Priorities in the Prevention Strategies for Medication Error Using the Analytical Hierarchy Process Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Decomposition of the Structure
2.2. Comparison of Judgements
2.3. Hierarchical Composition of Priorities
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Coordination Council for Medication Error Reporting and Prevention. Medication Error Definition. Available online: http://www.nccmerp.org/about-medication-errors (accessed on 10 February 2022).
- Morimoto, T.; Gandhi, T.K.; Seger, A.C.; Hsieh, T.C.; Bates, D.W. Adverse drug events and medication errors: Detection and classification methods. Qual. Saf. Health Care 2004, 13, 306–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aspden, P.; Wolcott, J.; Bootman, J.L.; Cronenwett, L.R. Preventing Medication Errors; National Academies Press: Washington, DC, USA, 2007. [Google Scholar]
- Bates, D.W.; Cullen, D.J.; Laird, N.; Petersen, L.A.; Small, S.D.; Servi, D.; Laffel, G.; Sweitzer, B.J.; Shea, B.F.; Hallisey, R.; et al. Incidence of adverse drug events and potential adverse drug events: Implications for prevention. JAMA 1995, 274, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, S.; Suh, H.S. Current State of Medication Error in Korea: Analysis of Medication Injury Relief in Korea Consumer Agency. J. Health Technol. Assess 2019, 7, 88–93. [Google Scholar]
- Elliott, R.A.; Camacho, E.; Jankovic, D.; Sculpher, M.J.; Faria, R. Economic analysis of the prevalence and clinical and economic burden of medication error in England. BMJ Qual. Saf. 2021, 30, 96–105. [Google Scholar] [CrossRef]
- Kohn, L.T.; Corrigan, J.M.; Donaldson, M.S. To Err Is Human: Building a Safer Health System; National Academies Press: Washington, DC, USA, 2000; Volume 6. [Google Scholar]
- World Health Organization. Reporting and Learning Systems for Medication Errors: The Role of Pharmacovigilance Centres; WHO: Geneva, Switzerland, 2014.
- FDA. Postmarketing Surveillance Programs. Available online: https://www.fda.gov/drugs/surveillance/postmarketing-surveillance-programs (accessed on 10 February 2022).
- NHS. Guide for General Practice Staff on Reporting Patient Safety Incidents to the National Reporting and Learning System. Available online: https://www.england.nhs.uk/wp-content/uploads/2015/02/gp-nrls-rep-guide.pdf (accessed on 10 February 2022).
- Basheti, I.A.; Reddel, H.K.; Armour, C.L.; Bosnic-Anticevich, S.Z. Improved asthma outcomes with a simple inhaler technique intervention by community pharmacists. J. Allergy Clin. Immunol. 2007, 119, 1537–1538. [Google Scholar] [CrossRef]
- Kjome, R.L.; Granas, A.G.; Nerhus, K.; Sandberg, S. Quality assessment of patients’ self-monitoring of blood glucose in community pharmacies. Pharm. Pract. 2010, 8, 62. [Google Scholar] [CrossRef] [Green Version]
- Pereles, L.; Romonko, L.; Murzyn, T.; Hogan, D.; Silvius, J.; Stokes, E.; Long, S.; Fung, T. Evaluation of a self-medication program. J. Am. Geriatr. Soc. 1996, 44, 161–165. [Google Scholar]
- Thokala, P.; Devlin, N.; Marsh, K.; Baltussen, R.; Boysen, M.; Kalo, Z.; Longrenn, T.; Mussen, F.; Peacock, S.; Watkins, J.; et al. Multiple Criteria Decision Analysis for Health Care Decision Making—An Introduction: Report 1 of the ISPOR MCDA Emerging Good Practices Task Force. Value Health 2016, 19, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Saaty, T.L. A scaling method for priorities in hierarchical structures. J. Math. Psychol. 1977, 15, 234–281. [Google Scholar] [CrossRef]
- Reddy, B.P.; Kelly, M.P.; Thokala, P.; Walters, S.J.; Duenas, A. Prioritising public health guidance topics in the National Institute for Health and Care Excellence using the Analytic Hierarchy Process. Public Health 2014, 128, 896–903. [Google Scholar] [CrossRef] [Green Version]
- Liberatore, M.J.; Nydick, R.L. The analytic hierarchy process in medical and health care decision making: A literature review. Eur. J. Oper. Res. 2008, 189, 194–207. [Google Scholar] [CrossRef]
- AHRQ. Development of Common Formats. Available online: https://pso.ahrq.gov/common/development (accessed on 10 February 2022).
- AHRQ. Advancing Patient Safety through Data-Driven Safety Improvement. Available online: https://pso.ahrq.gov/sites/default/files/wysiwyg/npsd_data_brief_0715.pdf (accessed on 10 February 2022).
- CEC. Root Cause Analysis. Available online: http://www.cec.health.nsw.gov.au/programs/patient-safety/root-cause-analysis#rcatemps (accessed on 11 October 2015).
- Commission, T.T.J. Sentinel Event Data Root Causes by Event Type 2004—2Q2015. Available online: http://www.jointcommission.org/assets/1/18/Root_Causes_Event_Type_2004-2Q_2015.pdf (accessed on 11 October 2015).
- EMA. Pharmacovigilance Risk Assessment Committee (PRAC). Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/about_us/general/general_content_000537.jsp&mid=WC0b01ac058058cb18 (accessed on 11 October 2015).
- EMA. EudraVigilance Training Programme. Available online: http://eudravigilance.ema.europa.eu/human/training.asp (accessed on 10 February 2022).
- EMA. Access to EudraVigilance Data. Available online: https://www.ema.europa.eu/en/human-regulatory/research-development/pharmacovigilance/eudravigilance/access-eudravigilance-data (accessed on 10 February 2022).
- EMA. Operational Definition of Medication Error for EU Reporting Requirements. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Presentation/2013/03/WC500139871.pdf (accessed on 10 February 2022).
- EMA. Medication Errors. Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/special_topics/general/general_content_000570.jsp (accessed on 10 February 2022).
- FDA. FAERS Reporting by Healthcare Providers and Consumers by Year. Available online: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/ucm070456.htm (accessed on 10 February 2022).
- Patient Safety Resource Centre—The Health Foundation. Patient Safety First 2008 to 2010—The Campaign Review. Available online: http://patientsafety.health.org.uk/resources/patient-safety-first-2008-2010-campaign-review (accessed on 11 October 2015).
- Gallagher, T.H.; Garbutt, J.M.; Waterman, A.D.; Flum, D.R.; Larson, E.B.; Waterman, B.M.; Dunagan, W.C.; Fraser, V.J.; Levinson, W. Choosing your words carefully: How physicians would disclose harmful medical errors to patients. Arch. Intern. Med. 2006, 166, 1585–1593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Department of Health. Never Events Policy Framework Review. Available online: https://www.engage.england.nhs.uk/consultation/never-events-policy-framework-review (accessed on 10 February 2022).
- ISMP. Historical Timeline. Available online: http://www.ismp.org/about/timeline.asp (accessed on 6 October 2015).
- ISMP. Reducing Medication Errors through Failure Mode & Effects Analysis. Available online: http://onlinestore.ismp.org/shop/item.aspx?itemid=103 (accessed on 11 October 2015).
- NHS. National Patient Safety Alerting System Guide. Available online: https://www.england.nhs.uk/patientsafety/psa/national-psa-system/ (accessed on 11 October 2015).
- NHS. Serious Incident Framework. Available online: https://www.england.nhs.uk/patientsafety/serious-incident/ (accessed on 11 October 2015).
- NPS. Living Well with Chronic Heart Failure. Available online: http://www.nps.org.au/health-professionals/for-your-patients/resources/heart-failure (accessed on 11 October 2015).
- NPSA. Seven Steps to Patient Safety for Primary Care. Available online: http://www.nrls.npsa.nhs.uk/resources/collections/seven-steps-to-patient-safety/entryid45=59804 (accessed on 11 October 2015).
- NPSF. RCA2: Improving Root Cause Analyses and Actions to Prevent Harm—National Patient Safety Foundation. Available online: http://www.npsf.org/?page=RCA2 (accessed on 11 October 2015).
- NSW. Incident Management Policy. Available online: http://www.health.nsw.gov.au/policies/pd/2014/PD2014_004.html (accessed on 11 October 2015).
- Smith, D.S.; Haig, K. Reduction of adverse drug events and medication errors in a community hospital setting. Nurs. Clin. N. Am. 2005, 40, 25–32. [Google Scholar] [CrossRef]
- Wachter, R.M. Patient safety at ten: Unmistakable progress, troubling gaps. Health Aff. 2010, 29, 165–173. [Google Scholar] [CrossRef]
- Dolan, J.G. Involving patients in decisions regarding preventive health interventions using the analytic hierarchy process. Health Expect. Int. J. Public Particip. Health Care Health Policy 2000, 3, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Choi, H.D.; Kim, S.; Han, S.; Lee, I.H.; Suh, H.S. Types of Medication Error to Be Used in Korea. J. Health Technol. Assess. 2017, 5, 31–41. [Google Scholar]
- Kim, S.; Han, S.; Suh, H.S. The Analysis of Definition and Types for Medication Error Used in Foreign Countries. J. Health Technol. Assess. 2016, 4, 35–43. [Google Scholar]
- Saaty, T.L. How to make a decision: The analytic hierarchy process. Eur. J. Oper. Res. 1990, 48, 9–26. [Google Scholar] [CrossRef]
- Miller, G.A. The magical number seven plus or minus two: Some limits on our capacity for processing information. Psychol. Rev. 1956, 63, 81–97. [Google Scholar] [CrossRef] [Green Version]
- Saaty, T.L. Transport planning with multiple criteria: The analytic hierarchy process applications and progress review. J. Adv. Transp. 1995, 29, 81–126. [Google Scholar] [CrossRef]
- Saaty, T.L. The Analytic Hierarchy Process: Planning, Priority Setting, Resource Allocation; McGraw-Hill: New York, NY, USA, 1980. [Google Scholar]
- Saaty, T.L.; Vargas, L.G. Prediction, Projection, and Forecasting: Applications of the Analytic Hierarchy Process in Economics, Finance, Politics, Games, and Sports; Kluwer Academic Publishers: Norwell, MA, USA, 1991. [Google Scholar]
- Saaty, T.L. Decision Making with Dependence and Feedback: The Analytic Network Process; RWS publications Pittsburgh: Pittsburgh, PA, USA, 1996; Volume 4922. [Google Scholar]
- Forman, E.H.; Selly, M.A. Decision by Objectives: How to Convince Others That You Are Right; World Scientific: Singapore, 2001. [Google Scholar]
- Wu, W.-H.; Chiang, C.-T.; Lin, C.-T. Comparing the aggregation methods in the analytic hierarchy process when uniform distribution. WSEAS Trans. Bus. Econ. 2008, 5, 74–80. [Google Scholar]
- Aczél, J.; Saaty, T.L. Procedures for synthesizing ratio judgements. J. Math. Psychol. 1983, 27, 93–102. [Google Scholar] [CrossRef]
- Yang, J.M. A Study on New Selection Process of Research Proposals Using AHP (Analytic Hierarchy Process); National Research Foundation of Korea: Daejeon, Korea, 2007.
- Matti, N.; Nguyen, M.-N.R.; Mosel, C.; Grzeskowiak, L.E. Utilization of neonatal medication error prevention strategies: A clinical practice survey of Australian and New Zealand neonatal units. Ther. Adv. Drug Saf. 2018, 9, 609–617. [Google Scholar] [CrossRef] [Green Version]
- Fortescue, E.B.; Kaushal, R.; Landrigan, C.P.; McKenna, K.J.; Clapp, M.D.; Federico, F.; Goldmann, D.A.; Bates, D.W. Prioritizing strategies for preventing medication errors and adverse drug events in pediatric inpatients. Pediatrics 2003, 111, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Kahraman, C.; Cebeci, U.; Ulukan, Z. Multi-criteria supplier selection using fuzzy AHP. Logist. Inf. Manag. 2003, 16, 382–394. [Google Scholar] [CrossRef]
- Hsieh, M.-C.; Wang, E.M.-Y.; Lee, W.-C.; Li, L.-W.; Hsieh, C.-Y.; Tsai, W.; Wang, C.-P.; Huang, J.-L.; Liu, T.-C. Application of HFACS, fuzzy TOPSIS, and AHP for identifying important human error factors in emergency departments in Taiwan. Int. J. Ind. Ergon. 2018, 67, 171–179. [Google Scholar] [CrossRef]
- Singh, S.; Dolan, J.G.; Centor, R.M. Optimal management of adults with pharyngitis–a multi-criteria decision analysis. BMC Med. Inform. Decis. Mak. 2006, 6, 14. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-J.; Kim, Y.-J.; Park, D.-J.; Liew, D.; Rhee, Y. Multiple Criteria Decision Analysis (MCDA) in health technology assessment: Review of literature on MCDA methodology and decision criteria. J. Health Technol. Assess. 2017, 5, 128–142. [Google Scholar]
- Frazão, T.D.; Camilo, D.G.; Cabral, E.L.; Souza, R.P. Multicriteria decision analysis (MCDA) in health care: A systematic review of the main characteristics and methodological steps. BMC Med. Inform. Decis. Mak. 2018, 18, 90. [Google Scholar] [CrossRef]
- Tseng, M.L.; Lin, Y.H. Selection of competitive advantages in TQM implementation using fuzzy AHP and sensitivity analysis. Asia Pac. Manag. Rev. 2008, 13, 583–599. [Google Scholar]
| Characteristics | Experts on Patient Safety Research (n = 5) | Experts on Clinical Pharmacotherapy (n = 5) |
|---|---|---|
| Female | 3 (60%) | 5 (100%) |
| Age (mean) | 47.8 years | 39.4 years |
| Specialty | ||
| Medicine | 2 (40%) | 0 (0%) |
| Pharmacy | 3 (60%) | 5 (100%) |
| Affiliation | ||
| Academy | 3 (60%) | 0 (0%) |
| Public institution | 1 (20%) | 0 (0%) |
| Medical institution | 1 (20%) | 5 (100%) |
| Work experience (mean) | 16.6 years | 13.2 years |
| Academic degrees | ||
| Bachelor’s degree | 0 (0%) | 2 (40%) |
| Masters degree | 0 (0%) | 1 (20%) |
| Doctoral degree | 5 (100%) | 2 (40%) |
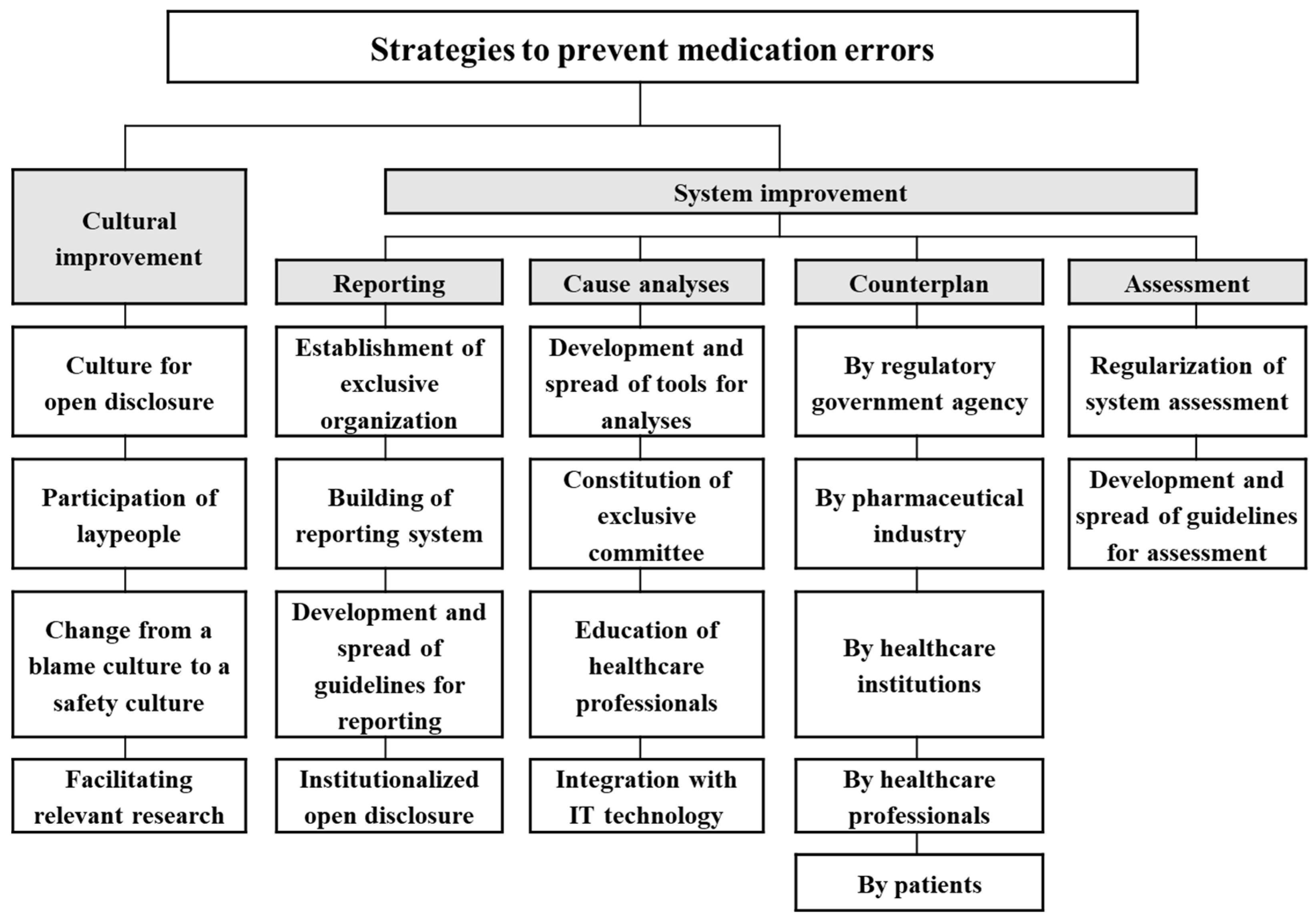
| Criteria | Alternatives | Definition |
|---|---|---|
| Cultural improvement | Culture for open disclosure | To establish a culture that enables open disclosure between healthcare professionals and patients |
| Participation of laypeople | To induce laypeople to participate actively in safe use by providing safe use information and running campaigns for spontaneous reporting of medication errors | |
| Change from a blame culture to a safety culture | To regard medication errors as a systemic problem and work together to find solutions instead of blaming an individual healthcare professional | |
| Facilitating relevant research | To encourage research on safety culture or safety policy | |
| System improvement in reporting | Establishment of exclusive organization | To establish an exclusive organization that manages medication error reporting and assesses the current status of medication error regularly, thus leading to system improvement |
| Building of reporting system | To establish a structured, national reporting system for patient safety event that encompasses adverse event and medication error | |
| Development and spread of guidelines for reporting | To develop and disseminate standardized guidelines for medication error reporting for specific population (e.g., the public, healthcare professionals, and the elderly) | |
| Institutionalized open disclosure | To develop and institutionalize guidelines for open disclosure (e.g., communication and discussion with patients and their families, apologies, and compensation without any penalty regarding disclosure) | |
| System improvement in cause analysis | Development and spread of tools for analyses | To develop and disseminate standardized tools for the cause analysis of safety events |
| Establishment of exclusive committee | To establish professional committees that take full responsibility of patient safety issues at a national or institutional level | |
| Education of healthcare professionals | To provide training to healthcare professionals (e.g., pharmacoepidemiology) to strengthen the individual professionals’ ability to cope with medication errors occurring in their institution | |
| Integration with IT technology | To develop IT technology such as data mining that detects signals of medication errors using patients medical record | |
| System improvement in counterplan | By regulatory government agency | To prepare government-level countermeasures such as the establishment of alarm systems, providing guidance for the pharmaceutical industry, dissemination of information regarding the safe use of drugs, establishment of a reimbursement system for error reporting (e.g., incentives for good reporting and legal liability for insufficient reporting), and development of a system that help institutions exchange patients information during patient transfer (e.g., medication reconciliation service) |
| By pharmaceutical industry | To establish industry-level countermeasures such as making patient brochure, restraint of making similar looking products, and production of pediatric-specific dosage formulation | |
| By healthcare professionals | To establish professional-level countermeasures such as developing an education program/materials, regular and mandatory education for professional knowledge, introducing courses related to patient safety (e.g., patient safety law and communication skill) in College of Medical, Nursing, and Pharmacy | |
| By healthcare institutions | To establish industry-level countermeasures such as staff training, regular discussion on errors occurring in the institution, the establishment of computerized physician order entry, improvements to the workflow and work environment, and developing guidelines for providing patient with medication information | |
| By patients | To establish patient-level countermeasures such as an education program on medication error and participation of patient/caregiver in patient safety committee | |
| System improvement in assessment | Regularization of system assessment | To regularly evaluate the system related to medication error and seek ways to improve the system |
| Development and spread of guidelines for assessment | To develop and disseminate the guidelines for assessment (e.g., design, criteria/indices, measurement, and analysis method) to acquire high-quality results |
| Cultural | System Reporting | System Cause Analyses | System Counterplan | System Assessment | Geometric Mean | Normalized Weights | |
|---|---|---|---|---|---|---|---|
| Cultural | 1.000 | 1.066 | 1.763 | 1.272 | 2.810 | 1.464 | 0.261 * |
| System reporting | 0.938 | 1.000 | 1.907 | 1.070 | 3.672 | 1.477 | 0.263 |
| System cause analyses | 0.567 | 0.524 | 1.000 | 0.411 | 2.946 | 0.815 | 0.145 |
| System counterplan | 0.786 | 0.935 | 2.432 | 1.000 | 3.753 | 1.463 | 0.261 * |
| System assessment | 0.356 | 0.272 | 0.339 | 0.266 | 1.000 | 0.388 | 0.069 |
| Total | 5.607 | 1.000 |
| Normalized Weights | Alternatives | Normalized Weights (within Criterion) | Normalized Weights (Overall) | |
|---|---|---|---|---|
| Cultural | 0.261 * | Culture for open disclosure | 0.243 | 0.063 |
| Participation of laypeople | 0.178 | 0.047 | ||
| Change from a blame culture to a safety culture | 0.445 | 0.116 | ||
| Facilitating relevant research | 0.134 | 0.035 | ||
| System reporting | 0.263 | Establishment of exclusive organization | 0.187 | 0.049 |
| Building of reporting system | 0.391 | 0.103 | ||
| Development and spread of guidelines for reporting | 0.160 | 0.042 | ||
| Institutionalized open disclosure | 0.262 | 0.069 | ||
| System cause analyses | 0.145 | Development and spread of tools for analyses | 0.299 | 0.044 |
| Constitution of exclusive committee | 0.158 | 0.023 | ||
| Education of healthcare professionals | 0.329 | 0.048 | ||
| Integration with IT technology | 0.214 | 0.031 | ||
| System counterplan | 0.261 * | By regulatory government agency | 0.118 | 0.031 |
| By pharmaceutical industry | 0.220 | 0.057 | ||
| By healthcare professionals | 0.158 | 0.041 | ||
| By healthcare institutions | 0.451 | 0.118 | ||
| By patients | 0.052 | 0.014 | ||
| System assessment | 0.069 | Regularization of system assessment | 0.558 | 0.039 |
| Development and spread of guidelines for assessment | 0.442 | 0.031 |
| Rank (Base-Case) | Factors | Weights | Rank (Sensitivity Analyses) | |
|---|---|---|---|---|
| Geometric Mean | Arithmetic Mean | |||
| Criteria | ||||
| 1 | System improvement in reporting | 0.263 | 1 | 3 |
| 2 | Cultural improvement | 0.261 * | 2 | 1 |
| 3 | System improvement in counterplan | 0.261 * | 3 | 2 |
| 4 | System improvement in cause analyses | 0.145 | 4 | 4 |
| 5 | System improvement in assessment | 0.069 | 5 | 5 |
| Alternatives | ||||
| 1 | Counterplan by healthcare institutions | 0.118 | 1 | 2 |
| 2 | Change from a blame culture to a safety culture | 0.116 | 2 | 1 |
| 3 | Building of reporting system | 0.103 | 3 | 4 |
| 4 | Institutionalized open disclosure | 0.069 | 4 | 6 |
| 5 | Culture for open disclosure | 0.063 | 6 | 3 |
| 6 | Counterplan by pharmaceutical industry | 0.057 | 5 | 7 |
| 7 | Establishment of exclusive organization for reporting | 0.049 | 7 | 11 |
| 8 | Education of healthcare professionals for cause analyses | 0.048 | 8 | 16 |
| 9 | Participation of laypeople | 0.047 | 12 | 5 |
| 10 | Development and spread of tools for cause analyses | 0.044 | 10 | 12 |
| 11 | Development and spread of guidelines for reporting | 0.042 | 11 | 15 |
| 12 | Counterplan by healthcare professionals | 0.041 | 9 | 10 |
| 13 | Regularization of system assessment | 0.039 | 13 | 8 |
| 14 | Facilitating relevant research | 0.035 | 15 | 9 |
| 15 | Integration of cause analyses and IT technology | 0.031 † | 17 | 17 |
| 16 | Counterplan by regulatory government agency | 0.031 † | 14 | 14 |
| 17 | Development and spread of guidelines for system assessment | 0.031 † | 16 | 13 |
| 18 | Constitution of exclusive committee for cause analyses | 0.023 | 18 | 18 |
| 19 | Counterplan by patients | 0.014 | 19 | 19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Kim, H.; Suh, H.S. Priorities in the Prevention Strategies for Medication Error Using the Analytical Hierarchy Process Method. Healthcare 2022, 10, 512. https://doi.org/10.3390/healthcare10030512
Kim S, Kim H, Suh HS. Priorities in the Prevention Strategies for Medication Error Using the Analytical Hierarchy Process Method. Healthcare. 2022; 10(3):512. https://doi.org/10.3390/healthcare10030512
Chicago/Turabian StyleKim, Siin, Hyungtae Kim, and Hae Sun Suh. 2022. "Priorities in the Prevention Strategies for Medication Error Using the Analytical Hierarchy Process Method" Healthcare 10, no. 3: 512. https://doi.org/10.3390/healthcare10030512






