Clinical Characteristics of ICI-Related Pancreatitis and Cholangitis Including Radiographic and Endoscopic Findings
Abstract
:1. Introduction
2. Results: ICI-Related Pancreatitis
2.1. Incidence
2.2. Diagnosis
2.2.1. Clinical Symptoms
2.2.2. Blood Examination
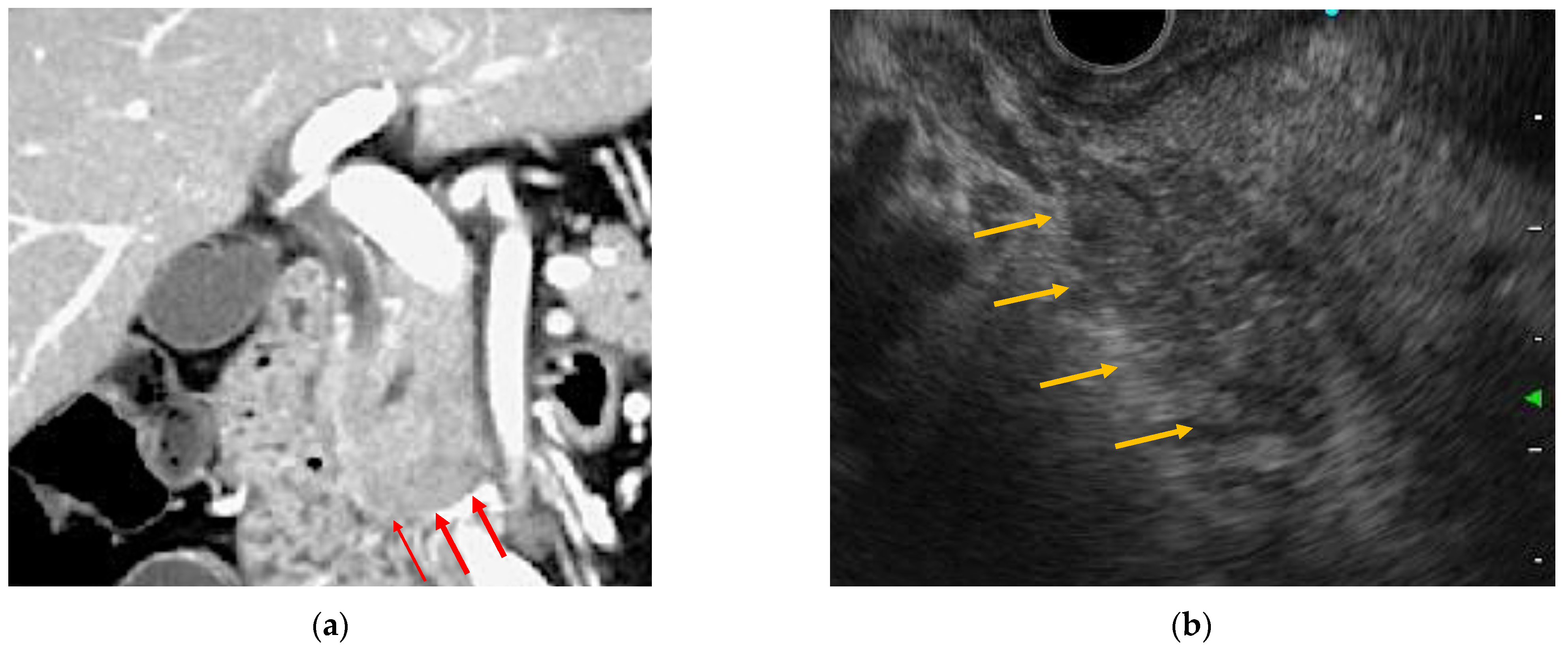
2.2.3. Radiology Images
2.3. Endoscopic Findings
2.3.1. EUS
2.3.2. ERCP
2.3.3. Histopathological Findings
2.4. Management
3. Results: ICI-Related Cholangitis
3.1. Incidence
3.2. Diagnosis
3.2.1. Clinical Symptoms
3.2.2. Blood Examination
3.2.3. Radiology Images
3.3. Endoscopic Findings
3.3.1. EUS
3.3.2. ERCP
3.3.3. Histopathological Findings
3.4. Management
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weber, J.S.; D’Angelo, S.P.; Minor, D.; Hodi, F.S.; Gutzmer, R.; Neyns, B.; Hoeller, C.; Khushalani, N.I.; Miller, W.H., Jr.; Lao, C.D.; et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015, 16, 375–384. [Google Scholar] [CrossRef]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, J.; Page, D.B.; Li, B.T.; Connell, L.C.; Schindler, K.; Lacouture, M.E.; Postow, M.A.; Wolchok, J.D. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann. Oncol. 2015, 26, 2375–2391. [Google Scholar] [CrossRef]
- Hofmann, L.; Forschner, A.; Loquai, C.; Goldinger, S.M.; Zimmer, L.; Ugurel, S.; Schmidgen, M.I.; Gutzmer, R.; Utikal, J.; Göppner, D.; et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur. J. Cancer 2016, 60, 190–209. [Google Scholar] [CrossRef]
- Postow, M.A. Managing Immune Checkpoint-Blocking Antibody Side Effects. Am. Soc. Clin. Oncol. Educ. Book 2015, 35, 76–83. [Google Scholar] [CrossRef]
- Kumar, V.; Chaudhary, N.; Garg, M.; Floudas, C.S.; Soni, P.; Chandra, A.B. Current Diagnosis and Management of Immune Related Adverse Events (irAEs) Induced by Immune Checkpoint Inhibitor Therapy. Front. Pharmacol. 2017, 8, 49. [Google Scholar] [CrossRef] [Green Version]
- Gangadhar, T.C.; Vonderheide, R.H. Mitigating the toxic effects of anticancer immunotherapy. Nat. Rev. Clin. Oncol. 2014, 11, 91–99. [Google Scholar] [CrossRef]
- Kong, Y.-C.M.; Flynn, J.C. Opportunistic Autoimmune Disorders Potentiated by Immune-Checkpoint Inhibitors Anti-CTLA-4 and Anti-PD-1. Front. Immunol. 2014, 5, 206. [Google Scholar] [CrossRef]
- Spain, L.; Diem, S.; Larkin, J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat. Rev. 2016, 44, 51–60. [Google Scholar] [CrossRef]
- Thompson, J.A.; Schneider, B.J.; Brahmer, J.; Andrews, S.; Armand, P.; Bhatia, S.; Budde, L.E.; Costa, L.; Davies, M.; Dunnington, D.; et al. NCCN Guidelines Insights: Management of Immunotherapy-Related Toxicities, Version 1.2020. J. Natl. Compr. Cancer Netw. 2020, 18, 230–241. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.K.; De Felice, K.M.; Loftus, E.V., Jr.; Khanna, S.K. Systematic review: Colitis associated with anti-CTLA-4 therapy. Aliment. Pharmacol. Ther. 2015, 42, 406–417. [Google Scholar] [CrossRef]
- Suzman, D.L.; Pelosof, L.; Rosenberg, A.; Avigan, M.I. Hepatotoxicity of immune checkpoint inhibitors: An evolving picture of risk associated with a vital class of immunotherapy agents. Liver Int. 2018, 38, 976–987. [Google Scholar] [CrossRef] [Green Version]
- Michot, J.-M.; Ragou, P.; Carbonnel, F.; Champiat, S.; Voisin, A.-L.; Mateus, C.; Lambotte, O.; Annereau, M. Significance of Immune-related Lipase Increase Induced by Antiprogrammed Death-1 or Death Ligand-1 Antibodies: A Brief Communication. J. Immunother. 2018, 41, 84–85. [Google Scholar] [CrossRef]
- Cramer, P.; Bresalier, R.S. Gastrointestinal and Hepatic Complications of Immune Checkpoint Inhibitors. Curr. Gastroenterol. Rep. 2017, 19, 3. [Google Scholar] [CrossRef]
- Tanaka, T.; Sakai, A.; Shiomi, H.; Masuda, A.; Kobayashi, T.; Tanaka, S.; Nakano, R.; Shigeoka, M.; Koma, Y.-I.; Kodama, Y. An autopsy case of severe acute pancreatitis induced by administration of pazopanib following nivolumab. Pancreatology 2021, 21, 21–24. [Google Scholar] [CrossRef]
- Tirumani, S.H.; Ramaiya, N.H.; Keraliya, A.R.; Bailey, N.D.; Ott, P.A.; Hodi, F.S.; Nishino, M. Radiographic Profiling of Immune-Related Adverse Events in Advanced Melanoma Patients Treated with Ipilimumab. Cancer Immunol. Res. 2015, 3, 1185–1192. [Google Scholar] [CrossRef] [Green Version]
- Friedman, C.F.; Clark, V.; Raikhel, A.V.; Barz, T.; Shoushtari, A.N.; Momtaz, P.; Callahan, M.K.; Wolchok, J.D.; Chapman, P.B.; Hellmann, M.D.; et al. Thinking Critically About Classifying Adverse Events: Incidence of Pancreatitis in Patients Treated With Nivolumab + Ipilimumab. JNCI J. Natl. Cancer Inst. 2016, 109, djw260. [Google Scholar] [CrossRef] [Green Version]
- Clamon, G.; Patel, R.; Mott, S. Pancreatitis associated with newer classes of antineoplastic therapies. J. Community Support. Oncol. 2017, 15, e135–e141. [Google Scholar] [CrossRef] [Green Version]
- George, J.; Bajaj, D.; Sankaramangalam, K.; Yoo, J.W.; Joshi, N.; Gettinger, S.; Price, C.; Farrell, J.J. Incidence of pancreatitis with the use of immune checkpoint inhibitors (ICI) in advanced cancers: A systematic review and meta-analysis. Pancreatol. 2019, 19, 587–594. [Google Scholar] [CrossRef]
- Ofuji, K.; Hiramatsu, K.; Nosaka, T.; Naito, T.; Takahashi, K.; Matsuda, H.; Ohtani, M.; Imamura, Y.; Ishizuka, T.; Nakamoto, Y. Pembrolizumab-induced autoimmune side effects of colon and pancreas in a patient with lung cancer. Clin. J. Gastroenterol. 2021, 14, 1692–1699. [Google Scholar] [CrossRef]
- Dehghani, L.; Mikail, N.; Kramkimel, N.; Soyer, P.; Lebtahi, R.; Mallone, R.; Larger, E. Autoimmune pancreatitis after nivolumab anti–programmed death receptor-1 treatment. Eur. J. Cancer 2018, 104, 243–246. [Google Scholar] [CrossRef]
- Das, J.P.; Postow, M.A.; Friedman, C.F.; Do, R.K.; Halpenny, D.F. Imaging findings of immune checkpoint inhibitor associated pancreatitis. Eur. J. Radiol. 2020, 131, 109250. [Google Scholar] [CrossRef]
- Capurso, G.; Archibugi, L.; Tessieri, L.; Petrone, M.C.; Laghi, A.; Arcidiacono, P.G. Focal immune-related pancreatitis occurring after treatment with programmed cell death 1 inhibitors: A distinct form of autoimmune pancreatitis? Eur. J. Cancer 2018, 95, 123–126. [Google Scholar] [CrossRef]
- Saito, H.; Ono, K. Nivolumab-induced Pancreatitis: An Immune-related Adverse Event. Radiol. 2019, 293, 521. [Google Scholar] [CrossRef]
- Kakuwa, T.; Hashimoto, M.; Izumi, A.; Naka, G.; Takeda, Y.; Sugiyama, H. Pembrolizumab-related pancreatitis with elevation of pancreatic tumour markers. Respirol. Case Rep. 2020, 8, e00525. [Google Scholar] [CrossRef]
- Tanaka, T.; Sakai, A.; Kobayashi, T.; Masuda, A.; Shiomi, H.; Kodama, Y. Nivolumab-related pancreatitis with autoimmune pancreatitis-like imaging features. J. Gastroenterol. Hepatol. 2019, 34, 1274. [Google Scholar] [CrossRef] [Green Version]
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S.; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012: Revision of the Atlanta classification and definitions by international consensus. Gut 2013, 62, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Di Giacomo, A.M.; Danielli, R.; Guidoboni, M.; Calabrò, L.; Carlucci, D.; Miracco, C.; Volterrani, L.; Mazzei, M.A.; Biagioli, M.; Altomonte, M.; et al. Therapeutic efficacy of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: Clinical and immunological evidence from three patient cases. Cancer Immunol. Immunother. 2009, 58, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Abu-Sbeih, H.; Tang, T.; Lu, Y.; Thirumurthi, S.; Altan, M.; Jazaeri, A.A.; Dadu, R.; Coronel, E.; Wang, Y. Clinical characteristics and outcomes of immune checkpoint inhibitor-induced pancreatic injury. J. Immunother. Cancer 2019, 7, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vissers, R.J.; Abu-Laban, R.B.; McHugh, D.F. Amylase and lipase in the emergency department evaluation of acute pancreatitis. J. Emerg. Med. 1999, 17, 1027–1037. [Google Scholar] [CrossRef]
- Janssens, L.; Takahashi, N.; Majumder, S. Pancreatic Atrophy in Nivolumab-Associated Pancreatitis Mimics Autoimmune Pancreatitis. Pancreas 2021, 50, e28–e29. [Google Scholar] [CrossRef]
- Song, Z.; Shih, J.; Seid, D.S. Rare Case of Nivolumab-Induced Chronic Pancreatitis. Clin. Gastroenterol. Hepatol. 2021. [Google Scholar] [CrossRef]
- Sugumar, A.; Levy, M.J.; Kamisawa, T.; Webster, G.J.; Kim, M.; Enders, F.; Amin, Z.; Baron, T.H.; Chapman, M.H.; I Church, N.; et al. Endoscopic retrograde pancreatography criteria to diagnose autoimmune pancreatitis: An international multicentre study. Gut 2011, 60, 666–670. [Google Scholar] [CrossRef]
- Wakabayashi, T.; Kawaura, Y.; Satomura, Y.; Watanabe, H.; Motoo, Y.; Okai, T.; Sawabu, N. Clinical and imaging features of autoimmune pancreatitis with focal pancreatic swelling or mass formation: Comparison with so-called tumor-forming pancreatitis and pancreatic carcinoma. Am. J. Gastroenterol. 2003, 98, 2679–2687. [Google Scholar] [CrossRef]
- Suda, T.; Kobayashi, M.; Kurokawa, K.; Matsushita, E. Simultaneous occurrence of autoimmune pancreatitis and sclerosing cholangitis as immune-related adverse events of pembrolizumab. BMJ Case Rep. 2021, 14, e243360. [Google Scholar] [CrossRef]
- Sznol, M.; Ferrucci, P.F.; Hogg, D.; Atkins, M.B.; Wolter, P.; Guidoboni, M.; Lebbé, C.; Kirkwood, J.M.; Schachter, J.; Daniels, G.A.; et al. Pooled Analysis Safety Profile of Nivolumab and Ipilimumab Combination Therapy in Patients With Advanced Melanoma. J. Clin. Oncol. 2017, 35, 3815–3822. [Google Scholar] [CrossRef] [Green Version]
- Gelsomino, F.; Vitale, G.; D’Errico, A.; Bertuzzi, C.; Andreone, P.; Ardizzoni, A. Nivolumab-induced cholangitic liver disease: A novel form of serious liver injury. Ann. Oncol. 2017, 28, 671–672. [Google Scholar] [CrossRef]
- Kawakami, H.; Tanizaki, J.; Tanaka, K.; Haratani, K.; Hayashi, H.; Takeda, M.; Kamata, K.; Takenaka, M.; Kimura, M.; Chikugo, T.; et al. Imaging and clinicopathological features of nivolumab-related cholangitis in patients with non-small cell lung cancer. Investig. New Drugs 2017, 35, 529–536. [Google Scholar] [CrossRef]
- Kashima, J.; Okuma, Y.; Shimizuguchi, R.; Chiba, K. Bile duct obstruction in a patient treated with nivolumab as second-line chemotherapy for advanced non-small-cell lung cancer: A case report. Cancer Immunol. Immunother. 2017, 67, 61–65. [Google Scholar] [CrossRef]
- Koya, Y.; Shibata, M.; Shinohara, N.; Nebuya, S.; Oe, S.; Honma, Y.; Senju, M.; Sato, N.; Harada, M. Secondary sclerosing cholangitis with hemobilia induced by pembrolizumab: Case report and review of published work. Hepatol. Res. 2019, 49, 950–956. [Google Scholar] [CrossRef]
- McClure, T.; Cui, W.; Asadi, K.; John, T.; Testro, A. Case of nivolumab-induced sclerosing cholangitis: Lessons from long-term follow-up. BMJ Open Gastroenterol. 2020, 7, e000487. [Google Scholar] [CrossRef]
- Sato, K.; Hayashi, M.; Abe, K.; Fujita, M.; Takahashi, A.; Ohira, H. Pembrolizumab-induced sclerosing cholangitis in a lung adenocarcinoma patient with a remarkable response to chemotherapy: A case report. Clin. J. Gastroenterol. 2020, 13, 1310–1314. [Google Scholar] [CrossRef]
- Cho, J.H.; Sun, J.-M.; Lee, S.-H.; Ahn, J.S.; Park, K.; Ahn, M.-J. Late-Onset Cholecystitis with Cholangitis after Avelumab Treatment in Non–Small Cell Lung Cancer. J. Thorac. Oncol. 2018, 13, e34–e36. [Google Scholar] [CrossRef] [Green Version]
- Yoshikawa, Y.; Imamura, M.; Yamaoka, K.; Kosaka, Y.; Murakami, E.; Morio, K.; Fujino, H.; Nakahara, T.; Okamoto, W.; Yamauchi, M.; et al. A case with life-threatening secondary sclerosing cholangitis caused by nivolumab. Clin. J. Gastroenterol. 2021, 14, 283–287. [Google Scholar] [CrossRef]
- Ogawa, K.; Kamimura, K.; Terai, S. Antiprogrammed Cell Death-1 Immunotherapy-Related Secondary Sclerosing Cholangitis. Hepatology 2018, 69, 914–916. [Google Scholar] [CrossRef] [Green Version]
- Onoyama, T.; Takeda, Y.; Kato, M.; Edano, M.; Tarumoto, R.; Matsumoto, K.; Isomoto, H. Peroral cholangioscopy of programmed cell death-1 inhibitor-related sclerosing cholangitis: Three case reports. Laryngo-Rhino-Otologie 2019, 51, E402–E403. [Google Scholar] [CrossRef] [Green Version]
- Tahboub Amawi, A.D.; Tremaine, W.J.; Venkatesh, S.K. Pembrolizumab-Induced Sclerosing Cholangitis. Clin. Gastroenterol. Hepatol. 2022, 20, e18. [Google Scholar] [CrossRef] [PubMed]
- Kuraoka, N.; Hara, K.; Terai, S.; Yatabe, Y.; Horio, Y. Peroral cholangioscopy of nivolumab-related (induced) ulcerative cholangitis in a patient with non-small cell lung cancer. Laryngo-Rhino-Otologie 2018, 50, E259–E261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamoir, C.; De Vos, M.; Clinckart, F.; Nicaise, G.; Komuta, M.; Lanthier, N. Hepatobiliary and Pancreatic: Nivolumab-related cholangiopathy. J. Gastroenterol. Hepatol. 2018, 33, 1695. [Google Scholar] [CrossRef] [PubMed]
- Kono, M.; Sakurai, T.; Okamoto, K.; Masaki, S.; Nagai, T.; Komeda, Y.; Kamata, K.; Minaga, K.; Yamao, K.; Takenaka, M.; et al. Efficacy and Safety of Chemotherapy Following Anti-PD-1 Antibody Therapy for Gastric Cancer: A Case of Sclerosing Cholangitis. Intern. Med. 2019, 58, 1263–1266. [Google Scholar] [CrossRef] [Green Version]
- Hirasawa, Y.; Yoshimura, K.; Matsui, H.; Kubota, Y.; Ishida, H.; Arai, J.; Sakaki, M.; Oguro, N.; Shida, M.; Taniguchi, M.; et al. A case report on severe nivolumab-induced adverse events similar to primary sclerosing cholangitis refractory to immunosuppressive therapy. Medicine 2021, 100, e25774. [Google Scholar] [CrossRef]
- Miura, F.; Okamoto, K.; Takada, T.; Strasberg, S.M.; Asbun, H.J.; Pitt, H.A.; Gomi, H.; Solomkin, J.; Schlossberg, D.; Han, H.-S.; et al. Tokyo Guidelines 2018: Initial management of acute biliary infection and flowchart for acute cholangitis. J. Hepato-Biliary-Pancreatic Sci. 2017, 25, 31–40. [Google Scholar] [CrossRef]
- Sawada, K.; Shonaka, T.; Nishikawa, Y.; Hasegawa, K.; Hayashi, H.; Hasebe, T.; Nakajima, S.; Ikuta, K.; Fujiya, M.; Furukawa, H.; et al. Successful Treatment of Nivolumab-related Cholangitis with Prednisolone: A Case Report and Review of the Literature. Intern. Med. 2019, 58, 1747–1752. [Google Scholar] [CrossRef] [Green Version]
- Talbot, S.; MacLaren, V.; Lafferty, H. Sclerosing cholangitis in a patient treated with nivolumab. BMJ Case Rep. 2021, 14, e241700. [Google Scholar] [CrossRef]
- Anderson, B.; Dawe, D.E. Nivolumab-Induced Secondary Sclerosing Cholangitis with Deterioration Despite Immunosuppression. J. Thorac. Oncol. 2019, 14, e205–e206. [Google Scholar] [CrossRef]
- Zen, Y.; Chen, Y.; Jeng, Y.; Tsai, H.; Yeh, M.M. Immune-related adverse reactions in the hepatobiliary system: Second-generation check-point inhibitors highlight diverse histological changes. Histopathology 2020, 76, 470–480. [Google Scholar] [CrossRef]

| No. | Ref. | Sex | Age | ICI | CT Findings | MRI Findings | EUS Findings | ERCP Findings | Imaging Type |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Ofuji et al. [21] | M | 82 | pembrolizumab | diffuse enlargement | diffuse restricted diffusion diffuse enlargement narrowing of the MPD | hypoechoic enlargement hyperechoic spots | NA | autoimmune pancreatitis |
| 2 | Dehghani et al. [22] | M | 63 | nivolumab | focal enlargement fat stranding | focal restricted diffusion late enhancement | NA | NA | autoimmune pancreatitis |
| 3 | Das et al. [23] | M | 47 | nivolumab | diffuse enlargement diffuse fat stranding | NA | NA | NA | acute interstitial pancreatitis |
| 4 | Das et al. [23] | F | 70 | nivolumab | focal enlargement subtle fat stranding | NA | NA | NA | acute interstitial pancreatitis |
| 5 | Das et al. [23] | F | 50 | pembrolizumab | NA | focal enlargement abrupt cut-off of the CBD | NA | NA | autoimmune pancreatitis |
| 6 | Das et al. [23] | F | 64 | nivolumab | diffuse enlargement heterogenous enhancement fat stranding | NA | NA | NA | acute interstitial pancreatitis |
| 7 | Das et al. [23] | F | 56 | ipilimumab nivolumab | NA | NA | NA | NA | autoimmune pancreatitis |
| 8 | Capurso et al. [24] | F | 76 | pembrolizumab | MPD dilation | MPD dilation focal restricted diffusion | hypoechoic solid lesion stiff at elastography stenosis of the MPD | NA | autoimmune pancreatitis |
| 9 | Saito et al. [25] | M | 72 | nivolumab | diffuse enlargement | NA | NA | NA | acute interstitial pancreatitis |
| 10 | Kakuwa et al. [26] | M | 70 | pembrolizumab | mild diffuse enlargement MPD dilation | NA | NA | NA | autoimmune pancreatitis |
| 11 | Tanaka et al. [27] | F | 70 | nivolumab | NA | diffuse enlargement focal restricted diffusion | diffuse hypoechoic enlargement | skipped narrowing of the MPD | autoimmune pancreatitis |
| No. | Ref. | Sex | Age | ICI | CT Findings | MRI Findings | EUS Findings | ERCP Findings | Imaging Type |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Kawakami et al. [39] | M | 64 | Nivolumab | E; dilation, hypertrophy I; NA G; hypertrophy | NA | E; dilation, diffuse hypertrophy I; normal G; hypertrophy | NA | IgG4-SC |
| 2 | Kawakami et al. [39] | F | 73 | Nivolumab | E; dilation I; NA G; hypertrophy | E; dilation I; NA G; NA | E, diffuse hypertrophy I; normal G; NA | E; dilation, distal stenosis I; normal G; NA | IgG4-SC |
| 3 | Kawakami et al. [39] | F | 82 | Nivolumab | E; dilation I; NA G; NA | E; dilation I; NA G; NA | E; dilation, diffuse hypertrophy I; normal G; NA | E; dilation, distal stenosis I; normal G; NA | IgG4-SC |
| 4 | Kashima et al. [40] | M | 63 | Nivolumab | E; dilation, beaking stenosis I; NA G; hypertrophy | E; dilation, beaking I; normal G; normal | NA | NA | IgG4-SC |
| 5 | Koya et al. [41] | M | 66 | Pembrolizumab | E; hypertrophy I; NA G; NA | E; normal I; multiple irregular narrowing G; NA | E; diffuse hypertrophy I; NA G; NA | E; irregularity of the bile duct I; multiple irregular narrowing G; NA | PSC |
| 6 | McClure et al. [42] | M | 79 | Nivolumab | NA | E; NA I; beaded appearance G; NA | NA | NA | PSC |
| 7 | Sato et al. [43] | M | 69 | Pembrolizumab | E; diffuse hypertrophy I; dilation G; hypertrophy | E; normal I; multiple irregular narrowing G; normal | NA | NA | PSC and IgG-SC |
| 8 | Cho et al. [44] | M | 69 | Avelumab | E; dilation, hypertrophy I; normal G; hypertrophy | NA | NA | NA | IgG4-SC |
| 9 | Yoshikawa et al. [45] | M | 75 | Nivolumab | NA | E; diffuse dilatation I; multifocal stenosis G; normal | NA | NA | PSC |
| 10 | Ogawa et al. [46] | M | 73 | Pembrolizumab | E; hypertrophy I; dilation G; hypertrophy | NA | E; irregular hypertrophy I; irregular hypertrophy G; NA | E; irregularity of the bile duct I; multiple irregular narrowing G; NA | PSC |
| 11 | Onoyama et al. [47] | M | 63 | Pembrolizumab | E; irregular hypertrophy I; NA G; NA | NA | E; irregular hypertrophy I; NA G; NA | E; irregularity of the bile duct I; normal G; NA | IgG4-SC |
| 12 | Tahboub et al. [48] | M | 67 | Pembrolizumab | E; diffuse hypertrophy I; dilation G; hypertrophy | E; normal I; multiple irregular narrowing G; normal | NA | NA | PSC |
| 13 | Kuraoka et al. [49] | M | 69 | Nivolumab | E; hypertrophy I; NA G; NA | NA | E; diffuse hypertrophy I; NA G; NA | E; irregularity of the bile duct I; multiple irregular narrowing G; NA | PSC and IgG-SC |
| 14 | Hamoir et al. [50] | M | 71 | Nivolumab | E; normal I; normal G; normal | E; normal I; multiple stenosis G; NA | NA | NA | PSC |
| 15 | Kono et al. [51] | F | 69 | Nivolumab | E; hypertrophy I; NA G; hypertrophy | E; NA I; dilation G; hypertrophy | E; NA I; dilation G; hypertrophy | E; stenosis I; multiple irregular narrowing G; NA | PSC and IgG-SC |
| 16 | Hirasawa et al. [52] | M | 64 | Nivolumab | E; diffuse hypertrophy I; dilation G; hypertrophy | E; hypertrophy I; beaded appearance G; normal | E; dilation I; NA G; hypertrophy | E; dilation I; NA G; NA | PSC and IgG-SC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakano, R.; Shiomi, H.; Fujiwara, A.; Yoshihara, K.; Yoshioka, R.; Kawata, S.; Ota, S.; Yuri, Y.; Takashima, T.; Aizawa, N.; et al. Clinical Characteristics of ICI-Related Pancreatitis and Cholangitis Including Radiographic and Endoscopic Findings. Healthcare 2022, 10, 763. https://doi.org/10.3390/healthcare10050763
Nakano R, Shiomi H, Fujiwara A, Yoshihara K, Yoshioka R, Kawata S, Ota S, Yuri Y, Takashima T, Aizawa N, et al. Clinical Characteristics of ICI-Related Pancreatitis and Cholangitis Including Radiographic and Endoscopic Findings. Healthcare. 2022; 10(5):763. https://doi.org/10.3390/healthcare10050763
Chicago/Turabian StyleNakano, Ryota, Hideyuki Shiomi, Aoi Fujiwara, Kohei Yoshihara, Ryota Yoshioka, Shoki Kawata, Shogo Ota, Yukihisa Yuri, Tomoyuki Takashima, Nobuhiro Aizawa, and et al. 2022. "Clinical Characteristics of ICI-Related Pancreatitis and Cholangitis Including Radiographic and Endoscopic Findings" Healthcare 10, no. 5: 763. https://doi.org/10.3390/healthcare10050763
APA StyleNakano, R., Shiomi, H., Fujiwara, A., Yoshihara, K., Yoshioka, R., Kawata, S., Ota, S., Yuri, Y., Takashima, T., Aizawa, N., Ikeda, N., Nishimura, T., Enomoto, H., & Iijima, H. (2022). Clinical Characteristics of ICI-Related Pancreatitis and Cholangitis Including Radiographic and Endoscopic Findings. Healthcare, 10(5), 763. https://doi.org/10.3390/healthcare10050763






