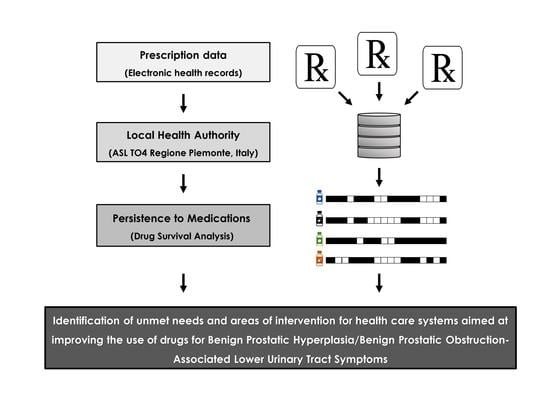
Persistence to Medications for Benign Prostatic Hyperplasia/Benign Prostatic Obstruction-Associated Lower Urinary Tract Symptoms in the ASL TO4 Regione Piemonte (Italy)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Population and Drugs
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Persistence to Medication
3.3. Prescription of Drugs for BPH/BPO-Associated LUTS
4. Discussion
4.1. Strengths and Limitations
4.2. Implications for Practice/Further Research
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roehrborn, C.G. Benign prostatic hyperplasia: An overview. Rev. Urol. 2005, 7, S3–S14. [Google Scholar] [PubMed]
- Speakman, M. Lower urinary tract symptoms suggestive of benign prostatic hyperplasia (LUTS/BPH): More than treating symptoms? Eur. Urol. Suppl. 2008, 7, 680–689. [Google Scholar] [CrossRef]
- Lerner, L.B.; McVary, K.T.; Barry, M.J.; Bixler, B.R.; Dahm, P.; Das, A.K.; Gandhi, M.C.; Kaplan, S.A.; Kohler, T.S.; Martin, L.; et al. Management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA Guideline Part I—Initial Work-Up and Medical Management. J. Urol. 2021, 206, 806–817. [Google Scholar] [CrossRef] [PubMed]
- Gravas, S.; Cornu, J.N.; Drake, M.J.; Gacci, M.; Gratzke, C.; Herrmann, T.R.W.; Madersbacher, S.; Tikkinen, K.A.O.; Karavitakis, M.; Kyriazis, I.; et al. Management of non-neurogenic male lower urinary tract symptoms (LUTS), incl. benign prostatic obstruction (BPO). EAU Guidelines, Edn. In Proceedings of the EAU Annual Congress, Amsterdam, The Netherlands, 1–4 July 2022. [Google Scholar]
- Welch, G.; Weinger, K.; Barry, M.J. Quality-of-life impact of lower urinary tract symptom severity: Results from the Health Professionals Follow-up Study. Urology 2002, 59, 245–250. [Google Scholar] [CrossRef]
- Roehrborn, C.G. BPH progression: Concept and key learning from MTOPS, ALTESS, COMBAT, and ALF-ONE. BJU Int. 2008, 101, 17–21. [Google Scholar] [CrossRef]
- Lepor, H.; Auerbach, S.; Puras-Baez, A.; Narayan, P.; Soloway, M.; Lowe, F.; Moon, T.; Leifer, G.; Madsen, P. A randomized, placebo-controlled multicenter study of the efficacy and safety of terazosin in the treatment of benign prostatic hyperplasia. J. Urol. 1992, 148, 1467–1474. [Google Scholar] [CrossRef]
- Mobley, D.F.; Kaplan, S.; Ice, K.; Gaffney, M.; Dias, N. Effect of doxazosin on the symptoms of benign prostatic hyperplasia: Results from three double-blind placebo-controlled studies. Int. J. Clin. Pract. 1997, 51, 282–288. [Google Scholar]
- McConnell, J.D.; Roehrborn, C.G.; Bautista, O.M.; Andriole, G.L., Jr.; Dixon, C.M.; Kusek, J.W.; Lepor, H.; McVary, K.T.; Nyberg, L.M., Jr.; Clarke, H.S.; et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N. Engl. J. Med. 2003, 349, 2387–2398. [Google Scholar] [CrossRef] [Green Version]
- Roehrborn, C.G.; Siami, P.; Barkin, J.; Damião, R.; Major-Walker, K.; Morrill, B.; Montorsi, F. The effects of dutasteride, tamsulosin and combination therapy on lower urinary tract symptoms in men with benign prostatic hyperplasia and prostatic enlargement: 2-year results from the CombAT study. J. Urol. 2008, 179, 616–621. [Google Scholar] [CrossRef]
- Goldfischer, E.; Kowalczyk, J.J.; Clark, W.R.; Brady, E.; Anne Shane, M.; Dgetluck, N.; Klise, S.R. Hemodynamic effects of once-daily tadalafil in men with signs and symptoms of benign prostatic hyperplasia on concomitant a1-adrenergic antagonist therapy: Results of a multicenter randomized, double-blind, placebo-controlled trial. Urology 2012, 79, 875–882. [Google Scholar] [CrossRef]
- Füllhase, C.; Chapple, C.; Cornu, J.N.; De Nunzio, C.; Gratzke, C.; Kaplan, S.A.; Marberger, M.; Montorsi, F.; Novara, G.; Oelke, M.; et al. Systematic review of combination drug therapy for non-neurogenic male lower urinary tract symptoms. Eur. Urol. 2013, 64, 228–243. [Google Scholar] [CrossRef] [PubMed]
- De Nunzio, C.; Tubaro, A. BPH: Unmet needs in managing LUTS—A European perspective. Nat. Rev. Urol. 2011, 9, 9–10. [Google Scholar] [CrossRef] [PubMed]
- Fourcade, R.O.; Lacoin, F.; Rouprêt, M.; Slama, A.; Le Fur, C.; Michel, E.; Sitbon, A.; Cotté, F. Outcomes and general health-related quality of life among patients medically treated in general daily practice for lower urinary tract symptoms due to benign prostatic hyperplasia. World J. Urol. 2012, 30, 419–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nichol, M.B.; Knight, T.K.; Wu, J.; Barron, R.; Penson, D.F. Evaluating use patterns of and adherence to medications for benign prostatic hyperplasia. J. Urol. 2009, 181, 2214–2221. [Google Scholar] [CrossRef] [PubMed]
- Gruschkus, S.; Poston, S.; Eaddy, M.; Chaudhari, S. Adherence to 5-alpha reductase inhibitor therapy for benign prostatic hyperplasia: Clinical and economic outcomes. Pharm. Ther. 2012, 37, 464–470. [Google Scholar]
- Lukacs, B.; Cornu, J.N.; Aout, M.; Tessier, N.; Hodée, C.; Haab, F.; Cussenot, O.; Merlière, Y.; Moysan, V.; Vicaut, E. Management of lower urinary tract symptoms related to benign prostatic hyperplasia in real-life practice in France: A comprehensive population study. Eur. Urol. 2013, 64, 493–501. [Google Scholar] [CrossRef]
- Koh, J.S.; Cho, J.; Kim, H.S.; Kim, J.C. Twelve-month medication persistence in men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. Int. J. Clin. Pract. 2014, 68, 197–202. [Google Scholar] [CrossRef]
- Cindolo, L.; Pirozzi, L.; Fanizza, C.; Romero, M.; Tubaro, A.; Autorino, R.; De Nunzio, C.; Schips, L. Drug adherence and clinical outcomes for patients under pharmacological therapy for lower urinary tract symptoms related to benign prostatic hyperplasia: Population-based cohort study. Eur. Urol. 2015, 68, 418–425. [Google Scholar] [CrossRef]
- Cindolo, L.; Pirozzi, L.; Sountoulides, P.; Fanizza, C.; Romero, M.; Castellan, P.; Antonelli, A.; Simeone, C.; Tubaro, A.; De Nunzio, C.; et al. Patient’s adherence on pharmacological therapy for benign prostatic hyperplasia (BPH)-associated lower urinary tract symptoms (LUTS) is different: Is combination therapy better than monotherapy. BMC Urol. 2015, 15, 96. [Google Scholar] [CrossRef] [Green Version]
- Drake, M.J.; Bowditch, S.; Arbe, E.; Hakimi, Z.; Gueòfucci, F.; Amri, I.; Nazir, J. A retrospective study of treatment persistence and adherence to α-blocker plus antimuscarinic combination therapies, in men with LUTS/BPH in the Netherlands. BMC Urol. 2017, 17, 36. [Google Scholar] [CrossRef] [Green Version]
- Eisen, C.; Lulic, Z.; Palacios-Moreno, J.M.; Adalig, B.; Hennig, M.; Cortes, V.; Gilg, F.; Kostev, K. Persistence and adherence to dutasteride/tamsulosin foxed-dose versus free-combination alpha blocker/5ARI therapy in patients with benign prostate hyperplasia in Germany. Int. J. Clin. Pharmacol. Ther. 2020, 58, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Yeaw, J.; Benner, J.S.; Walt, J.G.; Sian, S.; Smith, D.B. Comparing adherence and discontinuation across 6 chronic medication classes. J. Manag. Care Pharm. 2009, 15, 728–740. [Google Scholar] [CrossRef]
- Menditto, E.; Cahir, C.; Malo, S.; Aguilar-Palacio, I.; Almada, M.; Costa, E.; Giardini, A.; Gil Peinado, M.; Massot Mesquida, M.; Mucherino, S.; et al. Persistence as a robust indicator of medication adherence-related quality and performance. Int. J. Environ. Res. Public Health 2021, 18, 4872. [Google Scholar] [CrossRef] [PubMed]
- WHO Collaborating Centre for Drug Statistics Methodology. ATC Classification Index with DDDs, 2022; WHO: Oslo, Norway, 2021.
- Miglio, G.; Basso, L.; Armando, L.G.; Traina, S.; Benetti, E.; Diarassouba, A.; Baroetto Parisi, R.; Esiliato, M.; Rolando, C.; Remani, E.; et al. A network approach for the study of drug prescriptions: Analysis of administrative records from a local health unit (ASL TO4, Regione Piemonte, Italy). Int. J. Environ. Res. Public Health 2021, 18, 4859. [Google Scholar] [CrossRef] [PubMed]
- Pazzagli, L.; Brandt, L.; Linder, M.; Myers, D.; Mavros, P.; Andersen, M.; Bahmanyar, S. Methods for constructing treatment episodes and impact on exposure-outcome associations. Eur. J. Clin. Pharmacol. 2019, 76, 267–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emberton, M.; Cornel, E.B.; Bassi, P.F.; Fourcade, R.O.; Gómez, J.M.F.; Castro, R. Benign prostatic hyperplasia as a progressive disease: A guide to the risk factors and options for medical management. Int. J. Clin. Pract. 2008, 62, 1076–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strope, S.A.; Elliott, S.P.; Saigal, C.S.; Smith, A.; Wilt, T.J.; Wei, J.T. Urologic diseases in America project. Urologist compliance with AUA best practice guidelines for benign prostatic hyperplasia in Medicare population. Urology 2011, 78, 3–9. [Google Scholar] [CrossRef] [Green Version]
- The Medicines Utilization Monitoring Centre. National Report on Medicines Use in Italy. Year 2021; Italian Medicines Agency: Rome, Italy, 2021. [Google Scholar]
- Yu, Z.J.; Yan, H.L.; Xu, F.H.; Chao, H.C.; Deng, L.H.; Xu, X.D.; Huang, J.B.; Zeng, T. Efficacy and side effects of drugs commonly used for the treatment of lower urinary tract symptoms associated with benign prostatic hyperplasia. Front. Pharmacol. 2020, 11, 658. [Google Scholar] [CrossRef]
- Oelke, M.; Gericke, A.; Michel, M.C. Cardiovascular and ocular safety of α1-adrenoceptor antagonists in the treatment of male lower urinary tract symptoms. Expert Opin. Drug Saf. 2014, 13, 1187–1197. [Google Scholar] [CrossRef]
- Gacci, M.; Ficarra, V.; Sebastianelli, A.; Corona, G.; Serni, S.; Shariat, S.F.; Maggi, M.; Zattoni, F.; Carini, M.; Novara, G. Impact of medical treatments for male lower urinary tract symptoms due to benign prostatic hyperplasia on ejaculatory function: A systematic review and meta-analysis. J. Sex. Med. 2014, 11, 1554–1566. [Google Scholar] [CrossRef]
- Jung, J.H.; Kim, J.; MacDonald, R.; Reddy, B.; Kim, M.H.; Dahm, P. Silodosin for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia. Cochrane Database Syst. Rev. 2017, 11, CD012615. [Google Scholar] [PubMed]
- Yeung, H.E.L.; Sena, S.J.; Calopedos, R.J. Alfuzosin and its effect on ejaculatory dysfunction: A systematic review. World J. Men’s Health 2021, 39, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Radomski, S.; Chung, J.; Plazker, T.; Singer, S.; Slomovic, A.R. Intraoperative floppy-iris syndrome during cataract surgery in men using alpha-blockers for benign prostatic hypertrophy. J. Cataract Refract. Surg. 2007, 33, 1826–1827. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, S.A.; Chung, D.E.; Lee, R.K.; Scofield, S.; Te, A.E. A 5-year retrospective analysis of 5α-reductase inhibitors in men with benign prostatic hyperplasia: Finasteride has comparable urinary symptom efficacy and prostate volume reduction, but less sexual side effects and breast complications than dutasteride. Int. J. Clin. Pract. 2012, 66, 1052–1055. [Google Scholar] [CrossRef] [PubMed]
- Schröder, F.; Bangma, C.; Angulo, J.C.; Alcaraz, A.; Colombel, M.; McNicholas, T.; Tammela, T.L.; Nandy, I.; Castro, R. Dutasteride treatment over 2 years delays prostate-specific antigen progression in patients with biochemical failure after radical therapy for prostate cancer: Results from the randomised, placebo-controlled Avodart after Radical Therapy for Prostate Cancer Study (ARTS). Eur. Urol. 2013, 63, 779–787. [Google Scholar]
- Thompson, I.M.; Goodman, P.J.; Tangen, C.M.; Lucia, M.S.; Miller, G.J.; Ford, L.G.; Lieber, M.M.; Cespedes, D.R.; Atkins, J.N.; Lippman, S.M.; et al. The influence of finasteride on the development of prostate cancer. N. Engl. J. Med. 2003, 349, 215–224. [Google Scholar] [CrossRef]
- Barry, M.J. Evaluation of symptoms and quality of life in males with benign prostatic hyperplasia. Urology 2001, 58, 25–32. [Google Scholar] [CrossRef]
- Park, S.; Ryu, J.; Lee, M. Quality of life in older adults with benign prostatic hyperplasia. Healthcare 2020, 8, 158. [Google Scholar] [CrossRef]
- Mojon-Azzi, S.; Sousa-Poza, A.; Widmen, R. The effect of retirement on health: A panel analysis using data from the Swiss Household Panel. Swiss Med. Wkly. 2007, 137, 581–585. [Google Scholar] [CrossRef]
- Westerlund, H.; Vahtera, J.; Ferrie, J.E.; Singh-Manous, A.; Pentti, J.; Melchior, M.; Leineweber, C.; Jokela, M.; Siegrist, J.; Goldberg, M.; et al. Effect of retirement on major chronic conditions and fatigue: French GAZEL occupational cohort study. BMJ 2010, 341, c6149. [Google Scholar] [CrossRef] [Green Version]
- Oksanen, T.; Vahtera, J.; Westerlund, H.; Pentti, J.; Sjösten, N.; Virtanen, M.; Kawachi, I.; Kivimäki, M. Is retirement beneficial for mental health? Antidepressant use before and after retirement. Epidemiology 2011, 22, 553–559. [Google Scholar] [CrossRef] [Green Version]
- Kivimäki, M.; Nyberg, S.T.; Fransson, E.I.; Heikkilä, K.; Alfredsson, L.; Casini, A.; Clays, E.; De Bacquer, D.; Dragano, N.; Ferrie, J.E.; et al. Associations of job strain and lifestyle risk factors with risk of coronary artery disease: A meta-analysis of individual participant data. Can. Med. Assoc. J. 2013, 185, 763–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halava, H.; Westerlund, H.; Korhonen, M.J.; Pentti, J.; Kivimäki, M.; Kjeldgård, L.; Alexanderson, K.; Vahtera, J. Influence of retirement on adherence to statins in the insurance medicine all-Sweden total population data base. PLoS ONE 2015, 10, e0130901. [Google Scholar] [CrossRef]
- Madersbacher, S.; Marszalek, M.; Lackner, J.; Berger, P.; Schatzl, G. The long-term outcome of medical therapy for BPH. Eur. Urol. 2007, 51, 1522–1533. [Google Scholar] [CrossRef] [PubMed]
- de la Rosette, J.J.; Kortmann, B.B.; Rossi, C.; Sonke, G.S.; Floratos, D.L.; Kiemeney, L.A. Long-term risk of re-treatment of patients using alpha-blockers for lower urinary tract symptoms. J. Urol. 2002, 167, 1734–1739. [Google Scholar] [CrossRef] [PubMed]



| Group | N (%) |
|---|---|
| Study population | 4309 (100.0) |
| Age (years) | |
| 40–49 | 100 (2.3) |
| 50–59 | 525 (12.2) |
| 60–69 | 1229 (28.5) |
| 70–79 | 1533 (35.6) |
| 80–89 | 838 (19.4) |
| 90–100 | 84 (1.9) |
| Category | Percentage of Men Still on Treatment (95% CI) |
|---|---|
| ABs | 25.5 (24.2–26.9) |
| Alfuzosin | 43.8 (39.8–47.9) |
| Silodosin | 25.1 (22.5–27.7) |
| Tamsulosin | 23.4 (21.6–25.3) |
| Terazosin | 6.4 (3.2–9.6) |
| Doxazosin | 4.5 (0.2–8.9) |
| 5ARIs | 36.0 (33.6–38.5) |
| Dutasteride | 43.3 (40.3–46.3) |
| Finasteride | 17.3 (13.7–20.9) |
| Drug (N) | Median Age [IQR] (Years) | p-Value a |
|---|---|---|
| Overall (4380) | 71.0 [64.0–78.0] | |
| ABs (3273) | 70.0 [63.0–77.0] | NA |
| Alfuzosin (584) | 68.0 [61.0–75.0] | |
| Doxazosin (88) | 73.5 [64.0–78.3] | |
| Silodosin (1074) | 70.0 [63.0–77.0] | |
| Tamsulosin (2057) | 70.0 [63.0–77.0] | |
| Terazosin (219) | 68.0 [59.5–76.0] | |
| 5ARIs (1407) | 75.0 [68.0–81.0] | <0.001 |
| Dutasteride (1064) | 75.0 [68.0–80.0] | |
| Finasteride (416) | 75.0 [68.0–81.0] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armando, L.G.; Baroetto Parisi, R.; Remani, E.; Esiliato, M.; Rolando, C.; Vinciguerra, V.; Diarassouba, A.; Cena, C.; Miglio, G. Persistence to Medications for Benign Prostatic Hyperplasia/Benign Prostatic Obstruction-Associated Lower Urinary Tract Symptoms in the ASL TO4 Regione Piemonte (Italy). Healthcare 2022, 10, 2567. https://doi.org/10.3390/healthcare10122567
Armando LG, Baroetto Parisi R, Remani E, Esiliato M, Rolando C, Vinciguerra V, Diarassouba A, Cena C, Miglio G. Persistence to Medications for Benign Prostatic Hyperplasia/Benign Prostatic Obstruction-Associated Lower Urinary Tract Symptoms in the ASL TO4 Regione Piemonte (Italy). Healthcare. 2022; 10(12):2567. https://doi.org/10.3390/healthcare10122567
Chicago/Turabian StyleArmando, Lucrezia Greta, Raffaella Baroetto Parisi, Elisa Remani, Mariangela Esiliato, Cristina Rolando, Valeria Vinciguerra, Abdoulaye Diarassouba, Clara Cena, and Gianluca Miglio. 2022. "Persistence to Medications for Benign Prostatic Hyperplasia/Benign Prostatic Obstruction-Associated Lower Urinary Tract Symptoms in the ASL TO4 Regione Piemonte (Italy)" Healthcare 10, no. 12: 2567. https://doi.org/10.3390/healthcare10122567