Comparison of Different Self-Sampling Devices for Molecular Detection of Human Papillomavirus (HPV) and Other Sexually Transmitted Infections (STIs): A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Sample Collection
2.2. DNA Extraction; HPV and STI Detection
2.3. Statistical Analysis
3. Results
3.1. Study Population
3.2. HPV Detection and Typing
3.3. STI Prevalence
3.4. Patient Satisfaction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- World Health Organization. GLOBOCAN 2018: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2018. Available online: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx (accessed on 23 April 2021).
- Bosch, F.X.; Lorincz, A.; Muñoz, N.; Meijer, C.J.; Shah, K.V. The causal relation between human papillomavirus and cervical cancer. J. Clin. Pathol. 2002, 55, 244–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walboomers, J.M.; Jacobs, M.V.; Manos, M.M.; Bosch, F.X.; Kummer, J.A.; Shah, K.V.; Snijders, P.J.; Peto, J.; Meijer, C.J.; Muñoz, N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999, 189, 12–19. [Google Scholar] [CrossRef]
- Ronco, G.; Biggeri, A.; Confortini, M.; Naldoni, C.; Segnan, N.; Sideri, M.; Zappa, M.; Zorzi, M.; Calvia, M.; Accetta, G.; et al. Health technology assessment report: Ricerca del DNA di papillomavirus umano (HPV) come test primario per lo screening dei precursori del cancro del collo dell’utero [Health technology assessment report: HPV DNA based primary screening for cervical cancer precursors]. Epidemiol. Prev. 2012, 36 (Suppl. S1), e1-72. [Google Scholar] [PubMed]
- Mao, C.; Kulasingam, S.L.; Whitham, H.K.; Hawes, S.E.; Lin, J.; Kiviat, N.B. Clinician and Patient Acceptability of Self-Collected Human Papillomavirus Testing for Cervical Cancer Screening. J. Women’s Health 2017, 26, 609–615. [Google Scholar] [CrossRef]
- Gupta, S.; Palmer, C.; Bik, E.M.; Cardenas, J.P.; Nuñez, H.; Kraal, L.; Bird, S.W.; Bowers, J.; Smith, A.; Walton, N.A.; et al. Self-Sampling for Human Papillomavirus Testing: Increased Cervical Cancer Screening Participation and Incorporation in International Screening Programs. Front. Public Health 2018, 9, 77. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhu, L.; Li, H.; Ma, N.; Huang, H.; Zhang, X.; Li, Y.; Fang, J. Association between asymptomatic sexually transmitted infections and high-risk human papillomavirus in cervical lesions. J. Int. Med. Res. 2019, 47, 5548–5589. [Google Scholar] [CrossRef]
- Martinelli, M.; Musumeci, R.; Sechi, I.; Sotgiu, G.; Piana, A.; Perdoni, F.; Sina, F.; Fruscio, R.; Landoni, F.; Cocuzza, C.E. Prevalence of Human Papillomavirus (HPV) and Other Sexually Transmitted Infections (STIs) among Italian Women Referred for a Colposcopy. Int. J. Environ. Res. Public Health 2019, 16, 5000. [Google Scholar] [CrossRef] [Green Version]
- Martinelli, M.; Musumeci, R.; Rizzo, A.; Muresu, N.; Piana, A.; Sotgiu, G.; Landoni, F.; Cocuzza, C. Prevalence of Chlamydia trachomatis Infection, Serovar Distribution and Co-Infections with Seven High-Risk HPV Types among Italian Women with a Recent History of Abnormal Cervical Cytology. Int. J. Environ. Res. Public Health 2019, 16, 3354. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Shen, Z.; Luo, H.; Zhang, W.; Zhu, X. Chlamydia Trachomatis Infection-Associated Risk of Cervical Cancer: A Meta-Analysis. Medicine 2016, 95, e3077. [Google Scholar] [CrossRef]
- Schmauz, R.; Okong, P.; de Villiers, E.M.; Dennin, R.; Brade, L.; Lwanga, S.K.; Owor, R. Multiple infections in cases of cervical cancer from a high-incidence area in tropical Africa. Int. J. Cancer 1989, 15, 805–809. [Google Scholar] [CrossRef]
- Gaydos, C.A.; Rizzo-Price, P.A.; Barnes, M.; Dwyer, K.; Wood, B.J.; Hogan, M.T. The use of focus groups to design an internet-based program for chlamydia screening with self-administered vaginal swabs: What women want. Sex Health 2006, 3, 209–215. [Google Scholar] [CrossRef]
- Graseck, A.S.; Shih, S.L.; Peipert, J.F. Home versus clinic-based specimen collection for Chlamydia trachomatis and Neisseria gonorrhoeae. Expert. Rev. Anti. Infect Ther. 2011, 9, 183–194. [Google Scholar] [CrossRef] [Green Version]
- Lockhart, A.; Psioda, M.; Ting, J.; Campbell, S.; Mugo, N.; Kwatampora, J.; Chitwa, M.; Kimani, J.; Gakure, A.; Smith, J.S. Prospective Evaluation of Cervicovaginal Self- and Cervical Physician Collection for the Detection of Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis, and Mycoplasma genitalium Infections. Sex Transm. Dis. 2018, 45, 488–493. [Google Scholar] [CrossRef]
- Lunny, C.; Taylor, D.; Hoang, L.; Wong, T.; Gilbert, M.; Lester, R.; Krajden, M.; Ogilvie, G. Self-Collected versus Clinician-Collected Sampling for Chlamydia and Gonorrhea Screening: A Systemic Review and Meta-Analysis. PLoS ONE 2015, 10, e0132776. [Google Scholar] [CrossRef]
- Garrow, S.C.; Smith, D.W.; Harnett, G.B. The diagnosis of chlamydia, gonorrhoea, and trichomonas infections by self obtained low vaginal swabs, in remote northern Australian clinical practice. Sex Transm. Infect 2002, 78, 278–281. [Google Scholar] [CrossRef] [Green Version]
- Knox, J.; Tabrizi, S.N.; Miller, P.; Petoumenos, K.; Law, M.; Chen, S.; Garland, S.M. Evaluation of self-collected samples in contrast to practitioner-collected samples for detection of Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis by polymerase chain reaction among women living in remote areas. Sex Transm. Dis. 2002, 29, 647–654. [Google Scholar] [CrossRef]
- Nayar, R.; Wilbur, D.C. The Pap test and Bethesda 2014. Cancer Cytopathol. 2015, 123, 271–281. [Google Scholar] [CrossRef]
- EPICENTRO. Available online: https://www.epicentro.iss.it/passi/comunicazione/nazionali/nazionali (accessed on 23 April 2021).
- Marlow, L.A.V.; Waller, J.; Wardle, J. Barriers to cervical cancer screening among ethnic minority woman: A qualitative study. J. Fam. Plann. Reprod. Health Care 2015, 41, 248–254. [Google Scholar] [CrossRef] [Green Version]
- Arbyn, M.; Verdoodt, F.; Snijders, P.J.F.; Verhoef, V.M.J.; Suonio, E.; Dillner, L.; Minozzi, S.; Bellisario, C.; Banzi, R.; Zhao, F.-H.; et al. Accuracy of human papillomavirus testing on self-collected versus clinician-collected samples: A meta analysis. Lancet Oncol. 2014, 15, 172–183. [Google Scholar] [CrossRef]
- Cho, H.W.; Hong, J.H.; Min, K.J.; Ouh, Y.T.; Seong, S.J.; Moon, J.H.; Cho, S.H.; Lee, J.K. Performance and Diagnostic Accuracy of Human Papillomavirus Testing on Self-Collected Urine and Vaginal Samples in a Referral Population. Cancer Res. Treat. 2021, 53, 829–836. [Google Scholar] [CrossRef]
- Arbyn, M.; Smith, S.B.; Temin, S.; Sultana, F.; Castle, P. Collaboration on Self-Sampling and HPV Testing. Detecting cervical precancer and reaching underscreened women by using HPV testing on self-samples: Updated meta-analyses. BMJ 2018, 363, k4823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, F.H.; Lewkowitz, A.K.; Chen, F.; Lin, M.J.; Hu, S.Y.; Zhang, X.; Pan, Q.J.; Ma, J.F.; Niyazi, M.; Li, C.Q.; et al. Pooled analysis of a self-sampling HPV DNA Test as a cervical cancer primary screening method. J. Natl. Cancer Inst. 2012, 104, 178–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuriakose, S.; Sabeena, S.; Binesh, D.; Abdulmajeed, J.; Ravishankar, N.; Ramachandran, A.; Vijaykumar, B.; Ameen, N. Diagnostic accuracy of self-collected vaginal samples for HPV DNA detection in women from South India. Int. J. Gynaecol. Obstet. 2020, 149, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Magdi, R.; Elshafeey, F.; Elshebiny, M.; Kamel, M.; Abuelnaga, Y.; Ghonim, M.; Nabhan, A.; ECEBM Working Group. A systematic review and meta-analysis of diagnostic accuracy of HPV tests for the screening of cervical cancer in low-resource settings. Int. J. Gynaecol. Obstet. 2021, 152, 12–18. [Google Scholar] [CrossRef]
- Arbyn, M.; Peeters, E.; Benoy, I.; Vanden Broeck, D.; Bogers, J.; De Sutter, P.; Donders, G.; Tjalma, W.; Weyers, S.; Cuschieri, K.; et al. VALHUDES: A protocol for validation of human papillomavirus assays and collections devices for HPV testing on self-samples and urine samples. J. Clin. Virol. 2018, 107, 52–56. [Google Scholar] [CrossRef] [Green Version]
- Haguenoer, K.; Giraudeau, B.; Gaudy-Graffin, C.; de Pinieux, I.; Dubois, F.; Trignol-Viguier, N.; Viguier, J.; Marret, H.; Goudeau, A. Accuracy of dry vaginal self-sampling for detecting high-risk human papillomavirus infection in cervical cancer screening: A cross-sectional study. Gynecol. Oncol. 2014, 134, 302–308. [Google Scholar] [CrossRef]
- Ertik, F.C.; Kampers, J.; Hülse, F.; Stolte, C.; Böhmer, G.; Hillemanns, P.; Jentschke, M. CoCoss-Trial: Concurrent Comparison of Self-Sampling Devices for HPV-Detection. Int. J. Environ. Res. Public Health 2021, 18, 10388. [Google Scholar] [CrossRef]
- Bokan, T.; Ivanus, U.; Jerman, T.; Takac, I.; Arko, D. Long term results of follow-up after HPV self-sampling with devices Qvintip and HerSwab in women non-attending cervical screening programme. Radiol. Oncol. 2021, 55, 187–195. [Google Scholar] [CrossRef]
- Richman, A.R.; Brewer, N.T.; Liebman, A.K.; Rinas, A.C.; Smith, J.S. Optimising human papillomavirus self-testing for high risk women. Sex Transm. Infect 2011, 87, 118–122. [Google Scholar] [CrossRef]
- Mahomed, K.; Evans, D.; Sauls, C.; Richter, K.; Smith, J.; Firnhaber, C. Human papillomavirus (HPV) testing on self-collected specimens: Perceptions among HIV positive women attending rural and urban clinics in South Africa. Pan. Afr. Med. J. 2014, 17, 189. [Google Scholar] [CrossRef]
- Lippman, S.A.; Jones, H.E.; Luppi, C.G.; Pinho, A.A.; Veras, M.A.; van de Wijgert, J.H. Home-based self-sampling and self-testing for sexually transmitted infections: Acceptable and feasible alternatives to provider-based screening in low-income women in São Paulo, Brazil. Sex Transm. Dis. 2007, 34, 421–428. [Google Scholar] [CrossRef]
- McLarty, J.W.; Williams, D.L.; Loyd, S.; Hagensee, M.E. Cervical Human Papillomavirus Testing with Two Home Self-Collection Methods Compared With a Standard Clinically Collected Sampling Method. Sex Transm. Dis. 2019, 46, 670–675. [Google Scholar] [CrossRef]
- Bishop, E.; Katz, M.L.; Reiter, P.L. Acceptability of Human Papillomavirus Self-Sampling Among a National Sample of Women in the United States. BioRes. Open Access 2019, 8, 65–73. [Google Scholar] [CrossRef]
- Shin, H.Y.; Lee, B.; Hwang, S.H.; Lee, D.O.; Sung, N.Y.; Park, J.Y.; Jun, J.K. Evaluation of satisfaction with three different cervical cancer screening modalities: Clinician-collected Pap test vs. HPV test by self-sampling vs. HPV test by urine sampling. J. Gynecol. Oncol. 2019, 30, e76. [Google Scholar] [CrossRef]
- Abdullah, N.N.; Daud, S.; Wang, S.M.; Mahmud, Z.; Mohd Kornain, N.K.; Al-Kubaisy, W. Human Papilloma Virus (HPV) self-sampling: Do women accept it? J. Obstet. Gynaecol. 2018, 38, 402–407. [Google Scholar] [CrossRef]
- Virtanen, A.; Nieminen, P.; Niironen, M.; Luostarinen, T.; Anttila, A. Self-sampling experiences among non-attendees to cervical screening. Gynecol. Oncol. 2014, 135, 487–494. [Google Scholar] [CrossRef]



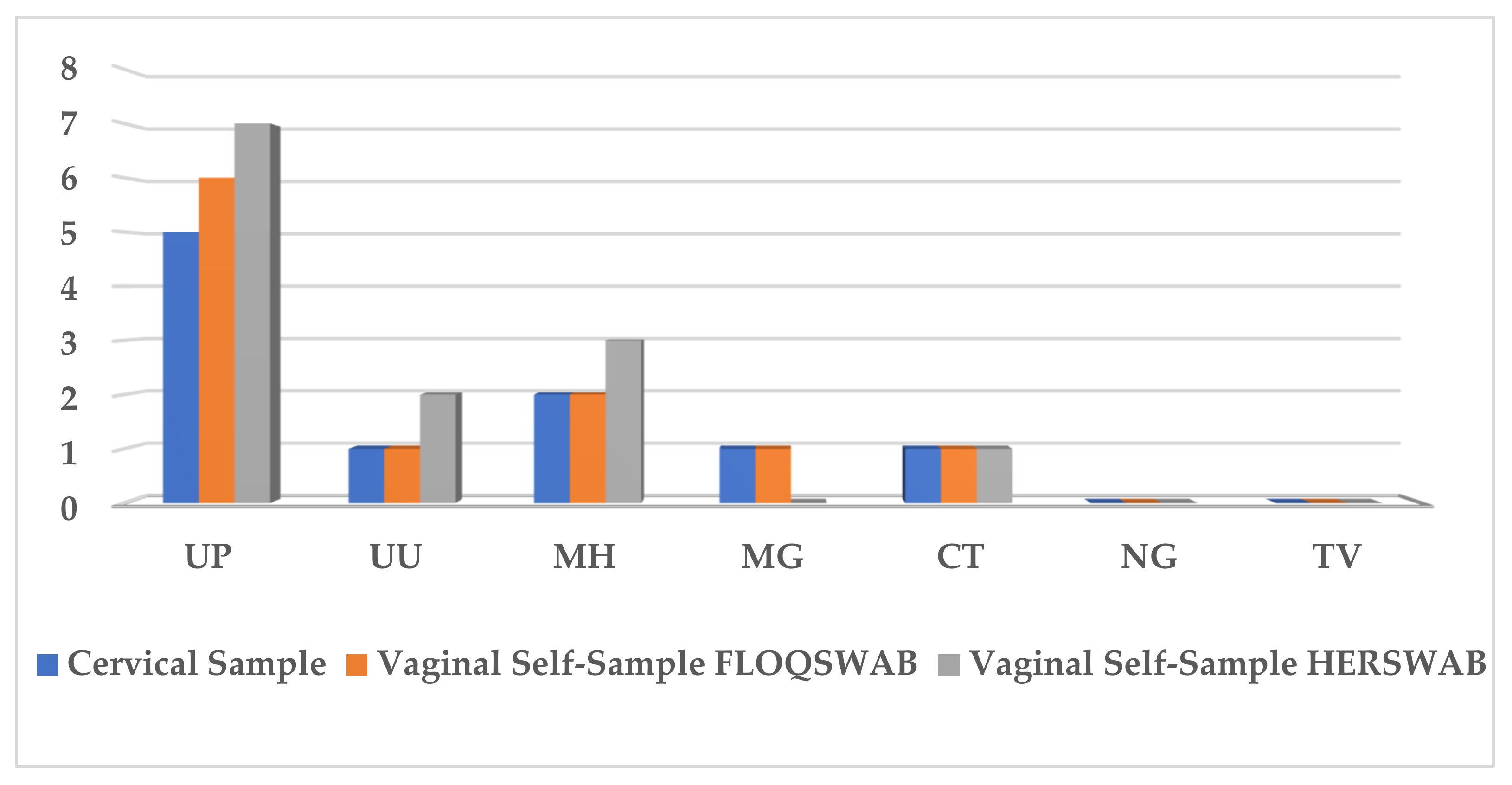
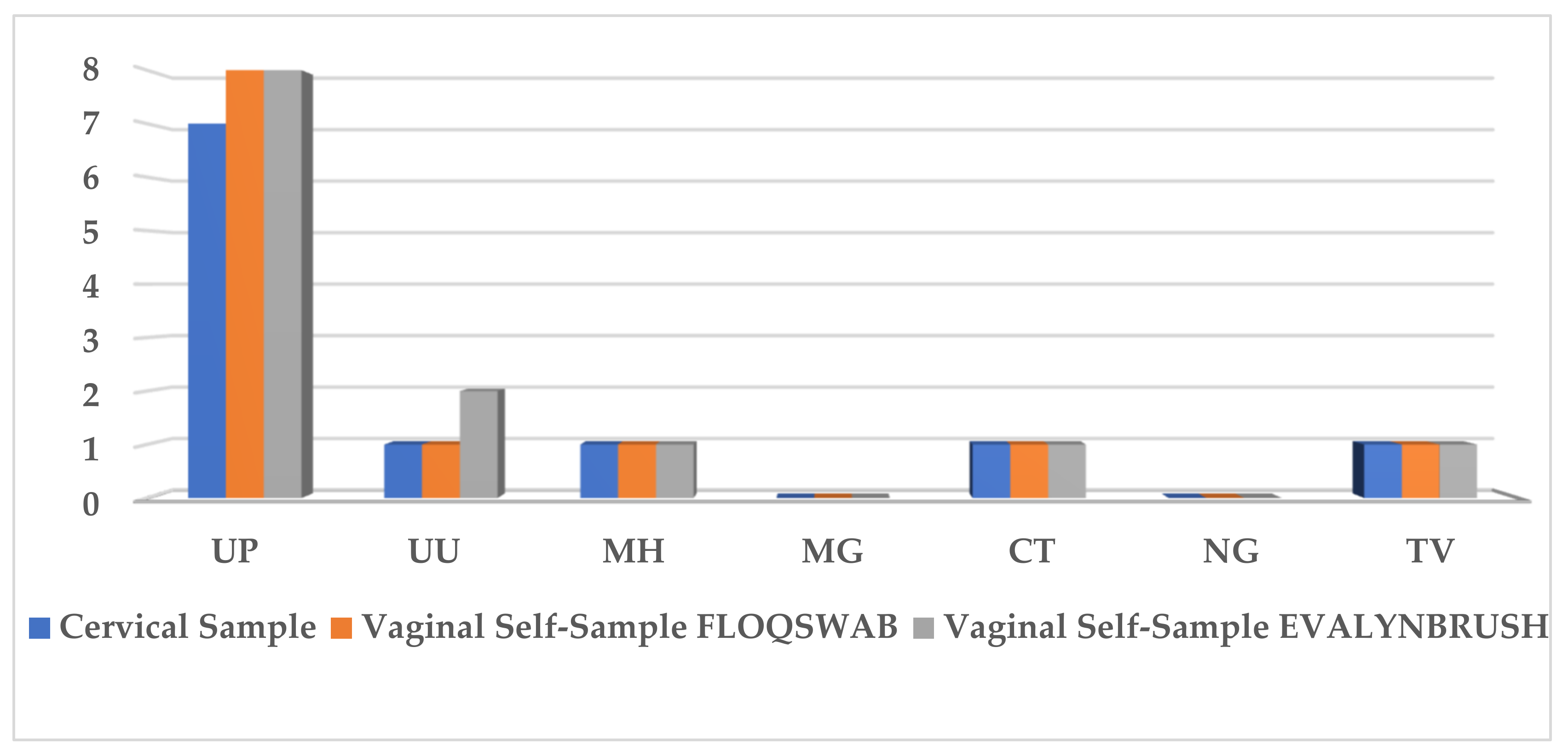
| Cytology | HSIL | n (%) | Total n |
| 5 (12.5) | 40 | ||
| ASCH | 1 (2.5) | ||
| LSIL | 16 (40) | ||
| AGC-NOS | 2 (5) | ||
| ASCUS | 16 (40) | ||
| Colposcopy examination | Cervical abnormality | 10 (25) | 40 |
| Negative | 30 (75) | ||
| Histology | CIN 1 | 2 (20) | 10 |
| CIN 3 | 4 (40) | ||
| Cervical Cancer | 1 (10) | ||
| Negative | 3 (30) |
| Cytology | Histology | hrHPV Types Detected | |||
|---|---|---|---|---|---|
| Cervical Sample Collection | FS Vaginal Self-Samples | HB Vaginal Self-Samples | EB Vaginal Self-Samples | ||
| LSIL | CIN 1 | 66 | 51, 66 | 51, 66 | - |
| AGCUS | Cervical Cancer | 16 | 16 | 16 | - |
| LSIL | CIN 1 | 16 | 16 | 16 | - |
| HSIL | CIN 3 | 16 | 16 | 16 | - |
| HSIL | CIN 3 | 16 | 16 | 16 | - |
| ASCUS | CIN 3 | 52 | 31, 52 | 31, 52 | - |
| HSIL | Negative | 16 | 16 | - | 16 |
| HSIL | CIN 3 | 16 | 16 | - | 16 |
| ASCH | Negative | 16 | 16, 18, 56 | - | 16, 18, 56 |
| ASCUS | Negative | 35 | 35 | - | 35 |
| FS (n: 40) | HS (n: 20) | EB (n: 20) | ||||
|---|---|---|---|---|---|---|
| YES | NO | YES | NO | YES | NO | |
| Did the swab cause discomfort during sample collection? | 2 (5%) | 38 (95%) | 0 (0%) | 20 (100%) | 0 (0%) | 20 (100%) |
| Did the swab cause bleeding during sample collection? | 2 (5%) | 38 (95%) | 2 (10%) | 18 (90%) | 0 (0%) | 20 (100%) |
| Were self-collection instructions clear? | 38 (95%) | 2 (5%) | 18 (90%) | 2 (10%) | 19 (95%) | 1 (5%) |
| Was self-collection acceptable and easy to perform? | 40 (100%) | 0 (0%) | 19 (95%) | 1 (5%) | 20 (100%) | 0 (0%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sechi, I.; Cocuzza, C.E.; Martinelli, M.; Muresu, N.; Castriciano, S.; Sotgiu, G.; Piana, A. Comparison of Different Self-Sampling Devices for Molecular Detection of Human Papillomavirus (HPV) and Other Sexually Transmitted Infections (STIs): A Pilot Study. Healthcare 2022, 10, 459. https://doi.org/10.3390/healthcare10030459
Sechi I, Cocuzza CE, Martinelli M, Muresu N, Castriciano S, Sotgiu G, Piana A. Comparison of Different Self-Sampling Devices for Molecular Detection of Human Papillomavirus (HPV) and Other Sexually Transmitted Infections (STIs): A Pilot Study. Healthcare. 2022; 10(3):459. https://doi.org/10.3390/healthcare10030459
Chicago/Turabian StyleSechi, Illari, Clementina Elvezia Cocuzza, Marianna Martinelli, Narcisa Muresu, Santina Castriciano, Giovanni Sotgiu, and Andrea Piana. 2022. "Comparison of Different Self-Sampling Devices for Molecular Detection of Human Papillomavirus (HPV) and Other Sexually Transmitted Infections (STIs): A Pilot Study" Healthcare 10, no. 3: 459. https://doi.org/10.3390/healthcare10030459
APA StyleSechi, I., Cocuzza, C. E., Martinelli, M., Muresu, N., Castriciano, S., Sotgiu, G., & Piana, A. (2022). Comparison of Different Self-Sampling Devices for Molecular Detection of Human Papillomavirus (HPV) and Other Sexually Transmitted Infections (STIs): A Pilot Study. Healthcare, 10(3), 459. https://doi.org/10.3390/healthcare10030459










