Role of Artificial Intelligence Interpretation of Colposcopic Images in Cervical Cancer Screening
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Patients and Terminology
2.2. Preparation of Machine Learning System
- (1)
- Satisfactory filtering module was introduced to differentiate whether the taken colposcopic image is adequately satisfied for screening. This module is implemented by a convolutional neural network (CNN)-based classification model, which was trained to yield binary results that consist of satisfactory and unsatisfactory.
- (2)
- Preprocessing and normalization module was applied to prepare and adjust the image before AI interpretation. Colposcopic images are usually captured in uncontrolled environments, which result in various quality of the taken images such as poor contrast, brightness, etc. To compensate and improve the quality of the images, an auto-adjustment algorithm was implemented to preprocess and normalize them by applying various thresholding and filtering methods.
- (3)
- Feature extraction and cervical cancer diagnosis module have an important role in exploring the regions of the colposcopic images which correspond to suspicious precancerous cervical lesions. This module is implemented by CNN-based multi-class detection model named AIDOTNet v1.2, which was trained with multi-category images that consists the location of low and high-grade lesions. AIDOTNet v1.2 utilizes a pre-trained model to extract the suspicious region from a given image for predicting the lesion location in the image. In other words, the model leverages the feature extraction from the pre-trained model to locate the suspicious lesion box in the image and finally classifies the detected box as CIN1, CIN2-3, or cancer lesion. However, if no suspicious lesion box is detected from the colposcopic image, the model will yield normal as the AI interpretation result.
2.3. Clinical Interpretation of Colposcopic Finding
2.4. Statistical Analysis
3. Results
3.1. Patient and Disease Characteristics
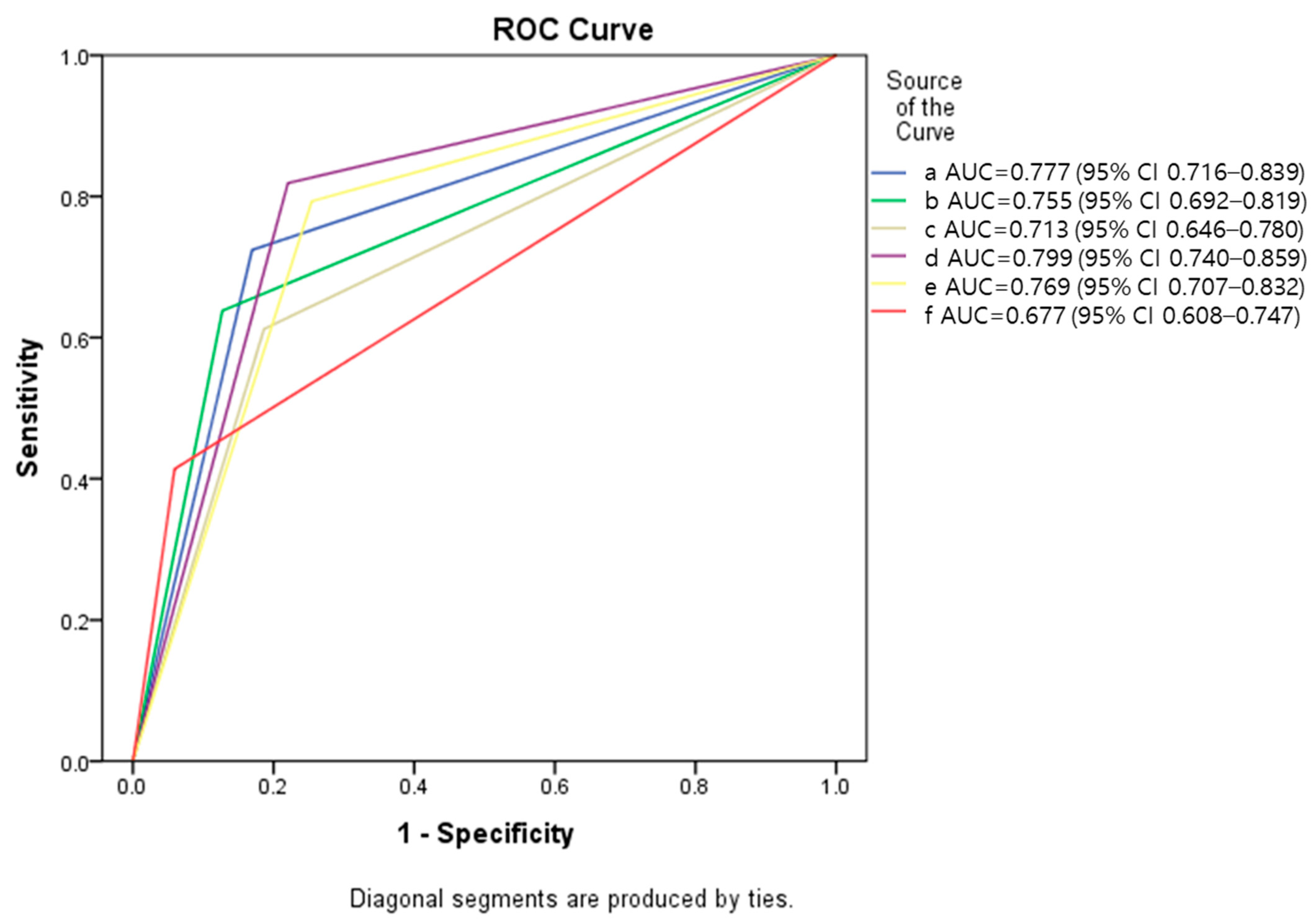
3.2. Evaluation of Diagnostic Accuracy
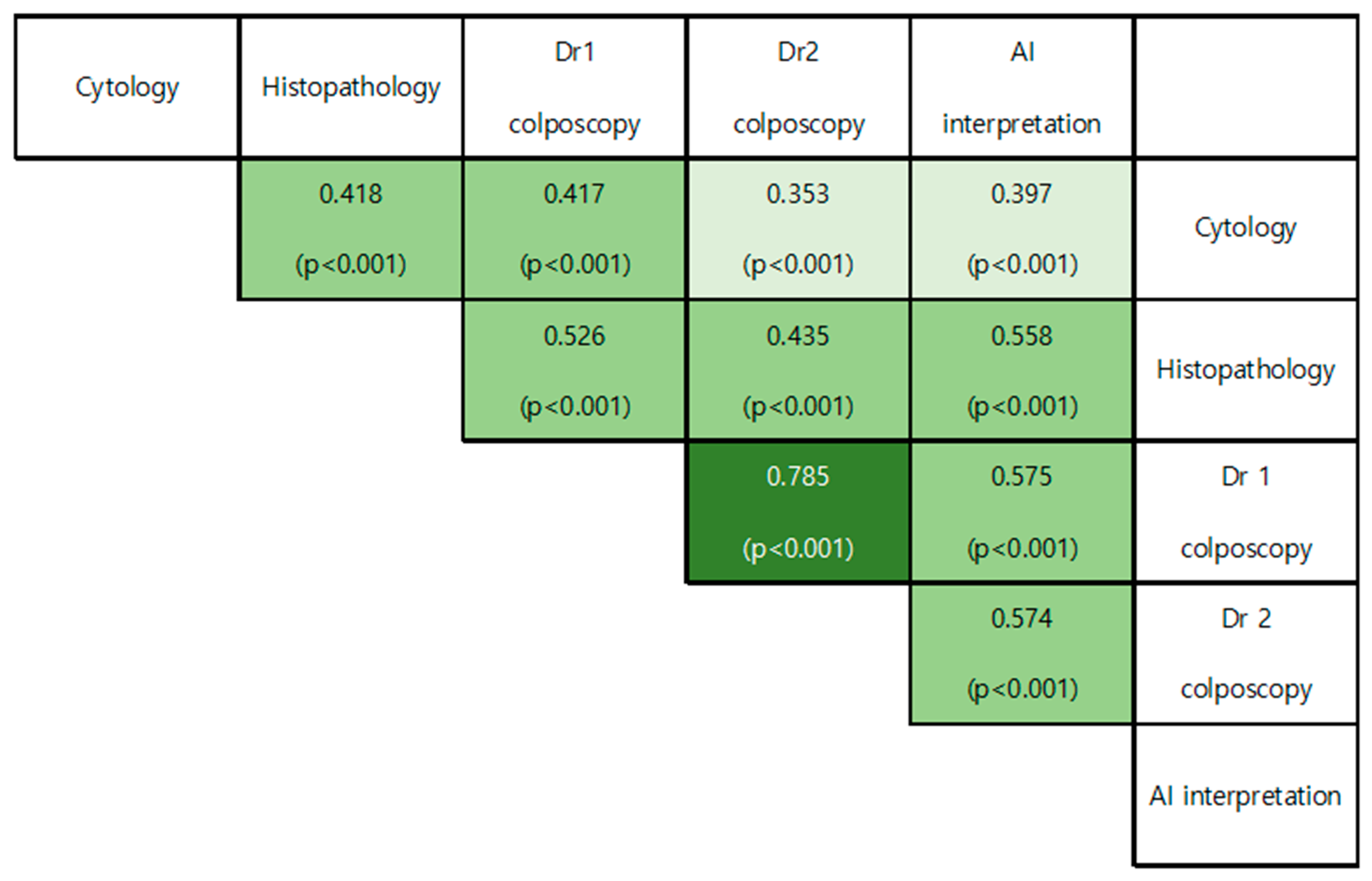
3.3. Correlation between Diagnostic Performances
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Guidelines for Treatment of Cervical Intraepithelial Neoplasia 2–3 and Adenocarcinoma in Situ: Cryotherapy, Large Loop Excision of the Transformation Zone, and Cold Knife Conization; WHO Guidelines Approved by the Guidelines Review Committee; WHO: Geneva, Switzerland, 2014.
- Chan, J.K.; Monk, B.J.; Brewer, C.; Keefe, K.A.; Osann, K.; McMeekin, S.; Rose, G.S.; Youssef, M.; Wilczynski, S.P.; Meyskens, F.L.; et al. HPV infection and number of lifetime sexual partners are strong predictors for ‘natural’ regression of CIN 2 and Br. J. Cancer 2003, 89, 1062–1066. [Google Scholar] [CrossRef] [PubMed]
- Ostör, A.G. Natural history of cervical intraepithelial neoplasia: A critical review. Int. J. Gynecol. Pathol. 1993, 12, 186–192. [Google Scholar] [CrossRef]
- Silver, M.I.; Andrews, J.; Cooper, C.K.; Gage, J.C.; Gold, M.A.; Khan, M.J.; Massad, L.S.; Parvu, V.; Perkins, R.B.; Schiffman, M.; et al. Risk of Cervical Intraepithelial Neoplasia 2 or Worse by Cytology, Human Papillomavirus 16/18, and Colposcopy Impression. Obstet. Gynecol. 2018, 132, 725–735. [Google Scholar] [CrossRef]
- Fontham, E.T.H.; Wolf, A.M.D.; Church, T.R.; Etzioni, R.; Flowers, C.R.; Herzig, A.; Guerra, C.E.; Oeffinger, K.C.; Shih, Y.T.; Walter, L.C.; et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J. Clin. 2020, 70, 321–346. [Google Scholar] [CrossRef] [PubMed]
- Goldie, S.J.; Gaffikin, L.; Goldhaber-Fiebert, J.D.; Gordillo-Tobar, A.; Levin, C.; Mahé, C.; Wright, T.C. Cost-Effectiveness of Cervical-Cancer Screening in Five Developing Countries. N. Engl. J. Med. 2005, 353, 2158–2168. [Google Scholar] [CrossRef] [PubMed]
- Hull, R.; Mbele, M.; Makhafola, T.; Hicks, C.; Wang, S.; Reis, R.M.; Mehrotra, R.; Mkhize-Kwitshana, Z.; Kibiki, G.; Bates, D.O.; et al. Cervical cancer in low and middle-income countries (Review). Oncol. Lett. 2020, 20, 2058–2074. [Google Scholar] [CrossRef]
- Gyawali, P.; Kc, S.; Ghimire, S. Role of Colposcopy in Detection of Dysplastic Cervical Lesion as a Screening Tool. J. Glob. Oncol. 2018, 4, 33s. [Google Scholar] [CrossRef]
- Barut, M.U.; Kale, A.; Kuyumcuoğlu, U.; Bozkurt, M.; Ağaçayak, E.; Özekinci, S.; Gül, T. Analysis of Sensitivity, Specificity, and Positive and Negative Predictive Values of Smear and Colposcopy in Diagnosis of Premalignant and Malignant Cervical Lesions. Med. Sci. Monit. 2015, 21, 3860–3867. [Google Scholar] [CrossRef]
- Karimi-Zarchi, M.; Zanbagh, L.; Shafii, A.; Shokouh, T.-Z.; Teimoori, S.; Yazdian-Anari, P. Comparison of Pap Smear and Colposcopy in Screening for Cervical Cancer in Patients with Secondary Immunodeficiency. Electron. Physician 2015, 7, 1542–1548. [Google Scholar] [CrossRef][Green Version]
- Stuebs, F.A.; Schulmeyer, C.E.; Mehlhorn, G.; Gass, P.; Kehl, S.; Renner, S.K.; Renner, S.P.; Geppert, C.; Adler, W.; Hartmann, A.; et al. Accuracy of colposcopy-directed biopsy in detecting early cervical neoplasia: A retrospective study. Arch. Gynecol. Obstet. 2018, 299, 525–532. [Google Scholar] [CrossRef]
- Waring, J.; Lindvall, C.; Umeton, R. Automated machine learning: Review of the state-of-the-art and opportunities for healthcare. Artif. Intell. Med. 2020, 104, 101822. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Luo, G. Progressive sampling-based Bayesian optimization for efficient and automatic machine learning model selection. Health Inf. Sci. Syst. 2017, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Bi, H.; Zhang, X.; Zhao, Y.; Dong, Y.; Luo, X.; Zhou, D.; You, Z.; Wu, Y.; Liu, Z.; et al. Artificial intelligence-assisted cytology for detection of cervical intraepithelial neoplasia or invasive cancer: A multicenter, clinical-based, observational study. Gynecol. Oncol. 2020, 159, 171–178. [Google Scholar] [CrossRef]
- Hu, L.; Bell, D.; Antani, S.; Xue, Z.; Yu, K.; Horning, M.P.; Gachuhi, N.; Wilson, B.; Jaiswal, M.S.; Befano, B.; et al. An Observational Study of Deep Learning and Automated Evaluation of Cervical Images for Cancer Screening. JNCI J. Natl. Cancer Inst. 2019, 111, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Xue, P.; Tang, C.; Li, Q.; Li, Y.; Shen, Y.; Zhao, Y.; Chen, J.; Wu, J.; Li, L.; Wang, W.; et al. Development and validation of an artificial intelligence system for grading colposcopic impressions and guiding biopsies. BMC Med. 2020, 18, 406. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Yao, Y.; Cheng, B.; Cheng, Y.; Li, Y.; Li, Y.; Liu, X.; Cheng, X.; Xie, X.; Wu, J.; et al. The application of deep learning based diagnostic system to cervical squamous intraepithelial lesions recognition in colposcopy images. Sci. Rep. 2020, 10, 11639. [Google Scholar] [CrossRef] [PubMed]
- Fan, A.; Wang, C.; Zhang, L.; Yan, Y.; Han, C.; Xue, F. Diagnostic value of the 2011 International Federation for Cervical Pathology and Colposcopy Terminology in predicting cervical lesions. Oncotarget 2018, 9, 9166–9176. [Google Scholar] [CrossRef]
- Mitchell, M.F. Colposcopy for the diagnosis of squamous intraepithelial lesions: A meta-analysis. Obstet. Gynecol. 1998, 91, 626–631. [Google Scholar] [CrossRef]
- Benedet, J.L. An analysis of 84,244 patients from the British Columbia cytology–colposcopy program. Gynecol. Oncol. 2004, 92, 127–134. [Google Scholar] [CrossRef]
- Cho, B.-J.; Choi, Y.J.; Lee, M.-J.; Kim, J.H.; Son, G.-H.; Park, S.-H.; Kim, H.-B.; Joo, Y.-J.; Cho, H.-Y.; Kyung, M.S.; et al. Classification of cervical neoplasms on colposcopic photography using deep learning. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Kuramoto, H.; Jobo, T. Utility of Colposcopy: Comparison of Colposcopic Abnormality with Histology and Cytology, with Colposcopic Findings Focusing on the Lesion in Cervical Canal. In Colposcopy and Cervical Pathology; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Khongthip, Y.; Manchana, T.; Oranratanaphan, S.; Lertkhachonsuke, R. Learning curve in colposcopic training among gynecologic oncology fellows. Eur. J. Gynaecol. Oncol. 2019, 40, 647–651. [Google Scholar]
- Egede, J.; Ajah, L.; Ibekwe, P.; Agwu, U.; Nwizu, E.; Iyare, F. Comparison of the Accuracy of Papanicolaou Test Cytology, Visual Inspection with Acetic Acid, and Visual Inspection with Lugol Iodine in Screening for Cervical Neoplasia in Southeast Nigeria. J. Glob. Oncol. 2018, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Vahedpoor, Z.; Behrashi, M.; Khamehchian, T.; Abedzadeh-Kalahroudi, M.; Moravveji, A.; Mohmadi-Kartalayi, M. Comparison of the diagnostic value of the visual inspection with acetic acid (VIA) and Pap smear in cervical cancer screening. Taiwan J. Obstet. Gynecol. 2019, 58, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Horie, K.; Hara, A.; Miyamoto, Y.; Kurihara, K.; Tomio, K.; Yokota, H. Application of deep learning to the classification of images from colposcopy. Oncol. Lett. 2018, 15, 3518–3523. [Google Scholar] [CrossRef]
- Simões, P.W.; Izumi, N.B.; Casagrande, R.S.; Venson, R.; Veronezi, C.D.; Moretti, G.P.; da Rocha, E.L.; Cechinel, C.; Ceretta, L.B.; Comunello, E.; et al. Classification of Images Acquired with Colposcopy Using Artificial Neural Networks. Cancer Inform. 2014, 13, S17948. [Google Scholar] [CrossRef] [PubMed]
- Scheiden, R.; Wagener, C.; Knolle, U.; Dippel, W.; Capesius, C. Atypical glandular cells in conventional cervical smears: Incidence and follow-up. BMC Cancer 2004, 4, 1–9. [Google Scholar] [CrossRef][Green Version]
- Arbyn, M.; Bergeron, C.; Klinkhamer, P.; Martin-Hirsch, P.; Siebers, A.G.; Bulten, J. Liquid compared with conventional cervical cytology: A systematic review and meta-analysis. Obstet. Gynecol. 2008, 111, 167–177. [Google Scholar] [CrossRef]
- Ovestad, I.T.; Vennestrøm, U.; Andersen, L.; Gudlaugsson, E.; Munk, A.C.; Malpica, A.; Feng, W.; Voorhorst, F.; Janssen, E.A.; Baak, J.P. Comparison of different commercial methods for HPV detection in follow-up cytology after ASCUS/LSIL, prediction of CIN2–3 in follow up biopsies and spontaneous regression of CIN2-3. Gynecol. Oncol. 2011, 123, 278–283. [Google Scholar] [CrossRef]


| Characteristics | Value |
|---|---|
| Age, years | 36.9 ± 8.9 |
| Cytological results | |
| Normal | 5 (2.1) |
| ASC-US | 107 (45.7) |
| LSIL | 67 (28.6) |
| ASC-H/HSIL | 52 (22.2) |
| SCC | 3 (1.3) |
| HPV status | |
| Positive for high-risk | 153 (65.4) |
| Positive for low-risk only or negative | 16 (6.8) |
| Not done | 65 (27.8) |
| Histopathology | |
| Benign | 52 (22.2) |
| CIN1 | 66 (28.2) |
| CIN2-3 | 110 (47.0) |
| Invasive cancer | 6 (2.6) |
| Treatment | |
| Observation and follow-up | 111 (47.4) |
| LEEP/Conization | 107 (45.7) |
| Extrafascial hysterectomy | 5 (2.1) |
| Radical hysterectomy | 4 (1.7) |
| Chemotherapy/Radiotherapy | 2 (0.9) |
| Refusal of treatment | 5 (2.1) |
| Cytology | Impression | Doctor 1 | Doctor 2 | AI | Histopathology |
|---|---|---|---|---|---|
| Normal | Non-specific/Benign | 2 | 2 | 3 | 4 |
| Minor/CIN1 | 2 | 3 | 2 | 0 | |
| Major/CIN2-3 | 1 | 0 | 0 | 1 | |
| ASC-US | Non-specific/Benign | 28 | 35 | 43 | 37 |
| Minor/CIN1 | 50 | 32 | 30 | 34 | |
| Major/CIN2-3 | 32 | 39 | 32 | 35 | |
| Suspicious for invasion/Cancer | 0 | 1 | 2 | 1 | |
| LSIL | Non-specific/Benign | 15 | 14 | 20 | 7 |
| Minor/CIN1 | 37 | 32 | 24 | 29 | |
| Major/CIN2-3 | 15 | 21 | 22 | 31 | |
| Suspicious for invasion/Cancer | 0 | 0 | 1 | 0 | |
| ASC-H/ HSIL | Non-specific/Benign | 4 | 4 | 7 | 4 |
| Minor/CIN1 | 6 | 9 | 5 | 3 | |
| Major/CIN2-3 | 41 | 38 | 37 | 43 | |
| Suspicious for invasion/Cancer | 1 | 1 | 3 | 2 | |
| SCC | Suspicious for invasion/Cancer | 3 | 3 | 3 | 3 |
| Method | Sensitivity | Specificity | PPV |
|---|---|---|---|
| Cytology | 41.38 | 94.07 | 87.27 |
| Doctor 1 | 71.55 | 87.29 | 84.69 |
| Doctor 2 | 69.83 | 81.36 | 78.64 |
| AI interpretation | 74.14 | 83.05 | 81.13 |
| Doctor 1 + AI | 84.48 | 77.97 | 79.03 |
| Doctor 2 + AI | 83.62 | 74.58 | 76.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Lee, H.; Lee, S.; Song, J.-Y.; Lee, J.-K.; Lee, N.-W. Role of Artificial Intelligence Interpretation of Colposcopic Images in Cervical Cancer Screening. Healthcare 2022, 10, 468. https://doi.org/10.3390/healthcare10030468
Kim S, Lee H, Lee S, Song J-Y, Lee J-K, Lee N-W. Role of Artificial Intelligence Interpretation of Colposcopic Images in Cervical Cancer Screening. Healthcare. 2022; 10(3):468. https://doi.org/10.3390/healthcare10030468
Chicago/Turabian StyleKim, Seongmin, Hwajung Lee, Sanghoon Lee, Jae-Yun Song, Jae-Kwan Lee, and Nak-Woo Lee. 2022. "Role of Artificial Intelligence Interpretation of Colposcopic Images in Cervical Cancer Screening" Healthcare 10, no. 3: 468. https://doi.org/10.3390/healthcare10030468
APA StyleKim, S., Lee, H., Lee, S., Song, J.-Y., Lee, J.-K., & Lee, N.-W. (2022). Role of Artificial Intelligence Interpretation of Colposcopic Images in Cervical Cancer Screening. Healthcare, 10(3), 468. https://doi.org/10.3390/healthcare10030468






