A Link between Handgrip Strength and Executive Functioning: A Cross-Sectional Study in Older Adults with Mild Cognitive Impairment and Healthy Controls
Abstract
:1. Introduction
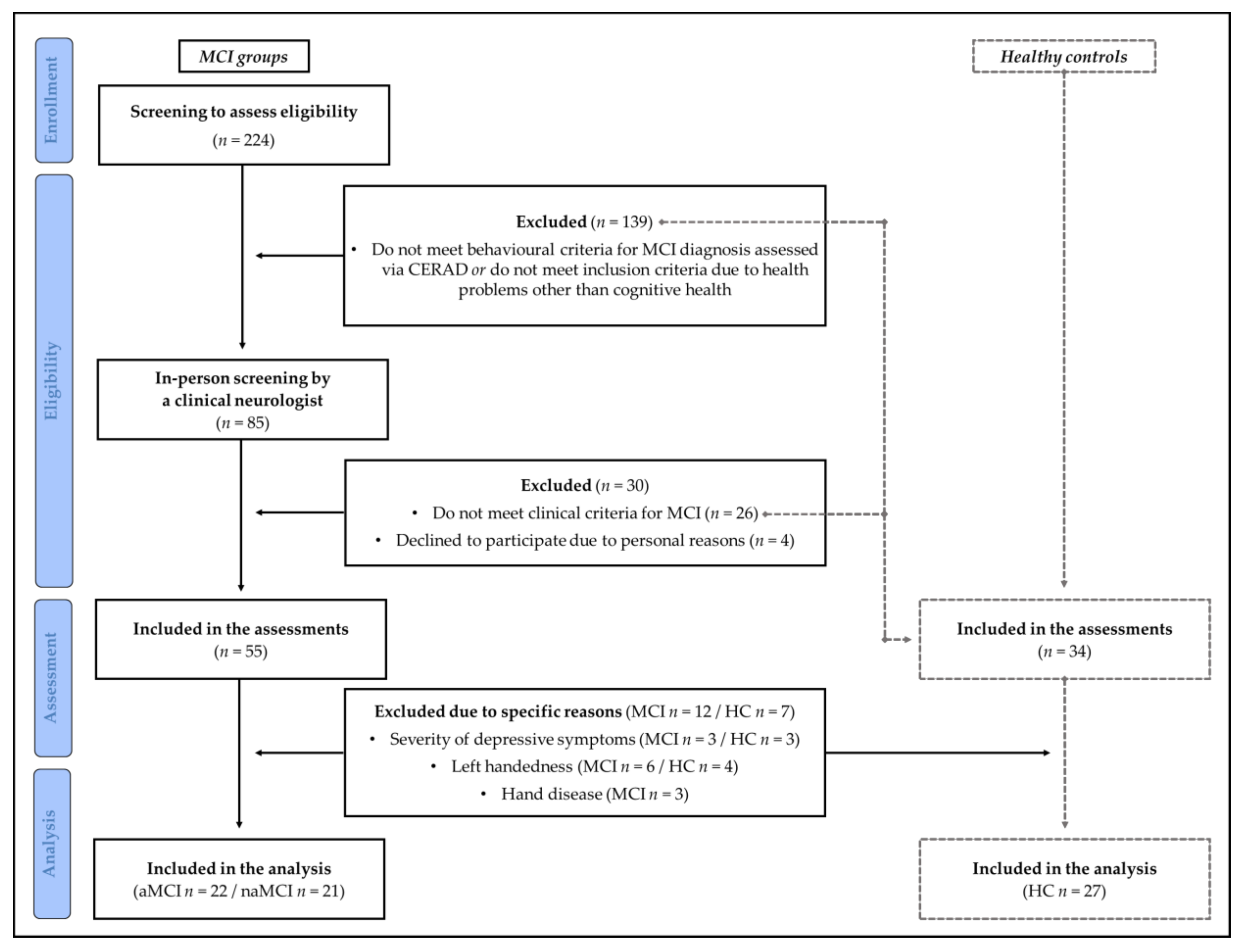
2. Materials and Methods
2.1. Participants
2.2. Assessment of Cognitive Performance and Handgrip Strength
2.3. Statistical Analysis
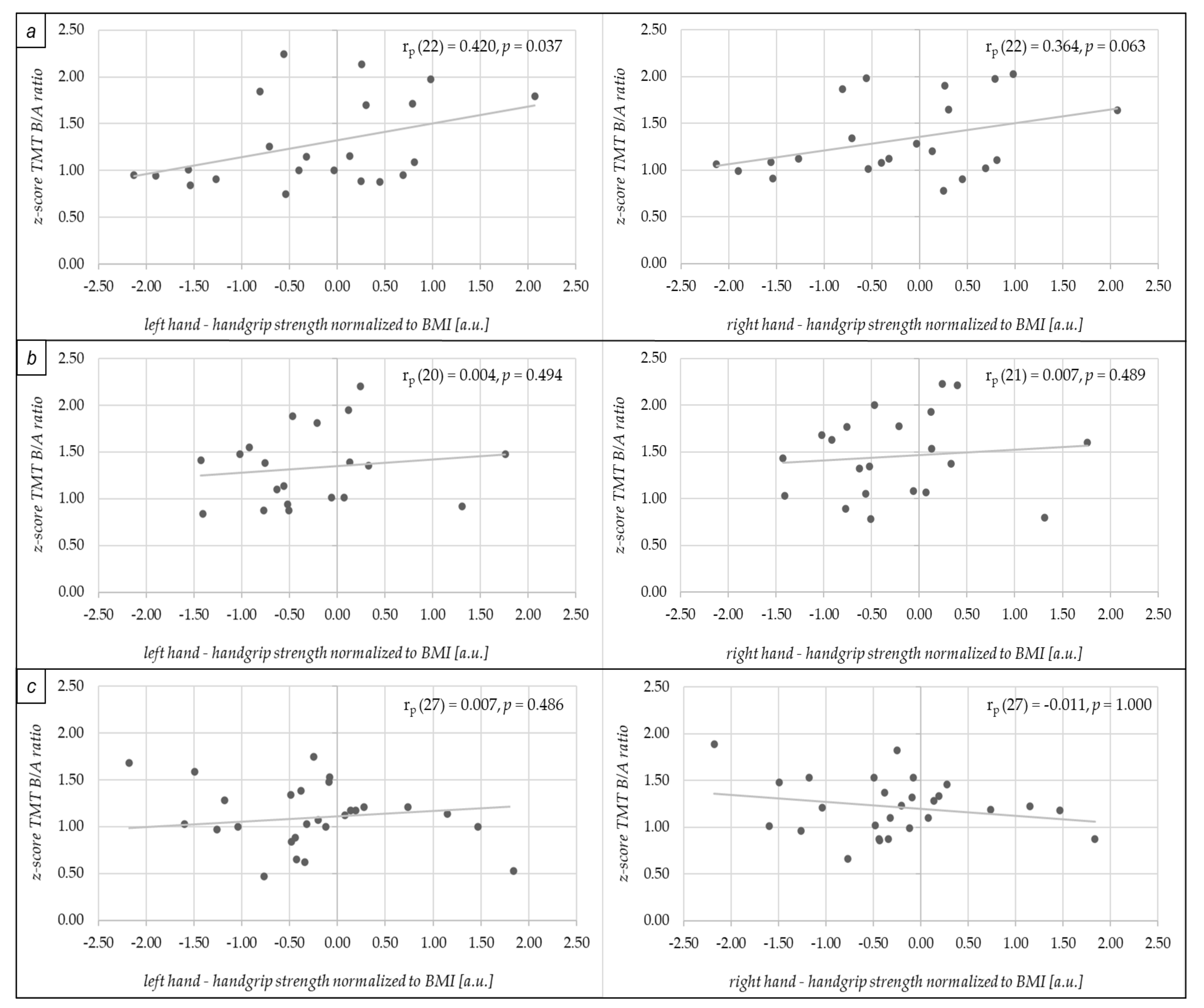
3. Results
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McGrath, R.; Johnson, N.; Klawitter, L.; Mahoney, S.; Trautman, K.; Carlson, C.; Rockstad, E.; Hackney, K.J. What are the association patterns between handgrip strength and adverse health conditions? A topical review. SAGE Open Med. 2020, 8, 2050312120910358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGrath, R.P.; Kraemer, W.J.; Snih, S.A.; Peterson, M.D. Handgrip Strength and Health in Aging Adults. Sports Med. 2018, 48, 1993–2000. [Google Scholar] [CrossRef] [PubMed]
- Soysal, P.; Hurst, C.; Demurtas, J.; Firth, J.; Howden, R.; Yang, L.; Tully, M.A.; Koyanagi, A.; Ilie, P.C.; López-Sánchez, G.F.; et al. Handgrip strength and health outcomes: Umbrella review of systematic reviews with meta-analyses of observational studies. J. Sport Health Sci. 2020, 10, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W. Grip Strength: An Indispensable Biomarker for Older Adults. Clin. Interv. Aging 2019, 14, 1681–1691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carson, R.G. Get a grip: Individual variations in grip strength are a marker of brain health. Neurobiol. Aging 2018, 71, 189–222. [Google Scholar] [CrossRef] [Green Version]
- Fritz, N.E.; McCarthy, C.J.; Adamo, D.E. Handgrip strength as a means of monitoring progression of cognitive decline—A scoping review. Ageing Res. Rev. 2017, 35, 112–123. [Google Scholar] [CrossRef]
- Shaughnessy, K.A.; Hackney, K.J.; Clark, B.C.; Kraemer, W.J.; Terbizan, D.J.; Bailey, R.R.; McGrath, R. A Narrative Review of Handgrip Strength and Cognitive Functioning: Bringing a New Characteristic to Muscle Memory. J. Alzheimers. Dis. 2020, 73, 1265–1278. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, A.K.; Jiwane, R.; Alam, T.; Kishanrao, S.S. Grip Strength and Impact on Cognitive Function in Healthy Kitchen Workers. Achiev. Life Sci. 2016, 10, 168–174. [Google Scholar] [CrossRef] [Green Version]
- Adamo, D.E.; Anderson, T.; Koochaki, M.; Fritz, N.E. Declines in grip strength may indicate early changes in cognition in healthy middle-aged adults. PLoS ONE 2020, 15, e0232021. [Google Scholar] [CrossRef]
- Firth, J.; Firth, J.A.; Stubbs, B.; Vancampfort, D.; Schuch, F.B.; Hallgren, M.; Veronese, N.; Yung, A.R.; Sarris, J. Association Between Muscular Strength and Cognition in People With Major Depression or Bipolar Disorder and Healthy Controls. JAMA Psychiatry 2018, 75, 740–746. [Google Scholar] [CrossRef]
- Firth, J.; Stubbs, B.; Vancampfort, D.; Firth, J.A.; Large, M.; Rosenbaum, S.; Hallgren, M.; Ward, P.B.; Sarris, J.; Yung, A.R. Grip Strength Is Associated With Cognitive Performance in Schizophrenia and the General Population: A UK Biobank Study of 476559 Participants. Schizophr. Bull. 2018, 44, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.Y.; Kim, J. Association between handgrip strength and cognitive impairment in elderly Koreans: A population-based cross-sectional study. J. Phys. Ther. Sci. 2015, 27, 3911–3915. [Google Scholar] [CrossRef] [Green Version]
- Pedrero-Chamizo, R.; Albers, U.; Tobaruela, J.L.; Meléndez, A.; Castillo, M.J.; González-Gross, M. Physical strength is associated with Mini-Mental State Examination scores in Spanish institutionalized elderly. Geriatr. Gerontol. Int. 2013, 13, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Ukegbu, U.; Maselko, J.; Malhotra, R.; Perera, B.; Ostbye, T. Correlates of handgrip strength and activities of daily living in elderly Sri Lankans. J. Am. Geriatr. Soc. 2014, 62, 1800–1801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zammit, A.R.; Piccinin, A.M.; Duggan, E.C.; Koval, A.; Clouston, S.; Robitaille, A.; Brown, C.L.; Handschuh, P.; Wu, C.; Jarry, V.; et al. A coordinated multi-study analysis of the longitudinal association between handgrip strength and cognitive function in older adults. J. Gerontol. B Psychol. Sci. Soc. Sci. 2019, 76, 229–241. [Google Scholar] [CrossRef] [Green Version]
- Noh, B.; Youm, C.; Lee, M.; Park, H. Associating Gait Phase and Physical Fitness with Global Cognitive Function in the Aged. Int. J. Environ. Res. Public Health 2020, 17, 4786. [Google Scholar] [CrossRef]
- Praetorius Björk, M.; Johansson, B.; Hassing, L.B. I forgot when I lost my grip-strong associations between cognition and grip strength in level of performance and change across time in relation to impending death. Neurobiol. Aging 2016, 38, 68–72. [Google Scholar] [CrossRef] [Green Version]
- Tou, N.X.; Wee, S.-L.; Pang, B.W.J.; Lau, L.K.; Jabbar, K.A.; Seah, W.T.; Chen, K.K.; Ng, T.P. Associations of fat mass and muscle function but not lean mass with cognitive impairment: The Yishun Study. PLoS ONE 2021, 16, e0256702. [Google Scholar] [CrossRef]
- Alfaro-Acha, A.; Al Snih, S.; Raji, M.A.; Kuo, Y.-F.; Markides, K.S.; Ottenbacher, K.J. Handgrip strength and cognitive decline in older Mexican Americans. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 859–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGrath, R.; Robinson-Lane, S.G.; Cook, S.; Clark, B.C.; Herrmann, S.; O’Connor, M.L.; Hackney, K.J. Handgrip Strength Is Associated with Poorer Cognitive Functioning in Aging Americans. J. Alzheimers. Dis. 2019, 70, 1187–1196. [Google Scholar] [CrossRef]
- Sternäng, O.; Reynolds, C.A.; Finkel, D.; Ernsth-Bravell, M.; Pedersen, N.L.; Dahl Aslan, A.K. Grip Strength and Cognitive Abilities: Associations in Old Age. J. Gerontol. B Psychol. Sci. Soc. Sci. 2016, 71, 841–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGrath, R.; Vincent, B.M.; Hackney, K.J.; Robinson-Lane, S.G.; Downer, B.; Clark, B.C. The Longitudinal Associations of Handgrip Strength and Cognitive Function in Aging Americans. J. Am. Med. Dir. Assoc. 2019, 21, 634–639. [Google Scholar] [CrossRef]
- Chou, M.-Y.; Nishita, Y.; Nakagawa, T.; Tange, C.; Tomida, M.; Shimokata, H.; Otsuka, R.; Chen, L.-K.; Arai, H. Role of gait speed and grip strength in predicting 10-year cognitive decline among community-dwelling older people. BMC Geriatr. 2019, 19, 186. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Park, S.K.; Lee, D.R.; Lee, J. The Relationship between Handgrip Strength and Cognitive Function in Elderly Koreans over 8 Years: A Prospective Population-Based Study Using Korean Longitudinal Study of Ageing. Korean J. Fam. Med. 2019, 40, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Viscogliosi, G.; Di Bernardo, M.G.; Ettorre, E.; Chiriac, I.M. Handgrip Strength Predicts Longitudinal Changes in Clock Drawing Test Performance. An Observational Study in a Sample of Older Non-Demented Adults. J. Nutr. Health Aging 2017, 21, 593–596. [Google Scholar] [CrossRef] [PubMed]
- McGrath, R.; Cawthon, P.M.; Cesari, M.; Al Snih, S.; Clark, B.C. Handgrip Strength Asymmetry and Weakness Are Associated with Lower Cognitive Function: A Panel Study. J. Am. Geriatr. Soc. 2020, 68, 2051–2058. [Google Scholar] [CrossRef] [PubMed]
- Vancampfort, D.; Stubbs, B.; Firth, J.; Smith, L.; Swinnen, N.; Koyanagi, A. Associations between handgrip strength and mild cognitive impairment in middle-aged and older adults in six low- and middle-income countries. Int. J. Geriatr. Psychiatry 2019, 34, 609–616. [Google Scholar] [CrossRef]
- Boyle, P.A.; Buchman, A.S.; Wilson, R.S.; Leurgans, S.E.; Bennett, D.A. Physical frailty is associated with incident mild cognitive impairment in community-based older persons. J. Am. Geriatr. Soc. 2010, 58, 248–255. [Google Scholar] [CrossRef]
- Cui, M.; Zhang, S.; Liu, Y.; Gang, X.; Wang, G. Grip Strength and the Risk of Cognitive Decline and Dementia: A Systematic Review and Meta-Analysis of Longitudinal Cohort Studies. Front. Aging Neurosci. 2021, 13, 625551. [Google Scholar] [CrossRef]
- Su, H.; Sun, X.; Li, F.; Guo, Q. Association between handgrip strength and cognition in a Chinese population with Alzheimer’s disease and mild cognitive impairment. BMC Geriatr. 2021, 21, 459. [Google Scholar] [CrossRef]
- Traykov, L.; Raoux, N.; Latour, F.; Gallo, L.; Hanon, O.; Baudic, S.; Bayle, C.; Wenisch, E.; Remy, P.; Rigaud, A.-S. Executive functions deficit in mild cognitive impairment. Cogn. Behav. Neurol. 2007, 20, 219–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crowell, T.A.; Luis, C.A.; Vanderploeg, R.D.; Schinka, J.A.; Mullan, M. Memory Patterns and Executive Functioning in Mild Cognitive Impairment and Alzheimer’s Disease. Aging Neuropsychol. Cogn. 2002, 9, 288–297. [Google Scholar] [CrossRef]
- Zheng, D.; Dong, X.; Sun, H.; Xu, Y.; Ma, Y.; Wang, X. The overall impairment of core executive function components in patients with amnestic mild cognitive impairment: A cross-sectional study. BMC Neurol. 2012, 12, 138. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.H.; Park, S.; Jang, H.; Cho, S.H.; Kim, S.J.; Kim, J.P.; Kim, S.T.; Na, D.L.; Seo, S.W.; Kim, H.J. Frontal-executive dysfunction affects dementia conversion in patients with amnestic mild cognitive impairment. Sci. Rep. 2020, 10, 772. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, H.; Kato, M.; Hayashi, M.; Okubo, Y.; Takano, A.; Ito, H.; Suhara, T. Memory and frontal lobe functions; possible relations with dopamine D2 receptors in the hippocampus. NeuroImage 2007, 34, 1643–1649. [Google Scholar] [CrossRef] [PubMed]
- Reuben, A.; Brickman, A.M.; Muraskin, J.; Steffener, J.; Stern, Y. Hippocampal atrophy relates to fluid intelligence decline in the elderly. J. Int. Neuropsychol. Soc. 2011, 17, 56–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papp, K.V.; Kaplan, R.F.; Springate, B.; Moscufo, N.; Wakefield, D.B.; Guttmann, C.R.G.; Wolfson, L. Processing speed in normal aging: Effects of white matter hyperintensities and hippocampal volume loss. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 2014, 21, 197–213. [Google Scholar] [CrossRef]
- Firth, J.A.; Smith, L.; Sarris, J.; Vancampfort, D.; Schuch, F.; Carvalho, A.F.; Solmi, M.; Yung, A.R.; Stubbs, B.; Firth, J. Handgrip Strength Is Associated With Hippocampal Volume and White Matter Hyperintensities in Major Depression and Healthy Controls: A UK Biobank Study. Psychosom. Med. 2020, 82, 39–46. [Google Scholar] [CrossRef]
- Emmert, N.A.; Reiter, K.E.; Butts, A.; Janecek, J.K.; Agarwal, M.; Franczak, M.; Reuss, J.; Klein, A.; Wang, Y.; Umfleet, L.G. Hippocampal Volumes in Amnestic and Non-Amnestic Mild Cognitive Impairment Types Using Two Common Methods of MCI Classification. J. Int. Neuropsychol. Soc. 2021, 1–10, First view. [Google Scholar] [CrossRef]
- Grässler, B.; Herold, F.; Dordevic, M.; Gujar, T.A.; Darius, S.; Böckelmann, I.; Müller, N.G.; Hökelmann, A. Multimodal measurement approach to identify individuals with mild cognitive impairment: Study protocol for a cross-sectional trial. BMJ Open 2021, 11, e046879. [Google Scholar] [CrossRef]
- Gauggel, S.; Birkner, B. Validität und Reliabilität einer deutschen Version der Geriatrischen Depressionsskala (GDS). Z. Für Klin. Psychol. Psychother. 1999, 28, 18–27. [Google Scholar] [CrossRef]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Dragovic, M. Categorization and validation of handedness using latent class analysis. Acta Neuropsychiatr. 2004, 16, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Winblad, B.; Palmer, K.; Kivipelto, M.; Jelic, V.; Fratiglioni, L.; Wahlund, L.-O.; Nordberg, A.; Bäckman, L.; Albert, M.; Almkvist, O.; et al. Mild cognitive impairment--beyond controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment. J. Intern. Med. 2004, 256, 240–246. [Google Scholar] [CrossRef]
- Petersen, R.C.; Smith, G.E.; Waring, S.C.; Ivnik, R.J.; Tangalos, E.G.; Kokmen, E. Mild cognitive impairment: Clinical characterization and outcome. Arch. Neurol. 1999, 56, 303–308. [Google Scholar] [CrossRef]
- Morris, J.C.; Heyman, A.; Mohs, R.C.; Hughes, J.P.; van Belle, G.; Fillenbaum, G.; Mellits, E.D.; Clark, C. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assesment of Alzheimer′s disease. Neurology 1989, 39, 1159. [Google Scholar] [CrossRef]
- Creavin, S.T.; Wisniewski, S.; Noel-Storr, A.H.; Trevelyan, C.M.; Hampton, T.; Rayment, D.; Thom, V.M.; Nash, K.J.E.; Elhamoui, H.; Milligan, R.; et al. Mini-Mental State Examination (MMSE) for the detection of dementia in clinically unevaluated people aged 65 and over in community and primary care populations. Cochrane Database Syst. Rev. 2016, 1, CD011145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jessen, F.; Spottke, A.; Boecker, H.; Brosseron, F.; Buerger, K.; Catak, C.; Fliessbach, K.; Franke, C.; Fuentes, M.; Heneka, M.T.; et al. Design and first baseline data of the DZNE multicenter observational study on predementia Alzheimer’s disease (DELCODE). Alzheimers. Res. Ther. 2018, 10, 15. [Google Scholar] [CrossRef] [Green Version]
- Memory Clinic Basel. CERAD-Plus Online: Manual zum Auswertungsprogramm; Basel. 2018. Available online: https://www.memoryclinic.ch/fileadmin/user_upload/Memory_Clinic/CERAD-Plus/CERAD-Plus_Online_Benutzeranleitung_2018.pdf (accessed on 31 August 2021).
- Bowie, C.R.; Harvey, P.D. Administration and interpretation of the Trail Making Test. Nat. Protoc. 2006, 1, 2277–2281. [Google Scholar] [CrossRef]
- Crowe, S.F. The differential contribution of mental tracking, cognitive flexibility, visual search, and motor speed to performance on parts A and B of the trail making test. J. Clin. Psychol. 1998, 54, 585–591. [Google Scholar] [CrossRef]
- Tombaugh, T. Trail Making Test A and B: Normative data stratified by age and education. Arch. Clin. Neuropsychol. 2004, 19, 203–214. [Google Scholar] [CrossRef]
- Reitan, R.M. Trail Making Test. Manual for Administration, Scoring, and Interpretation; Reitan Neuropsychology Laboratory: Tucson, Arizona, 1992. [Google Scholar]
- Arbuthnott, K.; Frank, J. Trail Making Test, Part B as a Measure of Executive Control: Validation Using a Set-Switching Paradigm. J. Clin. Exp. Neuropsychol. 2000, 22, 518–528. [Google Scholar] [CrossRef]
- Hashimoto, R.; Meguro, K.; Lee, E.; Kasai, M.; Ishii, H.; Yamaguchi, S. Effect of age and education on the Trail Making Test and determination of normative data for Japanese elderly people: The Tajiri Project. Psychiatry Clin. Neurosci. 2006, 60, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Oosterman, J.M.; Vogels, R.L.C.; van Harten, B.; Gouw, A.A.; Poggesi, A.; Scheltens, P.; Kessels, R.P.C.; Scherder, E.J.A. Assessing mental flexibility: Neuroanatomical and neuropsychological correlates of the Trail Making Test in elderly people. Clin. Neuropsychol. 2010, 24, 203–219. [Google Scholar] [CrossRef]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing 2011, 40, 423–429. [Google Scholar] [CrossRef] [Green Version]
- McGrath, R.; Hackney, K.J.; Ratamess, N.A.; Vincent, B.M.; Clark, B.C.; Kraemer, W.J. Absolute and Body Mass Index Normalized Handgrip Strength Percentiles by Gender, Ethnicity, and Hand Dominance in Americans. Adv. Geriatr. Med. Res. 2020, 2, e200005. [Google Scholar] [CrossRef] [Green Version]
- Cawthon, P.M.; Travison, T.G.; Manini, T.M.; Patel, S.; Pencina, K.M.; Fielding, R.A.; Magaziner, J.M.; Newman, A.B.; Brown, T.; Kiel, D.P.; et al. Establishing the Link Between Lean Mass and Grip Strength Cut-points With Mobility Disability and Other Health Outcomes: Proceedings of the Sarcopenia Definition and Outcomes Consortium Conference. J. Gerontol. Ser. A 2019, 75, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Herold, F.; Behrendt, T.; Törpel, A.; Hamacher, D.; Müller, N.G.; Schega, L. Cortical hemodynamics as a function of handgrip strength and cognitive performance: A cross-sectional fNIRS study in younger adults. BMC Neurosci. 2021, 22, 10. [Google Scholar] [CrossRef]
- Lu, S.; Herold, F.; Zhang, Y.; Lei, Y.; Kramer, A.F.; Jiao, C.; Yu, Q.; Doig, S.; Li, J.; Yan, Z.; et al. Higher Handgrip Strength Is Linked to Better Cognitive Performance in Chinese Adults with Hypertension. Brain Sci. 2021, 11, 985. [Google Scholar] [CrossRef] [PubMed]
- The Jamovi Project. Jamovi (Version 1.6) [Computer Software]. Available online: https://www.jamovi.org (accessed on 1 November 2021).
- Barros, L.A.N.; Ferrari-Piloni, C.; Torres, E.M.; Estrella, C.; Valladares-Neto, J. Effect size: A statistical basis for clinical practice. Rev. Odonto Ciênc. 2018, 33, 84. [Google Scholar] [CrossRef]
- Zasadzka, E.; Pieczyńska, A.; Trzmiel, T.; Kleka, P.; Pawlaczyk, M. Correlation between Handgrip Strength and Depression in Older Adults-A Systematic Review and a Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 4823. [Google Scholar] [CrossRef]
- Zhu, W. p < 0.05, <0.01, <0.001, <0.0001, <0.00001, <0.000001, or <0.0000001 …. J. Sport Health Sci. 2016, 5, 77–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, W. Sadly, the earth is still round (p < 0.05). J. Sport Health Sci. 2012, 1, 9–11. [Google Scholar] [CrossRef] [Green Version]
- Diedenhofen, B.; Musch, J. cocor: A comprehensive solution for the statistical comparison of correlations. PLoS ONE 2015, 10, e0121945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerrero-Berroa, E.; Ravona-Springer, R.; Heymann, A.; Schmeidler, J.; Silverman, J.M.; Sano, M.; Koifmann, K.; Preiss, R.; Hoffman, H.; Schnaider Beeri, M. Decreased motor function is associated with poorer cognitive function in elderly with type 2 diabetes. Dement. Geriatr. Cogn. Dis. Extra 2014, 4, 103–112. [Google Scholar] [CrossRef]
- Hesseberg, K.; Tangen, G.G.; Pripp, A.H.; Bergland, A. Associations between Cognition and Hand Function in Older People Diagnosed with Mild Cognitive Impairment or Dementia. Dement. Geriatr. Cogn. Dis. Extra 2020, 10, 195–204. [Google Scholar] [CrossRef]
- Yaffe, K.; Petersen, R.C.; Lindquist, K.; Kramer, J.; Miller, B. Subtype of mild cognitive impairment and progression to dementia and death. Dement. Geriatr. Cogn. Disord. 2006, 22, 312–319. [Google Scholar] [CrossRef]
- Glynn, K.; O’Callaghan, M.; Hannigan, O.; Bruce, I.; Gibb, M.; Coen, R.; Green, E.; Lawlor, B.A.; Robinson, D. Clinical utility of mild cognitive impairment subtypes and number of impaired cognitive domains at predicting progression to dementia: A 20-year retrospective study. Int. J. Geriatr. Psychiatry 2021, 36, 31–37. [Google Scholar] [CrossRef]
- Csukly, G.; Sirály, E.; Fodor, Z.; Horváth, A.; Salacz, P.; Hidasi, Z.; Csibri, É.; Rudas, G.; Szabó, Á. The Differentiation of Amnestic Type MCI from the Non-Amnestic Types by Structural MRI. Front. Aging Neurosci. 2016, 8, 52. [Google Scholar] [CrossRef] [Green Version]
- Montero-Odasso, M.; Oteng-Amoako, A.; Speechley, M.; Gopaul, K.; Beauchet, O.; Annweiler, C.; Muir-Hunter, S.W. The motor signature of mild cognitive impairment: Results from the gait and brain study. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 1415–1421. [Google Scholar] [CrossRef]
- Funahashi, S.; Andreau, J.M. Prefrontal cortex and neural mechanisms of executive function. J. Physiol. Paris 2013, 107, 471–482. [Google Scholar] [CrossRef] [Green Version]
- Miller, E.K. The prefrontal cortex and cognitive control. Nat. Rev. Neurosci. 2000, 1, 59–65. [Google Scholar] [CrossRef]
- Müller, L.D.; Guhn, A.; Zeller, J.B.M.; Biehl, S.C.; Dresler, T.; Hahn, T.; Fallgatter, A.J.; Polak, T.; Deckert, J.; Herrmann, M.J. Neural correlates of a standardized version of the trail making test in young and elderly adults: A functional near-infrared spectroscopy study. Neuropsychologia 2014, 56, 271–279. [Google Scholar] [CrossRef]
- Shibuya-Tayoshi, S.; Sumitani, S.; Kikuchi, K.; Tanaka, T.; Tayoshi, S.; Ueno, S.-I.; Ohmori, T. Activation of the prefrontal cortex during the Trail-Making Test detected with multichannel near-infrared spectroscopy. Psychiatry Clin. Neurosci. 2007, 61, 616–621. [Google Scholar] [CrossRef]
- Zakzanis, K.K.; Mraz, R.; Graham, S.J. An fMRI study of the Trail Making Test. Neuropsychologia 2005, 43, 1878–1886. [Google Scholar] [CrossRef]
- Hagen, K.; Ehlis, A.-C.; Haeussinger, F.B.; Heinzel, S.; Dresler, T.; Mueller, L.D.; Herrmann, M.J.; Fallgatter, A.J.; Metzger, F.G. Activation during the Trail Making Test measured with functional near-infrared spectroscopy in healthy elderly subjects. NeuroImage 2014, 85 Pt 1, 583–591. [Google Scholar] [CrossRef]
- Kubo, M.; Shoshi, C.; Kitawaki, T.; Takemoto, R.; Kinugasa, K.; Yoshida, H.; Honda, C.; Okamoto, M. Increase in prefrontal cortex blood flow during the computer version trail making test. Neuropsychobiology 2008, 58, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Talwar, N.; Churchill, N.W.; Hird, M.A.; Tam, F.; Graham, S.J.; Schweizer, T.A. Functional magnetic resonance imaging of the trail-making test in older adults. PLoS ONE 2020, 15, e0232469. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, P.S.; Wen, W.; Christensen, H.; Jorm, A.F. White matter hyperintensities are related to physical disability and poor motor function. J. Neurol. Neurosurg. Psychiatry 2005, 76, 362–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herold, F.; Törpel, A.; Schega, L.; Müller, N.G. Functional and/or structural brain changes in response to resistance exercises and resistance training lead to cognitive improvements—A systematic review. Eur. Rev. Aging Phys. Act. 2019, 16, 1676. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Hong, K.-S.; Yoo, S.-H.; Kim, C.-S. Evaluation of Neural Degeneration Biomarkers in the Prefrontal Cortex for Early Identification of Patients with Mild Cognitive Impairment: An fNIRS Study. Front. Hum. Neurosci. 2019, 13, 367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, J.A.; Kong, I.J.; Choi, J.; Baek, J.Y.; Kim, E.J.; Shin, Y.-I.; Ko, M.-H.; Shin, Y.B.; Shin, M.J. Neural Compensatory Response During Complex Cognitive Function Tasks in Mild Cognitive Impairment: A Near-Infrared Spectroscopy Study. Neural Plast. 2019, 2019, 7845104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yap, K.H.; Ung, W.C.; Ebenezer, E.G.M.; Nordin, N.; Chin, P.S.; Sugathan, S.; Chan, S.C.; Yip, H.L.; Kiguchi, M.; Tang, T.B. Visualizing Hyperactivation in Neurodegeneration Based on Prefrontal Oxygenation: A Comparative Study of Mild Alzheimer’s Disease, Mild Cognitive Impairment, and Healthy Controls. Front. Aging Neurosci. 2017, 9, 459. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Sun, H.; Dong, X.; Liu, B.; Xu, Y.; Chen, S.; Song, L.; Zhang, H.; Wang, X. Executive dysfunction and gray matter atrophy in amnestic mild cognitive impairment. Neurobiol. Aging 2014, 35, 548–555. [Google Scholar] [CrossRef] [Green Version]
- Grambaite, R.; Selnes, P.; Reinvang, I.; Aarsland, D.; Hessen, E.; Gjerstad, L.; Fladby, T. Executive dysfunction in mild cognitive impairment is associated with changes in frontal and cingulate white matter tracts. J. Alzheimers. Dis. 2011, 27, 453–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allali, G.; Montembeault, M.; Saj, A.; Wong, C.H.; Cooper-Brown, L.A.; Bherer, L.; Beauchet, O. Structural Brain Volume Covariance Associated with Gait Speed in Patients with Amnestic and Non-Amnestic Mild Cognitive Impairment: A Double Dissociation. J. Alzheimers. Dis. 2019, 71, S29–S39. [Google Scholar] [CrossRef] [Green Version]
- Rubin, R.D.; Schwarb, H.; Lucas, H.D.; Dulas, M.R.; Cohen, N.J. Dynamic Hippocampal and Prefrontal Contributions to Memory Processes and Representations Blur the Boundaries of Traditional Cognitive Domains. Brain Sci. 2017, 7, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eichenbaum, H. Prefrontal-hippocampal interactions in episodic memory. Nat. Rev. Neurosci. 2017, 18, 547–558. [Google Scholar] [CrossRef]
- Junquera, A.; García-Zamora, E.; Olazarán, J.; Parra, M.A.; Fernández-Guinea, S. Role of Executive Functions in the Conversion from Mild Cognitive Impairment to Dementia. J. Alzheimers. Dis. 2020, 77, 641–653. [Google Scholar] [CrossRef]
- Tabert, M.H.; Manly, J.J.; Liu, X.; Pelton, G.H.; Rosenblum, S.; Jacobs, M.; Zamora, D.; Goodkind, M.; Bell, K.; Stern, Y.; et al. Neuropsychological prediction of conversion to Alzheimer disease in patients with mild cognitive impairment. Arch. Gen. Psychiatry 2006, 63, 916–924. [Google Scholar] [CrossRef] [Green Version]
- Schmidtke, K.; Hermeneit, S. High rate of conversion to Alzheimer’s disease in a cohort of amnestic MCI patients. Int. Psychogeriatr. 2008, 20, 96–108. [Google Scholar] [CrossRef]
- Jungwirth, S.; Zehetmayer, S.; Hinterberger, M.; Tragl, K.H.; Fischer, P. The validity of amnestic MCI and non-amnestic MCI at age 75 in the prediction of Alzheimer’s dementia and vascular dementia. Int. Psychogeriatr. 2012, 24, 959–966. [Google Scholar] [CrossRef]
- Ding, D.; Zhao, Q.; Guo, Q.; Liang, X.; Luo, J.; Yu, L.; Zheng, L.; Hong, Z. Progression and predictors of mild cognitive impairment in Chinese elderly: A prospective follow-up in the Shanghai Aging Study. Alzheimers. Dement. 2016, 4, 28–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.-K.; Pan, C.-Y.; Chen, F.-T.; Tsai, C.-L.; Huang, C.-C. Effect of Resistance-Exercise Training on Cognitive Function in Healthy Older Adults: A Review. J. Aging Phys. Act. 2012, 20, 497–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coelho-Junior, H.; Marzetti, E.; Calvani, R.; Picca, A.; Arai, H.; Uchida, M. Resistance training improves cognitive function in older adults with different cognitive status: A systematic review and Meta-analysis. Aging Ment. Health 2020, 26, 1–12. [Google Scholar] [CrossRef]
- Landrigan, J.-F.; Bell, T.; Crowe, M.; Clay, O.J.; Mirman, D. Lifting cognition: A meta-analysis of effects of resistance exercise on cognition. Psychol. Res. 2019, 84, 1167–1183. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Wang, X.; Ye, M.; Wang, L.; Zheng, G. Effect of regular resistance training on memory in older adults: A systematic review. Exp. Gerontol. 2021, 150, 111396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, B.; Yang, J.; Wang, F.; Tang, Q.; Wang, S. Meta-Analysis: Resistance Training Improves Cognition in Mild Cognitive Impairment. Int. J. Sports Med. 2020, 41, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Suo, C.; Singh, M.F.; Gates, N.; Wen, W.; Sachdev, P.; Brodaty, H.; Saigal, N.; Wilson, G.C.; Meiklejohn, J.; Singh, N.; et al. Therapeutically relevant structural and functional mechanisms triggered by physical and cognitive exercise. Mol. Psychiatry 2016, 21, 1633–1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broadhouse, K.M.; Singh, M.F.; Suo, C.; Gates, N.; Wen, W.; Brodaty, H.; Jain, N.; Wilson, G.C.; Meiklejohn, J.; Singh, N.; et al. Hippocampal plasticity underpins long-term cognitive gains from resistance exercise in MCI. NeuroImage: Clin. 2020, 25, 102182. [Google Scholar] [CrossRef]
- Rothman, K.J. No adjustments are needed for multiple comparisons. Epidemiology 1990, 1, 43–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bender, R.; Lange, S. Adjusting for multiple testing—when and how? J. Clin. Epidemiol. 2001, 54, 343–349. [Google Scholar] [CrossRef]
- Perneger, T.V. What’s wrong with Bonferroni adjustments. BMJ 1998, 316, 1236–1238. [Google Scholar] [CrossRef] [PubMed]
| General Characteristics of the Participants | Median ± Interquartile Range (Minimum to Maximum) | ||
|---|---|---|---|
| aMCI (n = 22) | naMCI (n = 21) | HC (n = 27) | |
| Female/Male (n) | 14/8 | 9/12 | 19/8 |
| Age (years) | 69 ± 9 (60 to 81) | 71 ± 8 (56 to 80) | 68 ± 10 (54 to 83) |
| Body height (cm) | 171.0 ± 11.0 * (150.0 to 184.0) | 173.0 ± 13.0 # (159.0 to 189.0) | 165.0 ± 9.5 (156.0 to 179.0) |
| Body mass (kg) | 72.0 ± 15.0 (61.0 to 93.0) | 77.0 ± 8.0 (54.4 to 94.4) | 67.0 ± 22.5 (50.0 to 94.0) |
| BMI (kg/m2) | 24.1 ± 4.2 (20.9 to 29.1) | 25.8 ± 1.6 (21.4 to 28.5) | 24.7 ± 5.9 (19.3 to 31.0) |
| Educational level (years) | 15 ± 4 (11 to 20) | 15 ± 3 (11 to 18) | 15 ± 3 (12 to 18) |
| GDS (total score) | 1.5 ± 3.0 (0.0 to 4.0) | 2.0 ± 2.0 # (0.0 to 5.0) | 1.0 ± 1.5 (0.0 to 3.0) |
| EHI (score) | 100.0 ± 23.3 (52.9 to 100.0) | 100.0 ± 0.0 (73.3 to 100.0) | 100.0 ± 21.1 (53.9 to 100.0) |
| nHGS left/right (a.u.) | 1.05 ± 0.76/1.12 ± 0.62/ (0.75 to 2.24/0.78 to 2.03) | 1.37 ± 0.51 a/1.43 ± 0.70 (0.84 to 2.20/0.79 to 2.23) | 1.12 ± 0.33/1.21 ± 0.42 (0.47 to 1.75/0.66 to 1.89) |
| TMT B/A (z-score) | −0.18 ± 1.20 (−2.13 to 2.07)) | −0.47 ± 0.89 (−1.43 to 1.76) | −0.32 ± 0.74 (−2.18 to 1.84) |
| MMSE (points) | 27.0 ± 1.8 * (25.0 to 30.0) | 27.0 ± 2.0 # (24.0 to 30.0) | 28.0 ± 1.0 (27.0 to 30.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herold, F.; Labott, B.K.; Grässler, B.; Halfpaap, N.; Langhans, C.; Müller, P.; Ammar, A.; Dordevic, M.; Hökelmann, A.; Müller, N.G. A Link between Handgrip Strength and Executive Functioning: A Cross-Sectional Study in Older Adults with Mild Cognitive Impairment and Healthy Controls. Healthcare 2022, 10, 230. https://doi.org/10.3390/healthcare10020230
Herold F, Labott BK, Grässler B, Halfpaap N, Langhans C, Müller P, Ammar A, Dordevic M, Hökelmann A, Müller NG. A Link between Handgrip Strength and Executive Functioning: A Cross-Sectional Study in Older Adults with Mild Cognitive Impairment and Healthy Controls. Healthcare. 2022; 10(2):230. https://doi.org/10.3390/healthcare10020230
Chicago/Turabian StyleHerold, Fabian, Berit K. Labott, Bernhard Grässler, Nicole Halfpaap, Corinna Langhans, Patrick Müller, Achraf Ammar, Milos Dordevic, Anita Hökelmann, and Notger G. Müller. 2022. "A Link between Handgrip Strength and Executive Functioning: A Cross-Sectional Study in Older Adults with Mild Cognitive Impairment and Healthy Controls" Healthcare 10, no. 2: 230. https://doi.org/10.3390/healthcare10020230
APA StyleHerold, F., Labott, B. K., Grässler, B., Halfpaap, N., Langhans, C., Müller, P., Ammar, A., Dordevic, M., Hökelmann, A., & Müller, N. G. (2022). A Link between Handgrip Strength and Executive Functioning: A Cross-Sectional Study in Older Adults with Mild Cognitive Impairment and Healthy Controls. Healthcare, 10(2), 230. https://doi.org/10.3390/healthcare10020230










