y.; Wang, C.; Wang, L.; Chen, J.-X.; Yang, H.-H.; Zhang, C.-Y.; Zhou, Y.; Guan, C.-X. Targeting ferroptosis for lung diseases: Exploring novel strategies in ferroptosis-associated mechanisms. Oxid. Med. Cell Longev. 2021, 2021, 1098970. [Google Scholar] [CrossRef]
- Scaglia, N.; Igal, R.A. Inhibition of Stearoyl-CoA Desaturase 1 expression in human lung adenocarcinoma cells impairs tumorigenesis. Int. J. Oncol. 2008, 33, 839–850. [Google Scholar]
- Zhang, X.; Yang, Q. Association between serum copper levels and lung cancer risk: A meta-analysis. J. Int. Med. Res. 2018, 46, 4863–4873. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Sun, Z.; Li, A.; Zhang, Y. Association between serum zinc levels and lung cancer: A meta-analysis of observational studies. World J. Surg. Oncol. 2019, 17, 78. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, C.; Mitra, P.K.; Saha, S.; Nayak, C.; Chakraborty, R. Effect of copper-hydroquinone complex on oxidative stress-related parameters in human erythrocytes (in vitro). Toxicol. Mech. Methods 2009, 19, 86–93. [Google Scholar] [CrossRef]
- Lapenna, D.; Mezzetti, A.; de Gioia, S.; Pierdomenico, S.D.; Daniele, F.; Cuccurullo, F. Plasma copper and lipid peroxidation in cigarette smokers. Free Radic. Biol. Med. 1995, 19, 849–852. [Google Scholar] [CrossRef] [PubMed]
- Thounaojam, M.C.; Jadeja, R.N.; Valodkar, M.; Nagar, P.S.; Devkar, R.V.; Thakore, S. Oxidative stress induced apoptosis of human lung carcinoma (A549) cells by a novel copper nanorod formulation. Food Chem. Toxicol. 2011, 49, 2990–2996. [Google Scholar] [CrossRef] [PubMed]
- Zabłocka-Słowińska, K.; Płaczkowska, S.; Prescha, A.; Pawełczyk, K.; Porębska, I.; Kosacka, M.; Pawlik-Sobecka, L.; Grajeta, H. Serum and whole blood Zn, Cu and Mn profiles and their relation to redox status in lung cancer patients. J. Trace Elem. Med. Biol. 2018, 45, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Kocdor, H.; Ates, H.; Aydin, S.; Cehreli, R.; Soyarat, F.; Kemanli, P.; Harmanci, D.; Cengiz, H.; Kocdor, M.A. Zinc supplementation induces apoptosis and enhances antitumor efficacy of docetaxel in non-small-cell lung cancer. Drug Des. Devel. Ther. 2015, 9, 3899–3909. [Google Scholar] [CrossRef] [Green Version]
- Kocyigit, A.; Erel, Q.; Gur, S. Effects of tobacco smoking on plasma selenium, zinc, copper and iron concentrations and related antioxidative enzyme activities. Clin. Biochem. 2001, 34, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Foronjy, R.F.; Mirochnitchenko, O.; Propokenko, O.; Lemaitre, V.; Jia, Y.; Inouye, M.; Okada, Y.; D’Armiento, J.M. Superoxide dismutase expression attenuates cigarette smoke- or elastase-generated emphysema in mice. Am. J. Respir. Crit. Care Med. 2006, 173, 623–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.H.; Kim, J.S.; Shin, H.S.; Keen, C.L. Influence of smoking on markers of oxidative stress and serum mineral concentrations in teenage girls in Korea. Nutrition 2003, 19, 240–243. [Google Scholar] [CrossRef]
- Cay, M.; Naziroğlu, M.; Köylü, H. Selenium and vitamin E modulates cigarette smoke exposure-induced oxidative stress in blood of rats. Biol. Trace Elem. Res. 2009, 131, 62–70. [Google Scholar] [CrossRef]
- Duan, L.; Shen, H.; Zhao, G.; Yang, R.; Cai, X.; Zhang, L.; Jin, C.; Huang, Y. Inhibitory effect of Disulfiram/copper complex on non-small cell lung cancer cells. Biochem. Biophys. Res. Commun. 2014, 446, 1010–1016. [Google Scholar] [CrossRef]
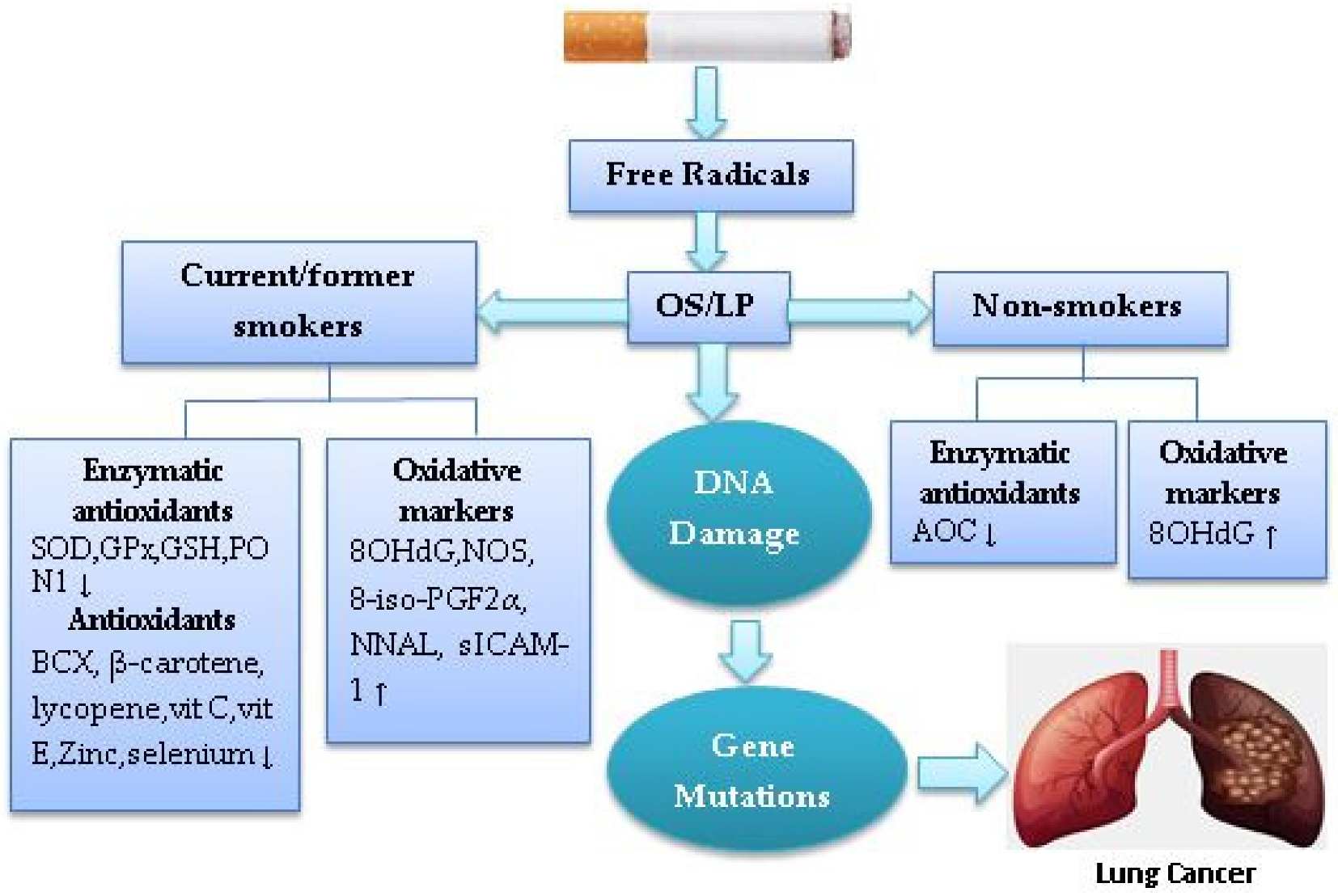
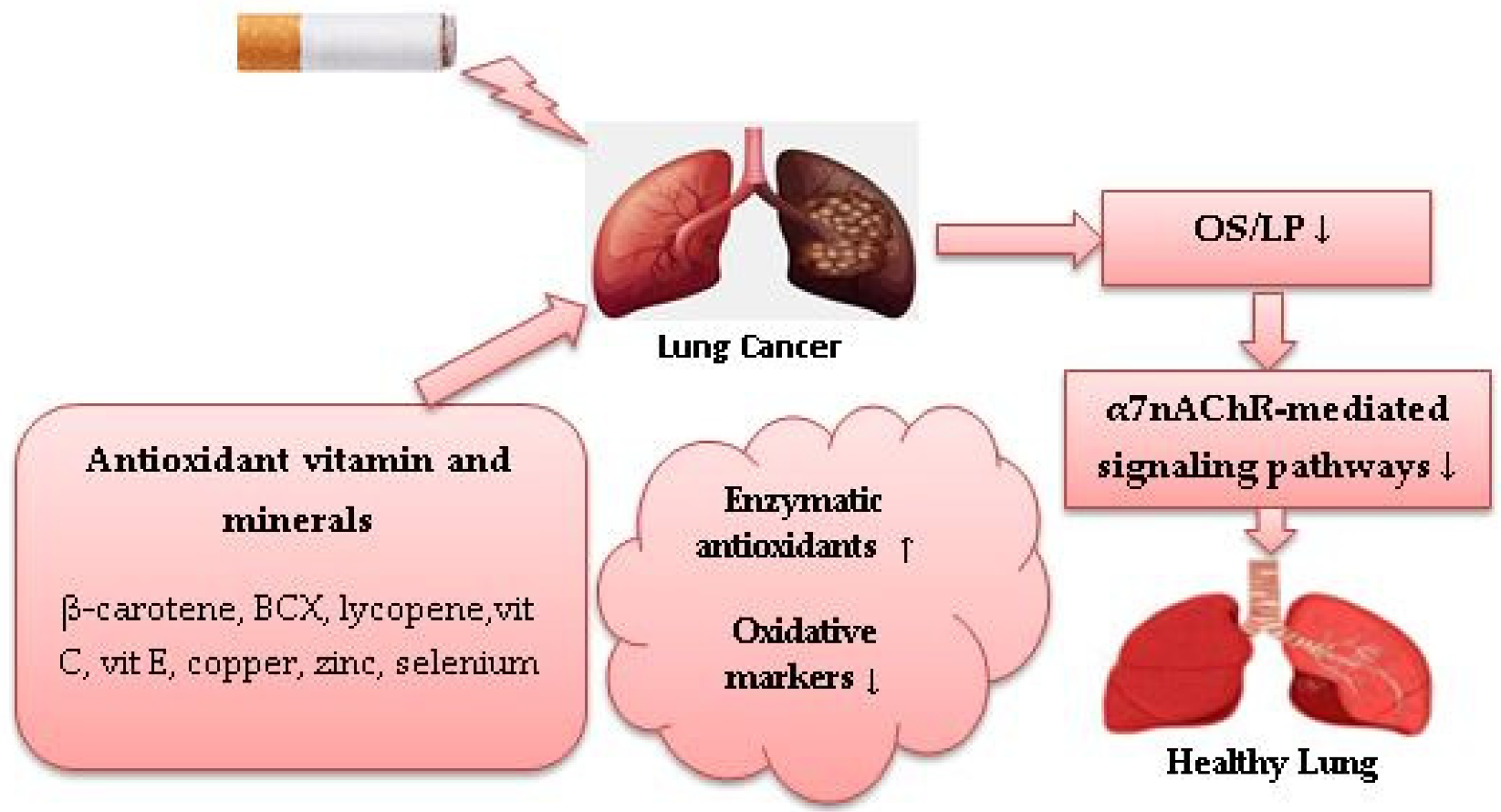
| Reference | Country | Research Design | Sample | Dietary Antioxidants | Findings |
|---|---|---|---|---|---|
| Männistö et al. [35] | Europe and North America | Meta-analysis of seven population-based cohort studies (follow-up duration of 7–16 years across studies) | Total subjects = 3155 LC patients Current smokers = 1915 Former smokers = 981 Non-smokers = 259 | Carotenoids | High intake of BCX (≥160 µg/day) was associated with reduced LC risk in current smokers (RR = 0.70, 95% CI = 0.60 to 0.81) |
| Zhu et al. [36] | US, Netherlands, China, Finland and Denmark | Meta-analysis of nine population-based cohort studies (follow-up duration of 4–20 years across studies) | Total subjects = 2768 LC patients Current smokers = 1930 Former smokers = 528 Non-smokers = 310 | Vitamin E (α-tocopherol) | Vitamin E intake (2 mg/day) was associated with reduced LC risk in current smokers (RR = 0.74, 95% CI = 0.61 to 0.89) |
| Luo et al. [40] | US, China, Netherlands, Uruguay and Canada | Meta-analysis of seven case-control and 14 population-based cohort studies | Total subjects = 2008 LC patients Current smokers = 1044 Former smokers = 702 Non-smokers = 262 | Vitamin C | High intake of vitamin C (100 mg/day) was not associated with LC risk in current/former smokers |
| Mahabir et al. [41] | US | Case-control study | Total subjects = 2502 LC cases = 1088 (current smokers = 438; former smokers = 417; non-smokers = 238) Healthy matched controls = 1414 (current smokers = 512; former smoker = 599; non-smokers = 303) Age = ≥60 years | Dietary tocopherols (α-, β-, γ-, and δ) | High intake of α-tocopherol (≥5.51 mg/day) was associated with reduced LC risk in current smokers (RR = 0.33, 95% CI = 0.18 to 0.62) and former smokers (RR = 0.46, 95% CI = 0.26 to 0.81) |
| Yong et al. [42] | US | Population-based cohort study (follow-up duration of 19 years) | Total subjects = 10,068 Current smokers = 3090 Former smokers = 1691 Non-smokers = 4261 Unknown smoking status = 1026 Age = 25–74 years | Vitamins A, C and E, carotenoids | High intake of carotenoids (>2289.87 IU/day) (RR = 0.49, 95% CI = 0.29 to 0.84), vitamin E (>6.71 mg/day) (RR = 0.36, 95% CI = 0.16 to 0.83) and vitamin C (>113.05 mg/day) (RR = 0.55, 95% CI = 0.32 to 0.95) were associated with reduced LC risk in current smokers |
| Narita et al. [43] | Japan | Population-based cohort study (average 15.5 years follow-up) | Total subjects = 1896 LC patients Current smokers (male = 641, female = 28) Former smokers (male = 109, female = 0) Non-smokers (male = 109, female = 289) Light smokers (male = 37, female = 0) Heavy smokers (male = 713, female = 0) Age = 40–69 years | Retinol, vitamin C, vitamin E and carotenoids | Retinol intake (10 mcg/day) was associated with increased LC risk in male current smokers (HR = 1.22, 95% CI = 0.99 to 1.50), and decreased LC risk in female current smokers(HR = 0.51, 95% CI = 0.18 to 1.40) α-carotene intake (>2064 µg/day) was associated with reduced LC risk in male light smokers (OR = 0.29, 95% CI = 0.09 to 0.93) No other associations with LC were observed |
| Shareck et al. [44] | Canada | Case-control study | Total subjects = 2554 LC cases = 1105 (Male current smokers = 465; former smokers = 69; non-smokers = 156, Female current smoker = 304; former smokers = 36, non-smokers = 75) Healthy matched controls = 1449 (Male current smokers = 249; former smoker = 81; non-smokers = 540, Female current smokers = 127; former smokers = 48; non-smokers = 404) Age = 35–75 years | Vitamin C and carotenoids | High intake of BCX (>178 µg/day) (OR = 0.58, 95% CI = 0.34 to 0.89), β-carotene (>6760 µg/day) (OR = 0.49, 95% CI = 0.28 to 0.83), α-carotene (>2064 µg/day) (OR = 0.53, 95% CI = 0.31 to 0.89) and lycopene (>19,281 µg/day) (OR = 0.48, 95% CI = 0.29 to 0.79) were associated with reduced LC risk in male heavy-intensity smokers High intake of vitamin C (≥79 mg/day) (OR = 0.45, 95% CI = 0.21 to 0.95) was associated with reduced LC risk in female heavy-intensity smokers |
| Zhou et al. [45] | US | Case-control study | Total subjects = 2048 LC cases = 923 (current smokers = 378; former smokers = 489; non-smokers = 56) Healthy matched controls = 1125 (current smokers = 213; former smoker = 518; non-smokers = 394) Age = ≥18 years | Iron and zinc | Iron intake (≥16.24 mg/d) was associated with increased LC risk in current smokers (OR = 4.03, 95% CI = 1.89 to 8.75) Zinc intake (≥12.88 mg/d) was associated with reduced LC risk in current smokers (OR = 0.41, 95% CI = 0.19 to 0.88) |
| Mahabir et al. [46] | US | Case-control study | Total subjects = 3352 LC cases = 1676 (current smokers = 747; former smokers = 693; non-smokers = 256) Healthy matched controls = 1676 (current smokers = 584; former smoker = 779; non-smokers = 313) Age = ≥60 years | Zinc, copper and selenium | Zinc intake (>12.31 mg/d) was associated with reduced LC risk in current smokers (OR = 0.36, 95% CI = 0.22 to 0.57) Copper intake (>1.56 mg/d) was associated with reduced LC risk in current smokers (OR = 0.38, 95% CI = 0.24 to 0.60) Selenium intake was not associated with LC risk |
| Ward et al. [47] | Denmark, Greece, Italy, France, Germany, Netherlands, Norway, Spain, Sweden and the UK | Multi-centre prospective cohort study (follow-up duration of 8 years) | Total subjects = 83,348 Current smokers = 21,754 Former smokers = 20,171 Non-smokers = 41,423 Age = ≥49 years | Iron | Total iron intake (mg/1000 kcal), but not haem and non-haem iron intake was associated with reduced LC risk in former smokers only (HR = 0.90, 95% CI = 0.83 to 0.97) |
| Mayne et al. [48] | US | Population-based cohort study (follow-up duration of 3 years) | Total subjects = 413 non-smokers Age = ≥60 years | β-carotene and retinol | β-carotene intake was associated with reduced LC risk (OR = 0.70, 95% CI = 0.50 to 0.99) Retinol intake was not associated with LC risk |
| Wu et al. [49] | China | Population-based cohort study (follow-up duration of 12 years) | Total subjects = 72,829 female non-smokers Age = 40–70 years | Vitamin E (α-tocopherol) | High intake of α-tocopherol (≥14 mg/day) was associated with reduced LC risk in (HR = 0.78, 95% CI = 0.60 to 0.99) |
| Liu et al. [50] | Europe, North America and Asia | Meta-analysis of six case-control and 16 population-based cohort studies | Total subjects = 15,304 LC current, former and non-smokers | Vitamin D | Vitamin E intake was associated with reduced LC risk in non-smokers only (OR = 0.76, 95% CI = 0.65 to 0.88) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsharairi, N.A. Dietary Antioxidants and Lung Cancer Risk in Smokers and Non-Smokers. Healthcare 2022, 10, 2501. https://doi.org/10.3390/healthcare10122501
Alsharairi NA. Dietary Antioxidants and Lung Cancer Risk in Smokers and Non-Smokers. Healthcare. 2022; 10(12):2501. https://doi.org/10.3390/healthcare10122501
Chicago/Turabian StyleAlsharairi, Naser A. 2022. "Dietary Antioxidants and Lung Cancer Risk in Smokers and Non-Smokers" Healthcare 10, no. 12: 2501. https://doi.org/10.3390/healthcare10122501
APA StyleAlsharairi, N. A. (2022). Dietary Antioxidants and Lung Cancer Risk in Smokers and Non-Smokers. Healthcare, 10(12), 2501. https://doi.org/10.3390/healthcare10122501









