Association between Mineralocorticoid Receptor Antagonist and Mortality in SARS-CoV-2 Patients: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Inclusion Criteria
- (1)
- Randomized controlled trials (RCTs), cohort studies (prospective or retrospective), case-control studies, and cross-sectional studies that reported the incidence of mortality in patients who were infected with SARS-CoV-2 who were on MRA compared to those who were not on MRA therapy;
- (2)
- Odds ratio (OR), hazard ratio (HR), or risk ratio (RR), and its corresponding 95% confidence intervals and p-values or sufficient raw data for these calculations had to be provided.
2.3. Data Extraction
2.4. Quality Assessment of the Included Studies
2.5. Statistical Analysis
3. Results
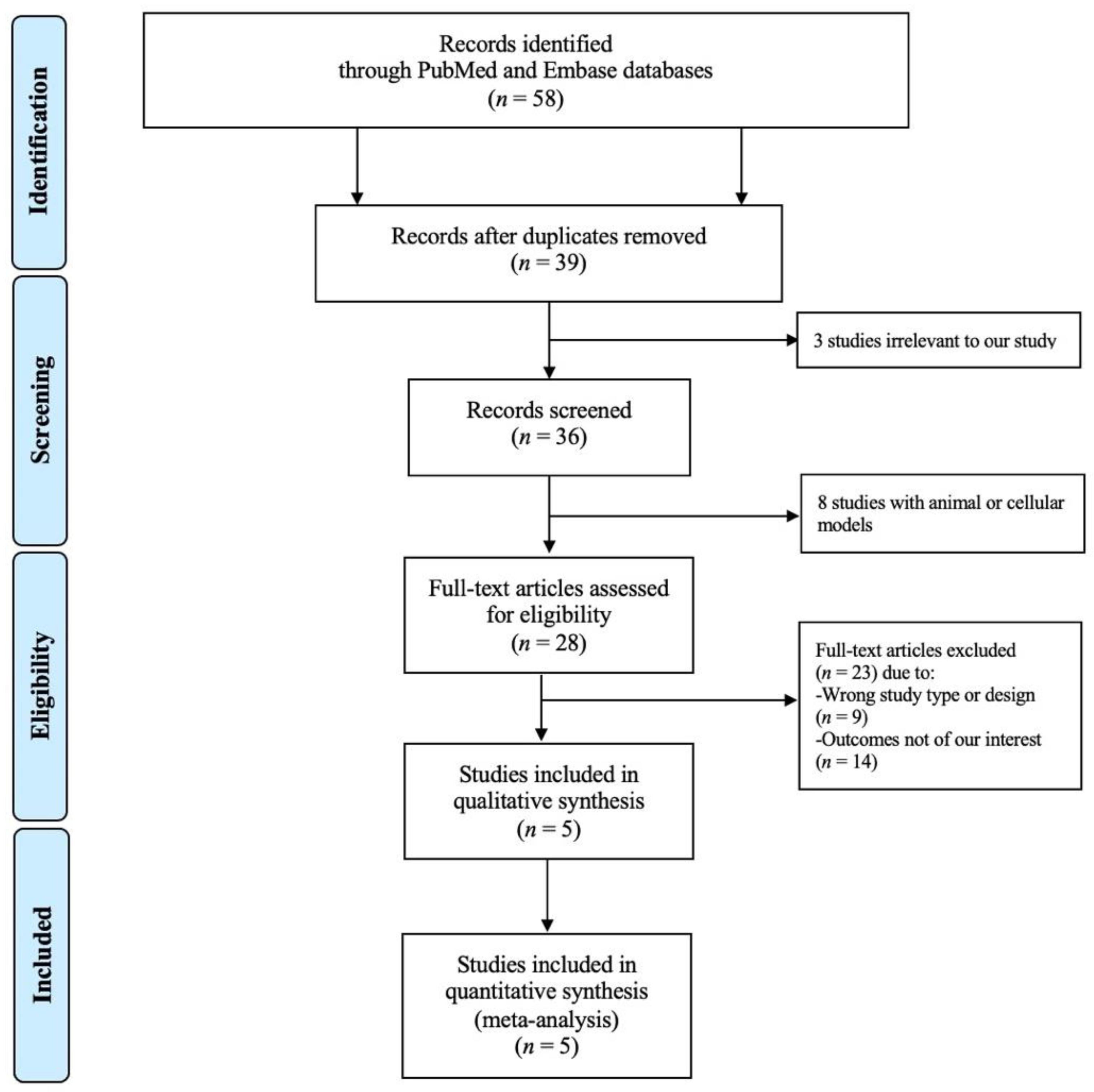
3.1. Study Search Results
3.2. Description of the Included Studies and Quality Assessment
3.3. Quantitative Meta-Analysis Results
3.4. Publication Bias
3.5. Sensitivity Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McFee, D.R.B. COVID-19 medical management including World Health Organization (WHO) suggested management strategies. Dis. Dm. 2020, 66, 101068. [Google Scholar] [CrossRef]
- Li, J.; Lai, S.; Gao, G.F.; Shi, W. The emergence, genomic diversity and global spread of SARS-CoV-2. Nature 2021, 600, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Sanders, J.M.; Monogue, M.L.; Jodlowski, T.Z.; Cutrell, J.B. Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 323, 1824–1836. [Google Scholar] [CrossRef] [PubMed]
- Kolkhof, P.; Bärfacker, L. 30 Years of the Mineralocorticoid Receptor: Mineralocorticoid receptor antagonists: 60 years of research and development. J. Endocrinol. 2017, 234, T125–T140. [Google Scholar] [CrossRef] [Green Version]
- Rico-Mesa, J.S.; White, A.; Ahmadian-Tehrani, A.; Anderson, A.S. Mineralocorticoid Receptor Antagonists: A Comprehensive Review of Finerenone. Curr. Cardiol. Rep. 2020, 22, 140. [Google Scholar] [CrossRef] [PubMed]
- Colussi, G.; Catena, C.; Sechi, L.A. Spironolactone, eplerenone and the new aldosterone blockers in endocrine and primary hypertension. J. Hypertens. 2013, 31, 3–15. [Google Scholar] [CrossRef]
- Moher, D. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 264. [Google Scholar] [CrossRef] [Green Version]
- Lo, C.K.L.; Mertz, D.; Loeb, M. Newcastle-Ottawa Scale: Comparing reviewers’ to authors’ assessments. BMC Med. Res. Methodol. 2014, 14, 45. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Patsopoulos, N.A.; Evangelou, E.; Ioannidis, J.P.A. Sensitivity of between-study heterogeneity in meta-analysis: Proposed metrics and empirical evaluation. Int. J. Epidemiol. 2008, 37, 1148–1157. [Google Scholar] [CrossRef] [Green Version]
- Kocayigit, I.; Kocayigit, H.; Yaylaci, S.; Can, Y.; Erdem, A.F.; Karabay, O. Impact of antihypertensive agents on clinical course and in-hospital mortality: Analysis of 169 hypertensive patients hospitalized for COVID-19. Rev. Da Assoc. Med. Bras. 2020, 66 (Suppl. 2), 71–76. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, F.; Adatorwovor, R.; Davarpanah, M.A.; Mansoori, Y.; Hajiani, M.; Azodi, F.; Sefidbakht, S.; Davoudi, S.; Rezaei, F.; Mohammadmoradi, S.; et al. A Randomized Trial of Combination Therapy, Sitagliptin and Spironolactone, in Hospitalized Adult Patients with COVID-19. J. Endocr. Soc. 2022, 6, bvac017. [Google Scholar] [CrossRef] [PubMed]
- Ersoy, A.; Güven, B.B.; Ertürk, T.; Yurtseven, F.; Karaman, Z.; Güner, T.; Kömpe, Ö. COVID19 ARDS olgularında spironolaktonun etkinliğinin değerlendirilmesi Assessment of The Efficacy of Spironolactone For COVID-19 ARDS Patients. Aydin. Sağlik Derg. 2015, 7, 191–209. [Google Scholar] [CrossRef]
- Vicenzi, M.; Ruscica, M.; Iodice, S.; Rota, I.; Ratti, A.; Di Cosola, R.; Corsini, A.; Bollati, V.; Aliberti, S.; Blasi, F. The Efficacy of the Mineralcorticoid Receptor Antagonist Canrenone in COVID-19 Patients. J. Clin. Med. 2020, 9, 2943. [Google Scholar] [CrossRef]
- Savarese, G.; Benson, L.; Sundström, J.; Lund, L.H. Association between renin–angiotensin–aldosterone system inhibitor use and COVID-19 hospitalization and death: A 1.4 million patient nationwide registry analysis. Eur. J. Heart Fail. 2020, 23, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Nishimura, Y.; Kewcharoen, J.; Yess, J. Statin Use Can Attenuate the Decline in Left Ventricular Ejection Fraction and the Incidence of Cardiomyopathy in Cardiotoxic Chemotherapy Recipients: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 3731. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Debray, T.P.A.; Moons, K.G.M.; Riley, R.D. Detecting small-study effects and funnel plot asymmetry in meta-analysis of survival data: A comparison of new and existing tests: Detecting small-study effects. Res. Synth. Methods 2017, 9, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Simmonds, M. Quantifying the risk of error when interpreting funnel plots. Syst. Rev. 2015, 4, 24. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Haslbauer, J.D.; Stalder, A.; Zinner, C.; Bassetti, S.; Mertz, K.D.; Went, P.; Matter, M.; Tzankov, A. Immunohistochemical and Transcriptional Analysis of SARS-CoV-2 Entry Factors and Renin-Angiotensin-Aldosterone System Components in Lethal COVID-19. Pathobiology 2021, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Chen, C.; Yin, T.; Fang, Q.; Hong, Z.; Zhou, R.; Tang, H.; Dong, H. ACE2 and Innate Immunity in the Regulation of SARS-CoV-2-Induced Acute Lung Injury: A Review. Int. J. Mol. Sci. 2021, 22, 11483. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, L.; Lu, X. Regulation of Angiotensin-Converting Enzyme 2: A Potential Target to Prevent COVID-19? Front. Endocrinol. 2021, 12, 725967. [Google Scholar] [CrossRef]
- South, A.M.; Diz, D.I.; Chappell, M.C. COVID-19, ACE2, and the cardiovascular consequences. Am. J. Physiol.-Heart C 2020, 318, H1084–H1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.-W.; Tsai, C.-H.; Pan, C.-T.; Chou, C.-H.; Liao, S.-C.; Hung, C.-S.; Wu, V.-C.; Lin, Y.-H.; TAIPAI Study Group. Endothelial Dysfunction in Primary Aldosteronism. Int. J. Mol. Sci. 2019, 20, 5214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, S.; Rauf, A.; Khan, H.; Abu-Izneid, T. Renin-angiotensin-aldosterone (RAAS): The ubiquitous system for homeostasis and pathologies. Biomed. Pharmacother. 2017, 94, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Mascolo, A.; Scavone, C.; Rafaniello, C.; De Angelis, A.; Urbanek, K.; di Mauro, G.; Cappetta, D.; Berrino, L.; Rossi, F.; Capuano, A. The Role of Renin-Angiotensin-Aldosterone System in the Heart and Lung: Focus on COVID-19. Front. Pharmacol. 2021, 12, 667254. [Google Scholar] [CrossRef]
- Ferrari, F.; Martins, V.M.; Fuchs, F.D.; Stein, R. Renin-Angiotensin-Aldosterone System Inhibitors in COVID-19: A Review. Clin. Sao Paulo Braz. 2021, 76, e2342. [Google Scholar] [CrossRef]
- Kim, G.H.J.; Melgoza, A.; Jiang, F.; Guo, S. The effect of renin-angiotensin-aldosterone system inhibitors on organ-specific ace2 expression in zebrafish and its implications for COVID-19. Sci. Rep. 2021, 11, 23670. [Google Scholar] [CrossRef]
- Vergara, A.; Jacobs-Cachá, C.; Bosch, M.M.-V.D.; Domínguez-Báez, P.; Benito, B.; García-Carro, C.; Serón, D.; Soler, M.J. Effect of ramipril on kidney, lung and heart ACE2 in a diabetic mice model. Mol. Cell. Endocrinol. 2021, 529, 111263. [Google Scholar] [CrossRef]
- Reynolds, H.R.; Adhikari, S.; Pulgarin, C.; Troxel, A.; Iturrate, E.; Johnson, S.; Hausvater, A.; Newman, J.; Berger, J.S.; Bangalore, S.; et al. Renin-Angiotensin-Aldosterone System Inhibitors and Risk of COVID-19. N. Engl. J. Med. 2020, 382, 2441–2448. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Vardeny, O.; Michel, T.; McMurray, J.J.V.; Pfeffer, M.A.; Solomon, S.D. Renin-Angiotensin-Aldosterone System Inhibitors in Patients with COVID-19. N. Engl. J. Med. 2020, 382, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Zuo, Y.; Yalavarthi, S.; Hunker, K.L.; Knight, J.S.; Kanthi, Y.; Obi, A.T.; Ganesh, S.K. SARS-CoV-2 Spike Protein S1-Mediated Endothelial Injury and Pro-Inflammatory State Is Amplified by Dihydrotestosterone and Prevented by Mineralocorticoid Antagonism. Viruses 2021, 13, 2209. [Google Scholar] [CrossRef]
- Deng, Q.; Rasool, R.; Russell, R.M.; Natesan, R.; Asangani, I.A. Targeting androgen regulation of TMPRSS2 and ACE2 as a therapeutic strategy to combat COVID-19. Iscience 2021, 24, 102254. [Google Scholar] [CrossRef] [PubMed]
- Gebhard, C.; Regitz-Zagrosek, V.; Neuhauser, H.K.; Morgan, R.; Klein, S.L. Impact of sex and gender on COVID-19 outcomes in Europe. Biol. Sex. Differ. 2020, 11, 29. [Google Scholar] [CrossRef]
- Alkhouli, M.; Nanjundappa, A.; Annie, F.; Bates, M.C.; Bhatt, D.L. Sex Differences in Case Fatality Rate of COVID-19: Insights from a Multinational Registry. Mayo Clin. Proc. 2020, 95, 1613–1620. [Google Scholar] [CrossRef]
- Mohamed, M.S.; Moulin, T.C.; Schiöth, H.B. Sex differences in COVID-19: The role of androgens in disease severity and progression. Endocrine 2021, 71, 3–8. [Google Scholar] [CrossRef]
- McCoy, J.; Cadegiani, F.A.; Wambier, C.G.; Herrera, S.; Vaño-Galván, S.; Mesinkovska, N.A.; Ramos, P.M.; Shapiro, J.; Sinclair, R.; Tosti, A.; et al. 5-alpha-reductase inhibitors are associated with reduced frequency of COVID-19 symptoms in males with androgenetic alopecia. J. Eur. Acad. Dermatol. Venereol. 2020, 35, e243–e246. [Google Scholar] [CrossRef]
- Wambier, C.G.; Vaño-Galván, S.; McCoy, J.; Gomez-Zubiaur, A.; Herrera, S.; Hermosa-Gelbard, Á.; Moreno-Arrones, O.M.; Jiménez-Gómez, N.; Gonzalez-Cantero, Á.; Fonda-Pascual, P.; et al. Androgenetic alopecia present in the majority of patients hospitalized with COVID-19: The “Gabrin sign”. J. Am. Acad. Dermatol. 2020, 83, 680–682. [Google Scholar] [CrossRef]
- Goren, A.; Vaño-Galván, S.; Wambier, C.G.; McCoy, J.; Gomez-Zubiaur, A.; Moreno-Arrones, O.M.; Shapiro, J.; Sinclair, R.D.; Gold, M.H.; Kovacevic, M.; et al. A preliminary observation: Male pattern hair loss among hospitalized COVID-19 patients in Spain—A potential clue to the role of androgens in COVID-19 severity. J. Cosmet. Dermatol. 2020, 19, 1545–1547. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, A.L.; Tucker, M.D.; Bakouny, Z.; Labaki, C.; Hsu, C.-Y.; Shyr, Y.; Armstrong, A.J.; Beer, T.M.; Bijjula, R.R.; Bilen, M.A.; et al. Association Between Androgen Deprivation Therapy and Mortality Among Patients with Prostate Cancer and COVID-19. JAMA Netw. Open 2021, 4, e2134330. [Google Scholar] [CrossRef] [PubMed]
- Kotfis, K.; Lechowicz, K.; Drożdżal, S.; Niedźwiedzka-Rystwej, P.; Wojdacz, T.; Grywalska, E.; Biernawska, J.; Wiśniewska, M.; Parczewski, M. COVID-19—The Potential Beneficial Therapeutic Effects of Spironolactone during SARS-CoV-2 Infection. Pharmaceuticals 2021, 14, 71. [Google Scholar] [CrossRef] [PubMed]
- Liaudet, L.; Szabo, C. Blocking mineralocorticoid receptor with spironolactone may have a wide range of therapeutic actions in severe COVID-19 disease. Crit. Care Lond. Engl. 2020, 24, 318. [Google Scholar] [CrossRef] [PubMed]
- Cadegiani, F.A.; Zimerman, R.A.; Fonseca, D.N.; Correia, M.N.; Muller, M.P.; Bet, D.L.; Slaviero, M.R.; Zardo, I.; Benites, P.R.; Barros, R.N.; et al. Final Results of a Randomized, Placebo-Controlled, Two-Arm, Parallel Clinical Trial of Proxalutamide for Hospitalized COVID-19 Patients: A Multiregional, Joint Analysis of the Proxa-Rescue AndroCoV Trial. Cureus 2021, 13, e20691. [Google Scholar] [CrossRef] [PubMed]
- Zarehoseinzade, E.; Allami, A.; Ahmadi, M.; Bijani, B.; Mohammadi, N. Finasteride in hospitalized adult males with COVID-19: A risk factor for severity of the disease or an adjunct treatment: A randomized controlled clinical trial. Med. J. Islam. Repub. Iran 2021, 35, 30. [Google Scholar] [CrossRef]
- Wang, D.; Wilcox, C.S. Abstract MP30: Circulating SARS-CoV-2 Spike Protein 1 Causes Microarteriolar Oxidative Stress, Endothelial Dysfunction and Enhanced Thromboxane and Endothelin Contractility That Are Prevented by Spironolactone. Available online: https://www.ahajournals.org/doi/10.1161/hyp.78.suppl_1.MP30 (accessed on 27 August 2021).
- Barut, F.; Ozacmak, V.H.; Turan, I.; Sayan-Ozacmak, H.; Aktunc, E. Reduction of Acute Lung Injury by Administration of Spironolactone After Intestinal Ischemia and Reperfusion in Rats. Clin. Investig. Med. 2016, 39, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Wilcox, C.S.; Pitt, B. Is spironolactone the preferred renin-angiotensin-aldosterone inhibitor for protection against COVID-19? J. Cardiovasc. Pharm. 2020, 77, 323–331. [Google Scholar] [CrossRef]
- Jeon, D.; Son, M.; Choi, J. Effect of Spironolactone on COVID-19 in Patients with Underlying Liver Cirrhosis: A Nationwide Case-Control Study in South Korea. Front. Med. 2021, 8, 629176. [Google Scholar] [CrossRef]
- Moustafa, D.A.; Imran, Z.; Ismail, R.; Rayan, M.; Gadeau, A.-P.; Eldassouki, H.; Abdulrahman, N.; Mraiche, F. Evaluating the effects of sodium glucose co-transporter -2 inhibitors from a renin-angiotensin-aldosterone system perspective in patients infected with COVID-19: Contextualizing findings from the dapagliflozin in respiratory failure in patients with COVID-19 study. Mol. Biol. Rep. 2022, 49, 2321–2324. [Google Scholar] [CrossRef]
- Li, X.-T.; Zhang, M.-W.; Zhang, Z.-Z.; Cao, Y.-D.; Liu, X.-Y.; Miao, R.; Xu, Y.; Song, X.-F.; Song, J.-W.; Liu, Y.; et al. Abnormal apelin-ACE2 and SGLT2 signaling contribute to adverse cardiorenal injury in patients with COVID-19. Int. J. Cardiol. 2021, 336, 123–129. [Google Scholar] [CrossRef]
- Sainsbury, C.; Wang, J.; Gokhale, K.; Acosta-Mena, D.; Dhalla, S.; Byne, N.; Chandan, J.S.; Anand, A.; Cooper, J.; Okoth, K.; et al. Sodium-glucose-co-transporter-2 inhibitors and susceptibility to COVID-19: A population-based retrospective cohort study. Diabetes Obes. Metab. 2020, 23, 14203. [Google Scholar] [CrossRef] [PubMed]
- Sazgarnejad, S.; Yazdanpanah, N.; Rezaei, N. Anti-inflammatory effects of GLP-1 in patients with COVID-19. Expert Rev. Anti-Infect. 2021, 20, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H. Potential therapeutic effect of glucagon-like peptide-1 receptor agonists on COVID-19-induced pulmonary arterial hypertension. Med. Hypotheses 2022, 158, 110739. [Google Scholar] [CrossRef] [PubMed]

| Author | Country | Published Year | Study Type | MRA (n = 80,903) | No MRA (n = 1,307,275) | Mean Age (Years) | Male (%) | HTN (%) | DM (%) | HLD (%) | MRA Type |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abbasi | U.S. | 2021 | Randomized-controlled trial | 50 | 87 | 57.0 | 54.0 | 31.4 | 27.7 | NR | Spironolactone |
| Ersoy | Turkey | 2021 | Case control | 30 | 30 | 58.8 | 80.0 | NR | NR | NR | Spironolactone |
| Kocayigit | Turkey | 2020 | Cross-sectional | 5 | 161 | 65.8 | 46.7 | 100 | 34.9 | 16.6 | NR |
| Savarese | Sweden | 2020 | Cross-sectional | 80,788 | 1,306,958 | 73.5 | 52.1 | 79.8 | 28.7 | NR | NR |
| Vicenzi | Italy | 2020 | Case-control | 30 | 39 | 61.0 | 72.0 | 45.0 | NR | 20.0 | Canrenone |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Miyazaki, K.; Shah, P.; Kozai, L.; Kewcharoen, J. Association between Mineralocorticoid Receptor Antagonist and Mortality in SARS-CoV-2 Patients: A Systematic Review and Meta-Analysis. Healthcare 2022, 10, 645. https://doi.org/10.3390/healthcare10040645
Kim J, Miyazaki K, Shah P, Kozai L, Kewcharoen J. Association between Mineralocorticoid Receptor Antagonist and Mortality in SARS-CoV-2 Patients: A Systematic Review and Meta-Analysis. Healthcare. 2022; 10(4):645. https://doi.org/10.3390/healthcare10040645
Chicago/Turabian StyleKim, Jean, Kyle Miyazaki, Parthav Shah, Landon Kozai, and Jakrin Kewcharoen. 2022. "Association between Mineralocorticoid Receptor Antagonist and Mortality in SARS-CoV-2 Patients: A Systematic Review and Meta-Analysis" Healthcare 10, no. 4: 645. https://doi.org/10.3390/healthcare10040645
APA StyleKim, J., Miyazaki, K., Shah, P., Kozai, L., & Kewcharoen, J. (2022). Association between Mineralocorticoid Receptor Antagonist and Mortality in SARS-CoV-2 Patients: A Systematic Review and Meta-Analysis. Healthcare, 10(4), 645. https://doi.org/10.3390/healthcare10040645





