Usefulness of Novel Atherogenic Lipid Indices for the Evaluation of Metabolic Status Leading to Coronary Heart Disease in a Real-World Survey of the Japanese Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Measurements
2.3. Statistical Analyses
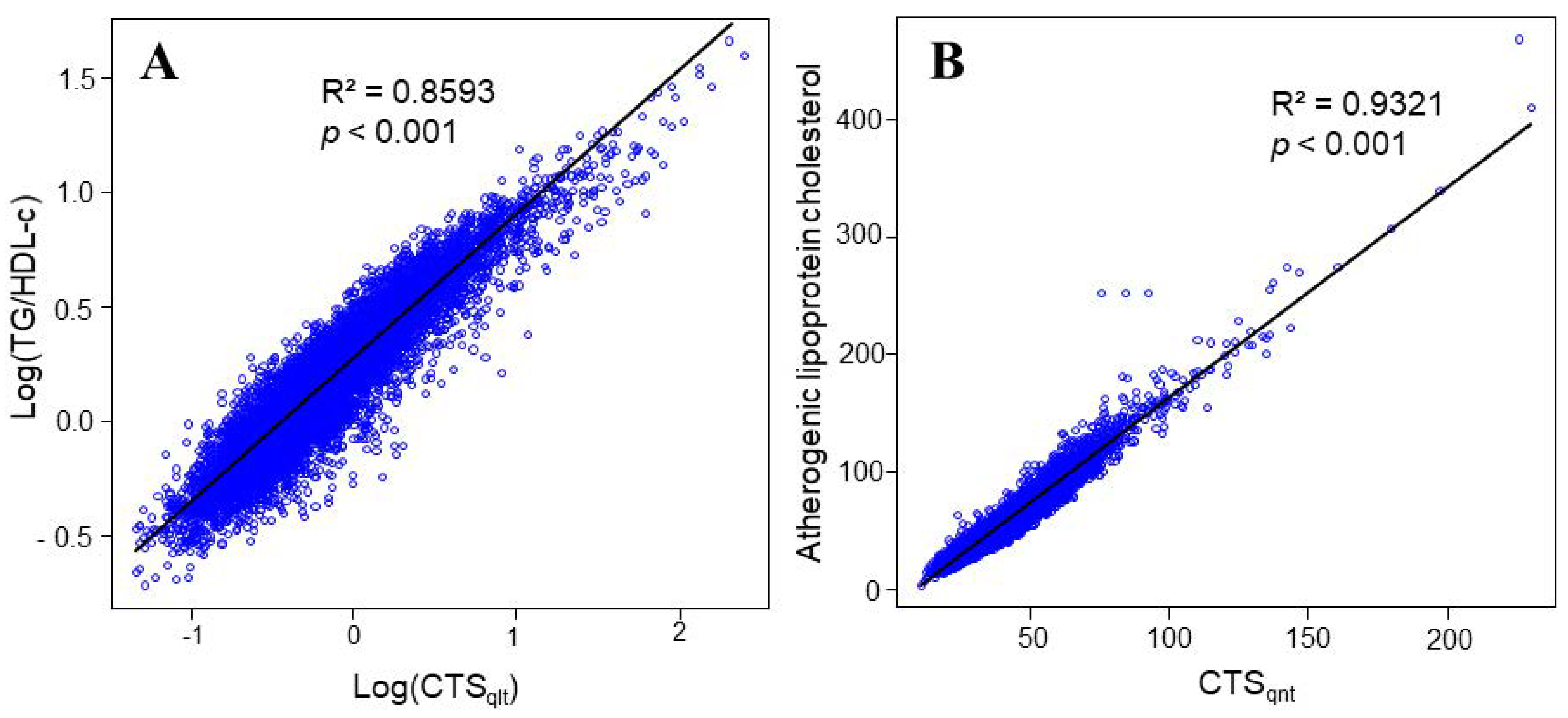
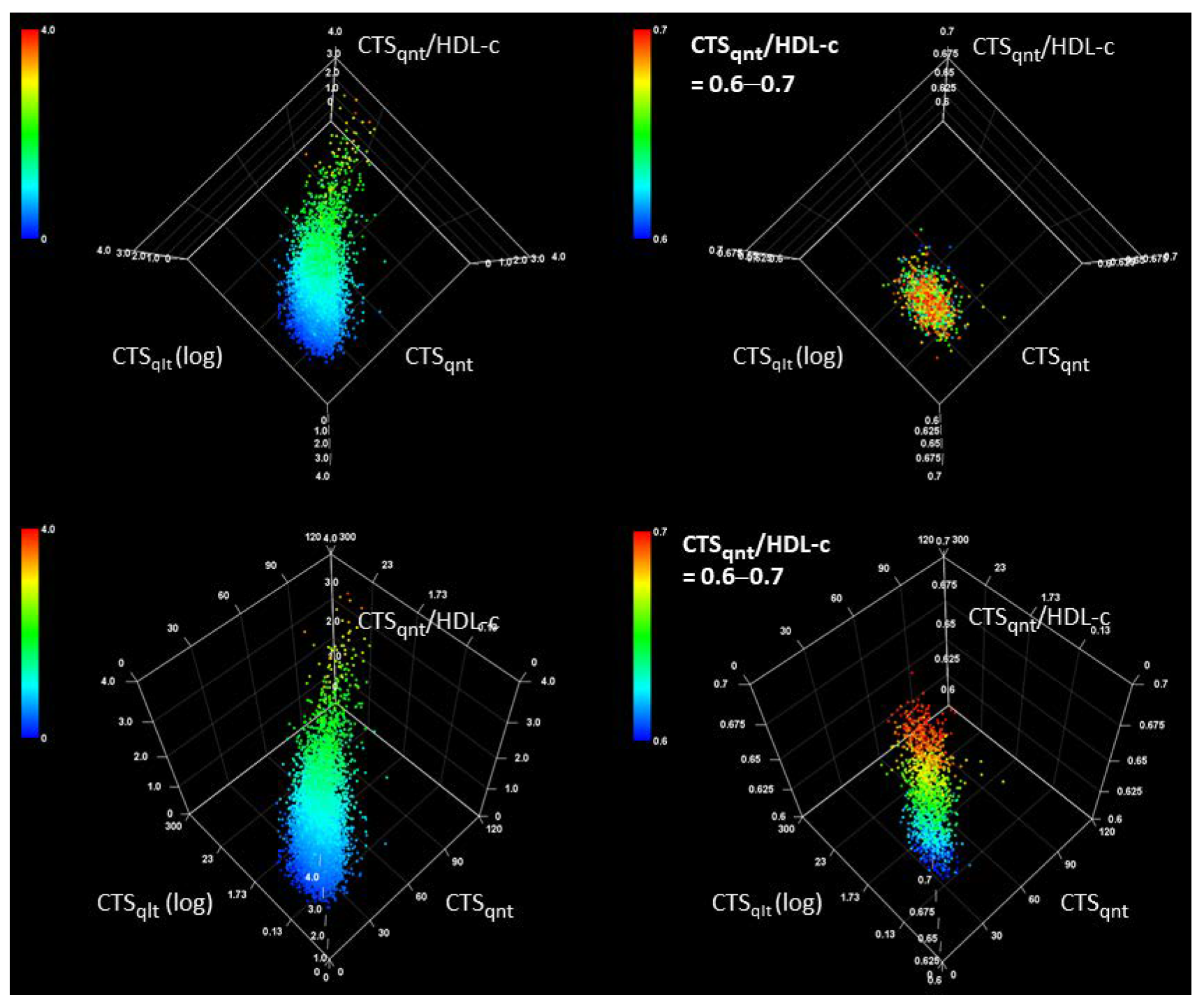
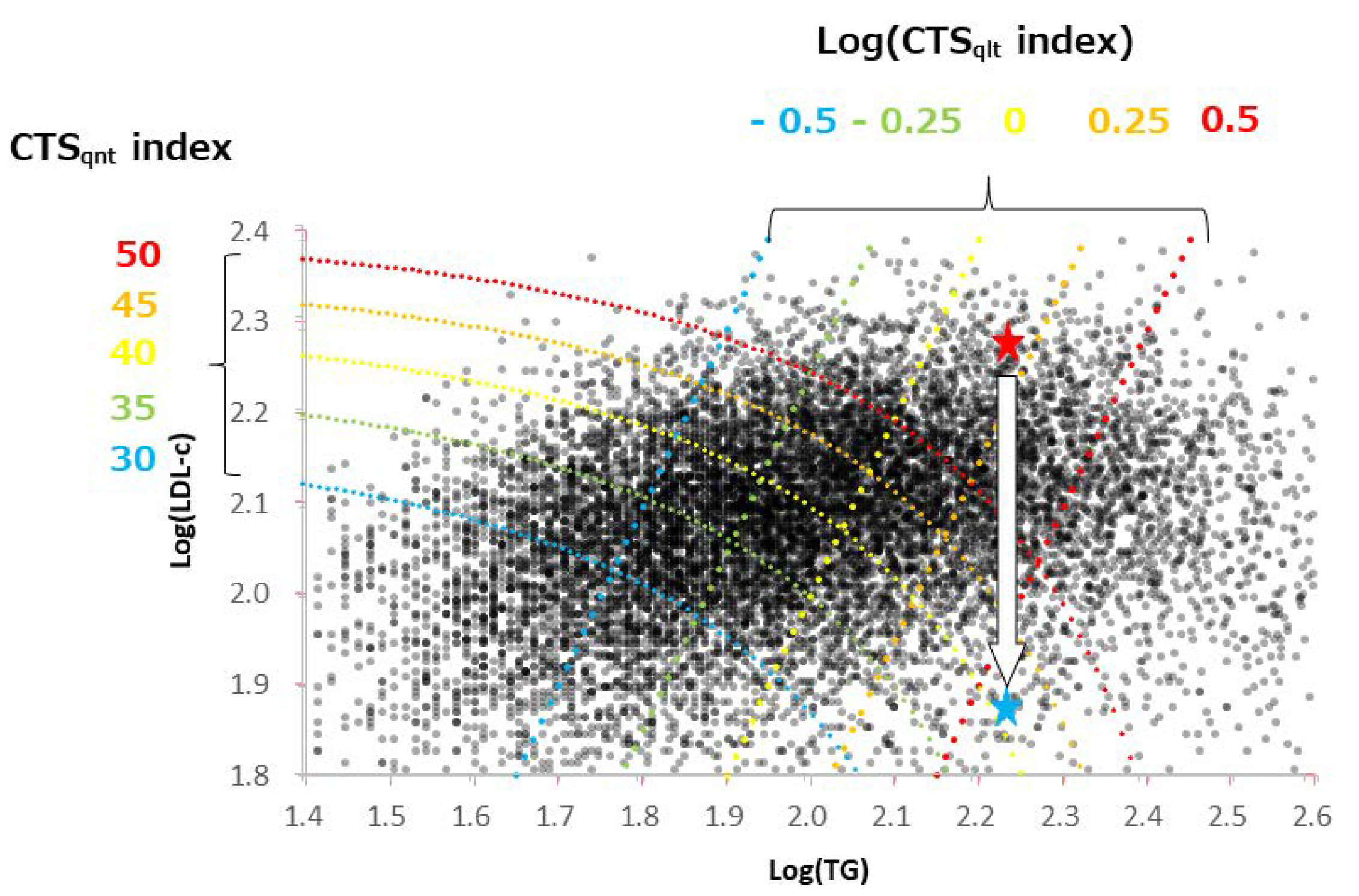
3. Results
3.1. Comparison of HRs for the Lipid Indices
3.2. Characteristics of CTS Indices
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zanoni, P.; Velagapudi, S.; Yalcinkaya, M.; Rohrer, L.; von Eckardstein, A. Endocytosis of lipoproteins. Atherosclerosis 2018, 275, 273–295. [Google Scholar] [CrossRef] [PubMed]
- Karathanasis, S.K.; Freeman, L.A.; Gordon, S.M.; Remaley, A.T. The Changing Face of HDL and the Best Way to Measure It. Clin. Chem. 2017, 63, 196–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Summerhill, V.I.; Grechko, A.V.; Yet, S.-F.; Sobenin, I.A.; Orekhov, A.N. The Atherogenic Role of Circulating Modified Lipids in Atherosclerosis. Int. J. Mol. Sci. 2019, 20, 3561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gisterå, A.; Hansson, G.K. The immunology of atherosclerosis. Nat. Rev. Nephrol. 2017, 13, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.I.; Yu, J.; Hayashi, T.; Han, S.H.; Koh, K.K. Strategies to Overcome Residual Risk During Statins Era. Circ. J. 2019, 83, 1973–1979. [Google Scholar] [CrossRef] [Green Version]
- Toth, P.P.; Fazio, S.; Wong, N.D.; Hull, M.; Nichols, G.A. Risk of cardiovascular events in patients with hypertriglyceridaemia: A review of real-world evidence. Diabetes Obes. Metab. 2019, 22, 279–289. [Google Scholar] [CrossRef]
- Packard, C.J.; Boren, J.; Taskinen, M.-R. Causes and Consequences of Hypertriglyceridemia. Front. Endocrinol. 2020, 11, 252. [Google Scholar] [CrossRef]
- Kajikawa, M.; Maruhashi, T.; Kishimoto, S.; Matsui, S.; Hashimoto, H.; Takaeko, Y.; Yusoff, F.M.; Kihara, Y.; Chayama, K.; Goto, C.; et al. Target of Triglycerides as Residual Risk for Cardiovascular Events in Patients With Coronary Artery Disease—Post Hoc Analysis of the FMD-J Study A. Circ. J. 2019, 83, 1064–1071. [Google Scholar] [CrossRef] [Green Version]
- Ivanova, E.A.; Myasoedova, V.A.; Melnichenko, A.A.; Grechko, A.; Orekhov, A.N. Small Dense Low-Density Lipoprotein as Biomarker for Atherosclerotic Diseases. Oxidative Med. Cell. Longev. 2017, 2017, 1273042. [Google Scholar] [CrossRef]
- Srisawasdi, P.; Chaloeysup, S.; Teerajetgul, Y.; Pocathikorn, A.; Sukasem, C.; Vanavanan, S.; Kroll, M.H. Estimation of Plasma Small Dense LDL Cholesterol From Classic Lipid Measures. Am. J. Clin. Pathol. 2011, 136, 20–29. [Google Scholar] [CrossRef]
- Box, G.E.; Cox, D.R. An Analysis of Transformations. J. R. Stat. Soc. Ser. B (Methodol.) 1964, 26, 211–252. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, R.; Li, M.; Wang, X.; Wang, S.; Li, S.; Tian, H.; Wu, Y.; Zhang, C. LDL-C/HDL-C ratio associated with carotid intima-media thickness and carotid plaques in male but not female patients with type 2 diabetes. Clin. Chim. Acta 2020, 511, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Liu, F.; Wang, Y.-H.; Lai, H.-M.; Luo, J.-Y.; Ma, Y.-T.; Li, X.-M.; Yang, Y.-N. LDL-C:HDL-C ratio and common carotid plaque in Xinjiang Uygur obese adults: A cross-sectional study. BMJ Open 2018, 8, e022757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesut, E.; Cihan, A.; Orhan, G. Is it possible to predict the complexity of peripheral artery disease with atherogenic index? Vascular 2020, 28, 513–519. [Google Scholar] [CrossRef]
- Anderson, J.L.; Bakker, S.J.; Tietge, U.J. The triglyceride to HDL-cholesterol ratio and chronic graft failure in renal transplantation. J. Clin. Lipidol. 2021. [Google Scholar] [CrossRef]
- Cai, G.; Shi, G.; Xue, S.; Lu, W. The atherogenic index of plasma is a strong and independent predictor for coronary artery disease in the Chinese Han population. Medicine 2017, 96, e8058. [Google Scholar] [CrossRef]
- Dobiášová, M.; Frohlich, J. The plasma parameter log (TG/HDL-C) as an atherogenic index: Correlation with lipoprotein particle size and esterification rate inapob-lipoprotein-depleted plasma (FERHDL). Clin. Biochem. 2001, 34, 583–588. [Google Scholar] [CrossRef]
- Varbo, A.; Benn, M.; Tybjærg-Hansen, A.; Jørgensen, A.B.; Frikke-Schmidt, R.; Nordestgaard, B.G. Remnant Cholesterol as a Causal Risk Factor for Ischemic Heart Disease. J. Am. Coll. Cardiol. 2012, 61, 427–436. [Google Scholar] [CrossRef] [Green Version]
- Varbo, A.; Freiberg, J.J.; Nordestgaard, B.G. Extreme Nonfasting Remnant Cholesterol vs Extreme LDL Cholesterol as Contributors to Cardiovascular Disease and All-Cause Mortality in 90000 Individuals from the General Population. Clin. Chem. 2015, 61, 533–543. [Google Scholar] [CrossRef]
- Samanta, B. Can calculated SdLDL serve as a substitute for estimated SdLDL? Asian J. Med. Sci. 2021, 12, 14–19. [Google Scholar] [CrossRef]
- Nordestgaard, B.G. Triglyceride-Rich Lipoproteins and Atherosclerotic Cardiovascular Disease. Circ. Res. 2016, 118, 547–563. [Google Scholar] [CrossRef] [PubMed]
| Category | Disease Grozup | Control Group | p | |
|---|---|---|---|---|
| Number | 131 | 12,242 | ||
| Sex (%) | Male | 104 (79.4) | 8103 (66.2) | 0.001 |
| Female | 27 (20.6) | 4139 (33.8) | ||
| Smoking history (%) | No | 30 (22.9) | 4640 (37.9) | <0.001 |
| Yes | 101 (77.1) | 7602 (62.1) | ||
| Blood pressure (%) | L | 63 (48.1) | 9343 (76.3) | <0.001 |
| M | 43 (32.8) | 2071 (16.9) | ||
| H | 25 (19.1) | 828 (6.8) | ||
| Blood sugar (%) | L | 97 (74.0) | 11,093 (90.6) | <0.001 |
| M | 12 (9.2) | 640 (5.2) | ||
| H | 22 (16.8) | 509 (4.2) | ||
| Age | 56.04 [49.95, 61.98] | 48.00 [40.99, 55.99] | <0.001 | |
| TC | 214.00 [192.00, 235.50] | 206.00 [185.00, 229.00] | 0.003 | |
| TG | 116.00 [84.50, 161.50] | 87.00 [61.00, 131.00] | <0.001 | |
| LDL-c | 129.00 [109.50, 149.50] | 121.00 [101.00, 142.00] | 0.002 | |
| HDL-c | 54.00 [45.50, 67.50] | 62.00 [51.00, 74.00] | <0.001 | |
| NonHDL-c | 156.00 [135.00, 182.00] | 142.00 [119.00, 167.00] | <0.001 | |
| LDL-c/HDL-c | 2.29 [1.78, 3.01] | 1.96 [1.48, 2.56] | <0.001 | |
| TG/HDL-c | 2.19 [1.44, 3.54] | 1.41 [0.86, 2.42] | <0.001 | |
| CTSqnt | 45.80 [36.60, 55.05] | 38.60 [31.40, 47.40] | <0.001 | |
| CTSqlt | 0.99 [0.60, 1.96] | 0.63 [0.32, 1.36] | <0.001 | |
| CTSqnt/HDL-c | 0.81 [0.58, 1.15] | 0.62 [0.44, 0.89] | <0.001 |
| Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|
| Index | λ *1 | HR (/1 SD) | 95% CI | p | HR (/1 SD) | 95% CI | p |
| CTSqnt | −0.2 | 1.595 | 1.352–1.881 | <0.001 | 1.354 | 1.131–1.622 | 0.001 |
| TG | 0.3 | 1.655 | 1.393–1.967 | <0.001 | 1.350 | 1.114–1.636 | 0.002 |
| NonHDL-c | 0.4 | 1.512 | 1.277–1.790 | <0.001 | 1.349 | 1.134–1.604 | <0.001 |
| CTSqlt | −0.2 | 1.587 | 1.338–1.893 | <0.001 | 1.281 *2 | 1.060–1.549 | 0011 |
| LDL-c | 0.6 | 1.325 | 1.117–1.568 | 0.001 | 1.270 | 1.078–1.506 | 0.005 |
| TC | 0.3 | 1.294 | 1.117–1.530 | 0.003 | 1.214 *3 | 1.024–1.439 | 0.025 |
| HDL-c | −0.1 | 0.673 | 0.567–0.798 | <0.001 | 0.741 | 0.616–0.891 | 0.001 |
| Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|
| Index | λ *1 | HR (/1 SD) | 95% CI | p | HR (/1 SD) | 95% CI | p |
| LDL-c/HDL-c | 0.3 | 1.571 | 1.323–1.866 | <0.001 | 1.454 | 1.212–1.744 | <0.001 |
| CTSqnt/HDL-c | −0.1 | 1.642 | 1.388–1.943 | <0.001 | 1.428 | 1.186–1.721 | <0.001 |
| TG/HDL-c | −0.3 | 1.685 | 1.416–2.004 | <0.001 | 1.411 | 1.161–1.714 | <0.001 |
| Groups *1 | ||||
|---|---|---|---|---|
| Index | G-1 | G-2 | G-3 | |
| CTSqlt | Min to Max | 0.038–0.412 | 0.412–1.019 | 1.019–252.001 |
| HR (vs. G-1) *2 | - | 2.331 | 2.295 | |
| (95% CI) | - | (1.321–4.112) | (1.299–4.056) | |
| p (vs. G-1) | - | 0.003 | 0.004 | |
| TG/HDL-c | Min to Max | 0.173–1.015 | 1.015–1.982 | 1.982–46.800 |
| HR (vs. G-1) *2 | - | 1.775 | 2.387 | |
| (95% CI) | - | (1.016–3.101) | (1.386–4.112) | |
| p (vs. G-1) | - | 0.044 | 0.002 | |
| CTSqnt | Min to Max | 11.2–33.8 | 33.9–44.1 | 44.2–230.4 |
| HR (vs. G-1) *2 | - | 1.446 | 2.190 | |
| (95% CI) | - | (0.849–2.461) | (1.332–3.601) | |
| p (vs. G-1) | - | 0.175 | 0.002 | |
| NonHDL-c | Min to Max | 39–127 | 128–158 | 159–378 |
| HR (vs. G-1) *2 | - | 1.451 | 1.828 | |
| (95% CI) | - | (0.893–2.902) | (1.152–2.902) | |
| p (vs. G-1) | - | 0.133 | 0.010 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsunaga, I.; Ando, M.; Tsubakimoto, Y.; Nagasawa, M.; Kurumi, Y. Usefulness of Novel Atherogenic Lipid Indices for the Evaluation of Metabolic Status Leading to Coronary Heart Disease in a Real-World Survey of the Japanese Population. Healthcare 2022, 10, 747. https://doi.org/10.3390/healthcare10040747
Matsunaga I, Ando M, Tsubakimoto Y, Nagasawa M, Kurumi Y. Usefulness of Novel Atherogenic Lipid Indices for the Evaluation of Metabolic Status Leading to Coronary Heart Disease in a Real-World Survey of the Japanese Population. Healthcare. 2022; 10(4):747. https://doi.org/10.3390/healthcare10040747
Chicago/Turabian StyleMatsunaga, Isamu, Miyuki Ando, Yuki Tsubakimoto, Miyuki Nagasawa, and Yoshimasa Kurumi. 2022. "Usefulness of Novel Atherogenic Lipid Indices for the Evaluation of Metabolic Status Leading to Coronary Heart Disease in a Real-World Survey of the Japanese Population" Healthcare 10, no. 4: 747. https://doi.org/10.3390/healthcare10040747
APA StyleMatsunaga, I., Ando, M., Tsubakimoto, Y., Nagasawa, M., & Kurumi, Y. (2022). Usefulness of Novel Atherogenic Lipid Indices for the Evaluation of Metabolic Status Leading to Coronary Heart Disease in a Real-World Survey of the Japanese Population. Healthcare, 10(4), 747. https://doi.org/10.3390/healthcare10040747






