Uterine Fibroids and Pregnancy: A Review of the Challenges from a Romanian Tertiary Level Institution
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pavone, D.; Clemenza, S.; Sorbi, F.; Fambrini, M.; Petraglia, F. Epidemiology and Risk Factors of Uterine Fibroids. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 46, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Parker, W.H. Etiology, Symptomatology, and Diagnosis of Uterine Myomas. Fertil. Steril. 2007, 87, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, E.; As-Sanie, S.; Marsh, E.E. Epidemiology and Management of Uterine Fibroids. Int. J. Gynecol. Obstet. 2020, 149, 3–9. [Google Scholar] [CrossRef]
- Wang, H.-M.; Tian, Y.-C.; Xue, Z.-F.; Zhang, Y.; Dai, Y.-M. Associations between Uterine Fibroids and Obstetric Outcomes in Twin Pregnancies. Int. J. Gynaecol. Obstet. 2016, 135, 22–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.J.; Norwitz, E.R.; Shaw, J. Contemporary Management of Fibroids in Pregnancy. Rev. Obstet. Gynecol. 2010, 3, 20–27. [Google Scholar] [PubMed]
- Benson, C.B.; Chow, J.S.; Chang-Lee, W.; Hill, J.A.; Doubilet, P.M. Outcome of Pregnancies in Women with Uterine Leiomyomas Identified by Sonography in the First Trimester. J. Clin. Ultrasound 2001, 29, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, K.E.; Velez Edwards, D.R.; Savitz, D.A.; Jonsson-Funk, M.L.; Wu, P.; Sundermann, A.C.; Baird, D.D. Prospective Cohort Study of Uterine Fibroids and Miscarriage Risk. Am. J. Epidemiol. 2017, 186, 1140–1148. [Google Scholar] [CrossRef]
- Klatsky, P.C.; Tran, N.D.; Caughey, A.B.; Fujimoto, V.Y. Fibroids and Reproductive Outcomes: A Systematic Literature Review from Conception to Delivery. Am. J. Obstet. Gynecol. 2008, 198, 357–366. [Google Scholar] [CrossRef]
- Abam, D.S.; Kasso, T. Uterine Fibroids and Pregnancy: A Review of the Challenges. In Obstetrics; Abduljabbar, H.S., Ed.; InTech: London, UK, 2017; ISBN 9789535137030. [Google Scholar]
- Morgan Ortiz, F.; Piña Romero, B.; Elorriaga García, E.; Báez Barraza, J.; Quevedo Castro, E.; de Peraza Garay, F.J. Uterine leiomyomas during pregnancy and its impact on obstetric outcome. Ginecol. Obstet. Mex. 2011, 79, 467–473. [Google Scholar]
- Vitale, S.G.; Tropea, A.; Rossetti, D.; Carnelli, M.; Cianci, A. Management of Uterine Leiomyomas in Pregnancy: Review of Literature. Updates Surg. 2013, 65, 179–182. [Google Scholar] [CrossRef]
- Yousra, K.; Erraghay, S.; Guennoun, A.; Mamouni, N.; Bouchikhi, C.; Banani, A. Myoma Praevia and Pregnancy. Pan. Afr. Med. J. 2019, 33, 216. [Google Scholar] [CrossRef] [PubMed]
- Chuang, J.; Tsai, H.-W.; Hwang, J.-L. Fetal Compression Syndrome Caused by Myoma in Pregnancy: A Case Report. Acta Obstet. Gynecol. Scand. 2001, 80, 472–473. [Google Scholar] [CrossRef] [PubMed]
- Sundermann, A.C.; Aldridge, T.D.; Hartmann, K.E.; Jones, S.H.; Torstenson, E.S.; Edwards, D.R.V. Uterine Fibroids and Risk of Preterm Birth by Clinical Subtypes: A Prospective Cohort Study. BMC Pregnancy Childbirth. 2021, 21, 560. [Google Scholar] [CrossRef]
- Day Baird, D.; Dunson, D.B.; Hill, M.C.; Cousins, D.; Schectman, J.M. High Cumulative Incidence of Uterine Leiomyoma in Black and White Women: Ultrasound Evidence. Am. J. Obstet. Gynecol. 2003, 188, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Shavell, V.I.; Thakur, M.; Sawant, A.; Kruger, M.L.; Jones, T.B.; Singh, M.; Puscheck, E.E.; Diamond, M.P. Adverse Obstetric Outcomes Associated with Sonographically Identified Large Uterine Fibroids. Fertil. Steril. 2012, 97, 107–110. [Google Scholar] [CrossRef]
- Egbe, T.O.; Badjang, T.G.; Tchounzou, R.; Egbe, E.-N.; Ngowe, M.N. Uterine Fibroids in Pregnancy: Prevalence, Clinical Presentation, Associated Factors and Outcomes at the Limbe and Buea Regional Hospitals, Cameroon: A Cross-Sectional Study. BMC Res. Notes 2018, 11, 889. [Google Scholar] [CrossRef] [Green Version]
- Dai, Y.; Xia, L.; Lin, J.; Xu, R.; You, W. Study on the Method of Enucleation of Anterior Uterine Fibroids by Transverse Incision of the Lower Uterine Segment during Cesarean Section. BMC Pregnancy Childbirth. 2021, 21, 744. [Google Scholar] [CrossRef]
- Al-Hendy, A.; Myers, E.R.; Stewart, E. Uterine Fibroids: Burden and Unmet Medical Need. Semin. Reprod. Med. 2017, 35, 473–480. [Google Scholar] [CrossRef] [Green Version]
- O’Sullivan, M.; Overton, C. Tailor Management to the Patient with Fibroids. Practitioner 2017, 261, 19–22. [Google Scholar]
- Benaglia, L.; Cardellicchio, L.; Filippi, F.; Paffoni, A.; Vercellini, P.; Somigliana, E.; Fedele, L. The Rapid Growth of Fibroids during Early Pregnancy. PLoS ONE 2014, 9, e85933. [Google Scholar] [CrossRef]
- Rosati, P.; Exacoustòs, C.; Mancuso, S. Longitudinal Evaluation of Uterine Myoma Growth during Pregnancy. A Sonographic Study. J. Ultrasound Med. 1992, 11, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Navid, S.; Arshad, S.; Qurat-ul-Ain; Meo, R.A. Impact of Leiomyoma in Pregnancy. J. Ayub. Med. Coll. Abbottabad 2012, 24, 90–92. [Google Scholar] [PubMed]
- Vergani, P.; Locatelli, A.; Ghidini, A.; Andreani, M.; Sala, F.; Pezzullo, J.C. Large Uterine Leiomyomata and Risk of Cesarean Delivery. Obstet. Gynecol. 2007, 109, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Coronado, G.D.; Marshall, L.M.; Schwartz, S.M. Complications in Pregnancy, Labor, and Delivery with Uterine Leiomyomas: A Population-Based Study. Obstet. Gynecol. 2000, 95, 764–769. [Google Scholar] [CrossRef]
- Okeji, N.A.; Eli, S.; Abam, D.S.; Nnaka, M.P. Inevitable caesarean myomectomy: Report of two cases. Gazette Med. 2015, 4, 378–381. [Google Scholar]
- Sparić, R. Uterine myomas in pregnancy, childbirth and puerperium. Srp. Arh. Celok. Lek. 2014, 142, 118–124. [Google Scholar] [CrossRef]
- Kotani, Y.; Tobiume, T.; Fujishima, R.; Shigeta, M.; Takaya, H.; Nakai, H.; Suzuki, A.; Tsuji, I.; Mandai, M.; Matsumura, N. Recurrence of Uterine Myoma after Myomectomy: Open Myomectomy versus Laparoscopic Myomectomy: Recurrence after Laparotomy vs LM. J. Obstet. Gynaecol. Res. 2018, 44, 298–302. [Google Scholar] [CrossRef] [Green Version]
- El-Refaie, W.; Hassan, M.; Abdelhafez, M.S. Myomectomy during Cesarean Section: A Retrospective Cohort Study. J. Gynecol. Obstet. Hum. Reprod. 2020, 49, 101900. [Google Scholar] [CrossRef]
- Pergialiotis, V.; Sinanidis, I.; Louloudis, I.-E.; Vichos, T.; Perrea, D.N.; Doumouchtsis, S.K. Perioperative Complications of Cesarean Delivery Myomectomy: A Meta-Analysis. Obstet. Gynecol. 2017, 130, 1295–1303. [Google Scholar] [CrossRef]
- Goyal, M.; Dawood, A.S.; Elbohoty, S.B.; Abbas, A.M.; Singh, P.; Melana, N.; Singh, S. Cesarean Myomectomy in the Last Ten Years; A True Shift from Contraindication to Indication: A Systematic Review and Meta-Analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 256, 145–157. [Google Scholar] [CrossRef]
- Awoleke, J.O. Myomectomy during Caesarean Birth in Fibroid-Endemic, Low-Resource Settings. Obstet. Gynecol. Int. 2013, 2013, 520834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dracea, L.; Codreanu, D. Vaginal Birth after Extensive Myomectomy during Pregnancy in a 39-Year-Old Nulliparous Woman. J. Obstet. Gynaecol. 2006, 26, 374–375. [Google Scholar] [CrossRef] [PubMed]
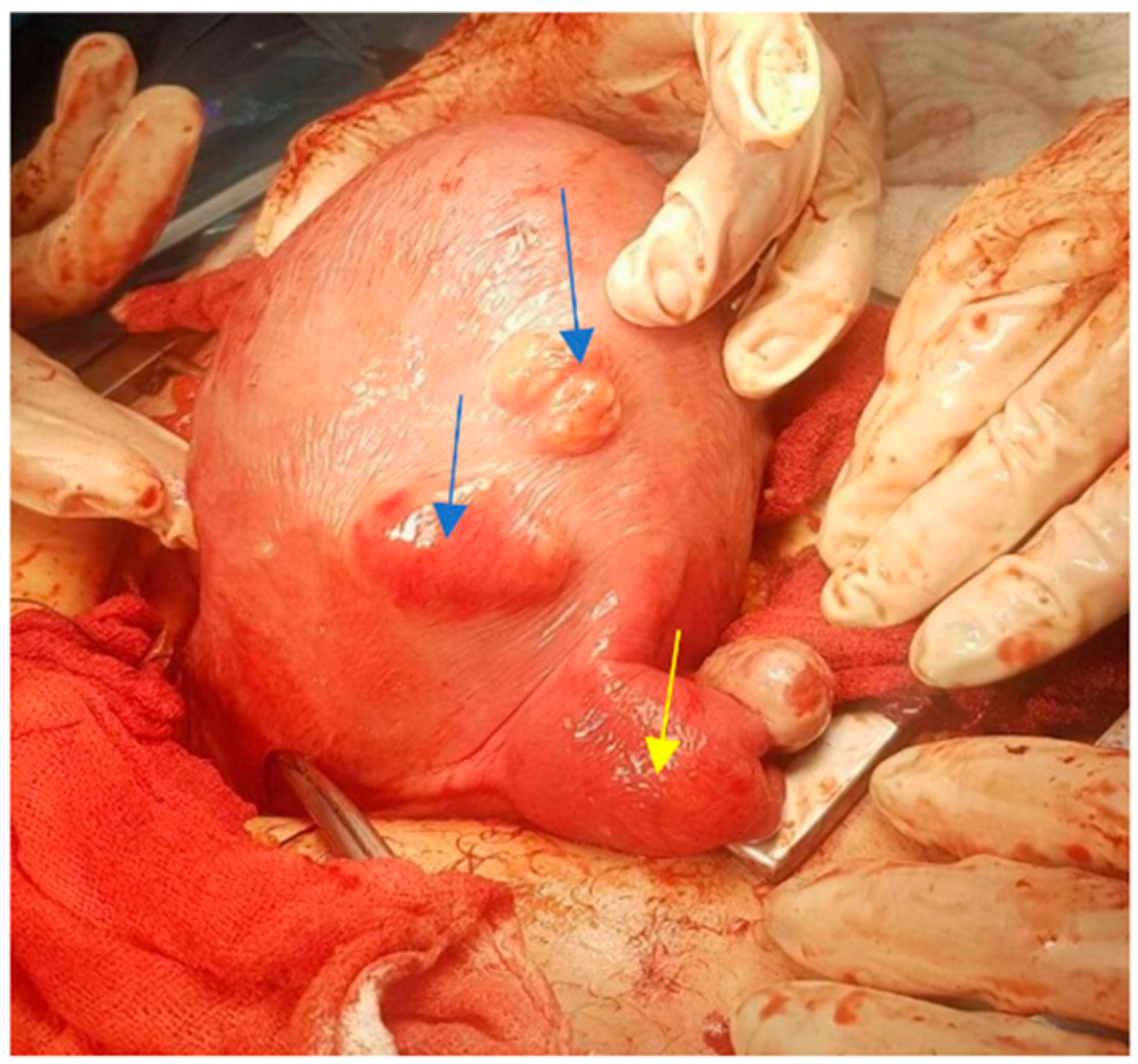









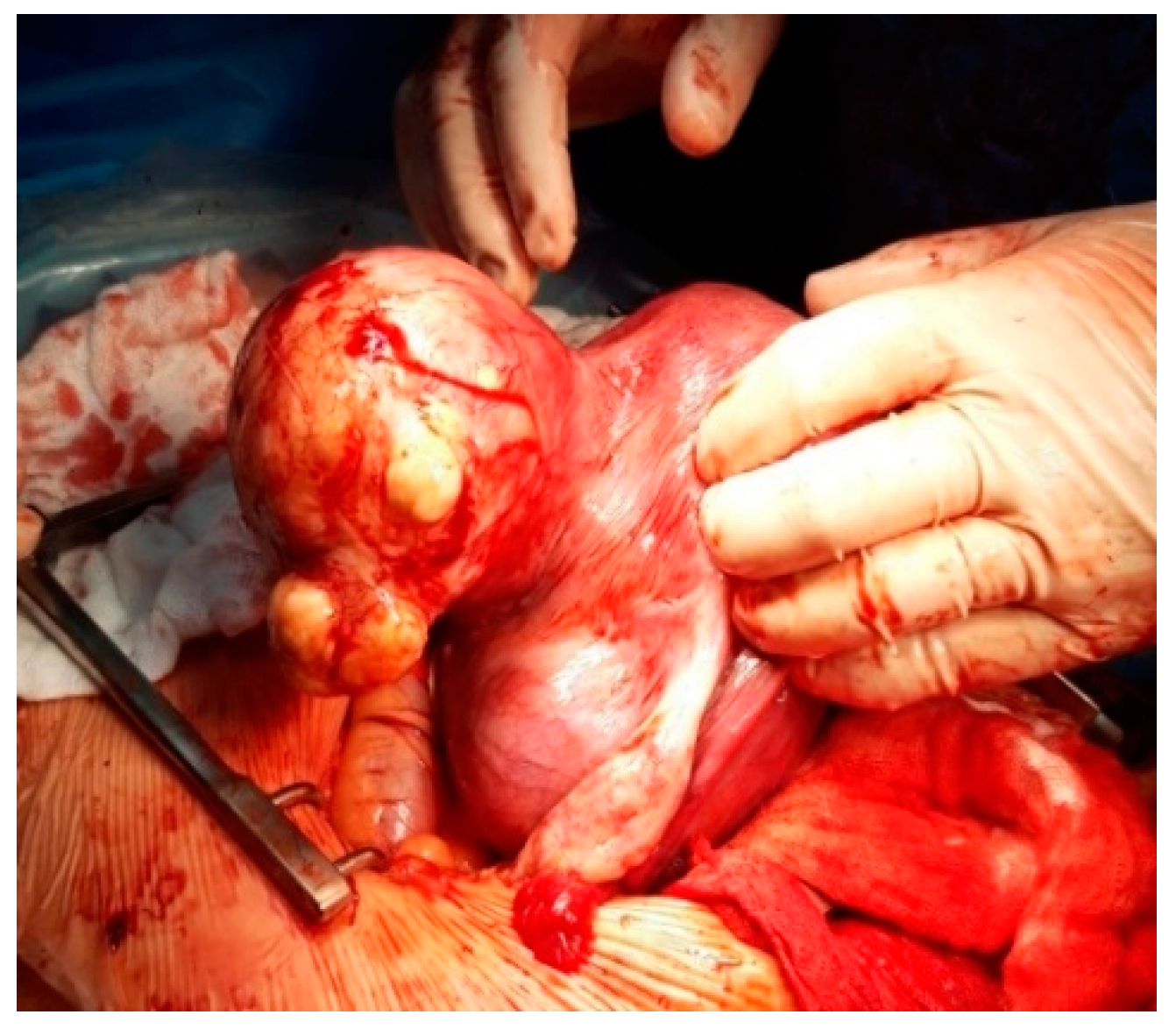





| Characteristics | Overall n = 57 | CS with Myomectomy n = 12 | CS without Myomectomy n = 37 | Abortions n = 4 | Normal Births n = 4 | p-Value |
|---|---|---|---|---|---|---|
| Age in years, (mean ± SD) § | 34.5 ± 4.2 | 34 ± 4.3 | 34.3 ± 3.7 | 38.3 ± 4.5 | 33.5 ± 3.7 | 0.4008 |
| Age, median (range) ** | 35 (31; 37) | 34 (30.5; 37.5) | 34 (31; 37) | 35 (31; 35) | 39 (34; 42) | |
| Environment, U/R, n(%) † | 46/11 (80.7/19.3) | 10/2 (83.3/16.7) | 31/6 (83.8/16.2) | 3/1 (75/25) | 2/2 (50/50) | 0.4262 |
| Previous abortions, n(%) | ||||||
| without one abortion more than two abortions | 40 (70.3) 7 (12.2) 10 (17.5) | 7 (58.4) 1 (8.3) 4 (33.3) | 27 (72) 6 (16.2) 4 (10.8) | 4 (100) - - | 2 (50) - 2 (50) | 0.2111 |
| Obese, Yes/No, n(%) | 11/46 (19.3/80.7) | 2/10 (16.7/83.3) | 6/31 (16.2/83.8) | 2/2 (50/50) | 1/3 (25/75) | 0.5198 |
| Previous births | ||||||
| without previous births single births multiple births | 37 (64.9) 17 (29.8) 3 (5.3) | 8 (66.7) 4 (33.3) - | 26 70.3) 9 (24.3) 2 (5.4) | 2 (50) 2 (50) - | 1 (25) 2 (50) 1 (25) | 0.4038 |
| Gestational age, weeks | ||||||
| mean ± SD median (range) | 38.2 ± 1.7 39 (38; 39) | 38.1 ± 1.2 38 (38; 39) | 38.3 ±1.6 39 (38; 39) | - | 37.3 ± 3.6 38 (35; 39) | 0.7464 |
| Intrauterine Growth Restriction, n(%) | 6 (10.5) | 2 (16.7) | 4 (10.8) | - | - | 0.7352 |
| Time of diagnosis with fibroids | ||||||
| before pregnancy during pregnancy | 26 (45.6) 31 (54.4) | 4 (33.3) 8 (66.7) | 18 (48.6) 19 (51.4) | 2 (50) 2 (50) | 2 (50) 2 (50) | 0.8184 |
| Number of fibroids | ||||||
| single fibroids multiple fibroids | 42 (73.7) 15 (26.3) | 8 (66.7) 4 (33.3) | 27 (73) 10 (37) | 3 (75) 1 (25) | 4 (100) - | 0.4324 |
| Type of fibroid | ||||||
| interstitial subserous submucous | - | 28 (49.12) 26 (45.62) 3 (5.26) | - | - | - | |
| Location of the myoma | ||||||
| anterior uterine wall fundic region posterior wall isthmic region right lateral walls left lateral walls | - | 29 (50.87) 11 (19.3) 7 (12.28) 6 (10.52) 1(1.75) 3 (5.26) | - | - | - |
| Size of Fibroid, mm | ||||
|---|---|---|---|---|
| Antepartum | 1st Trimester | 2nd Trimester | 3rd Trimester | |
| N | 55 | 49 | 50 | 53 |
| Mean | 40.02 | 44.51 p = 0.005 * | 54.76 p = 0.001 * | 61.58 p = 0.001 * |
| Difference from the previous average | - | +4.49 mm (+11.2%) | +10.25 mm (+23.0%) | +6.92 mm (+12.6%) |
| Median | 38 | 40 | 50 | 60 |
| Std. Deviation | 18.29 | 17.21 | 21.37 | 24.54 |
| Variance | 42.88 | 38.67 | 39.04 | 39.59 |
| Skewness | 0.970 | 0.733 | 0.664 | 1.636 |
| Std. Err. of Skewness | 0.322 | 0.340 | 0.337 | 0.327 |
| Minimum | 20 | 20 | 20 | 25 |
| Maximum | 90 | 90 | 100 | 160 |
| 25th percentile | 23 | 30 | 40 | 48 |
| 50th percentile | 40 | 40 | 50 | 60 |
| 75th percentile | 50 | 50 | 64 | 70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tîrnovanu, M.C.; Lozneanu, L.; Tîrnovanu, Ş.D.; Tîrnovanu, V.G.; Onofriescu, M.; Ungureanu, C.; Toma, B.F.; Cojocaru, E. Uterine Fibroids and Pregnancy: A Review of the Challenges from a Romanian Tertiary Level Institution. Healthcare 2022, 10, 855. https://doi.org/10.3390/healthcare10050855
Tîrnovanu MC, Lozneanu L, Tîrnovanu ŞD, Tîrnovanu VG, Onofriescu M, Ungureanu C, Toma BF, Cojocaru E. Uterine Fibroids and Pregnancy: A Review of the Challenges from a Romanian Tertiary Level Institution. Healthcare. 2022; 10(5):855. https://doi.org/10.3390/healthcare10050855
Chicago/Turabian StyleTîrnovanu, Mihaela Camelia, Ludmila Lozneanu, Ştefan Dragoş Tîrnovanu, Vlad Gabriel Tîrnovanu, Mircea Onofriescu, Carmen Ungureanu, Bogdan Florin Toma, and Elena Cojocaru. 2022. "Uterine Fibroids and Pregnancy: A Review of the Challenges from a Romanian Tertiary Level Institution" Healthcare 10, no. 5: 855. https://doi.org/10.3390/healthcare10050855
APA StyleTîrnovanu, M. C., Lozneanu, L., Tîrnovanu, Ş. D., Tîrnovanu, V. G., Onofriescu, M., Ungureanu, C., Toma, B. F., & Cojocaru, E. (2022). Uterine Fibroids and Pregnancy: A Review of the Challenges from a Romanian Tertiary Level Institution. Healthcare, 10(5), 855. https://doi.org/10.3390/healthcare10050855







