The Effects of Combined Physical and Cognitive Interventions on Direct and Indirect Fall Outcomes for the Elderly with Mild Cognitive Impairment: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Inclusion Criteria
2.2. Search Strategy
2.3. Study Selection and Quality
2.4. Data Extraction and Synthesis
3. Results
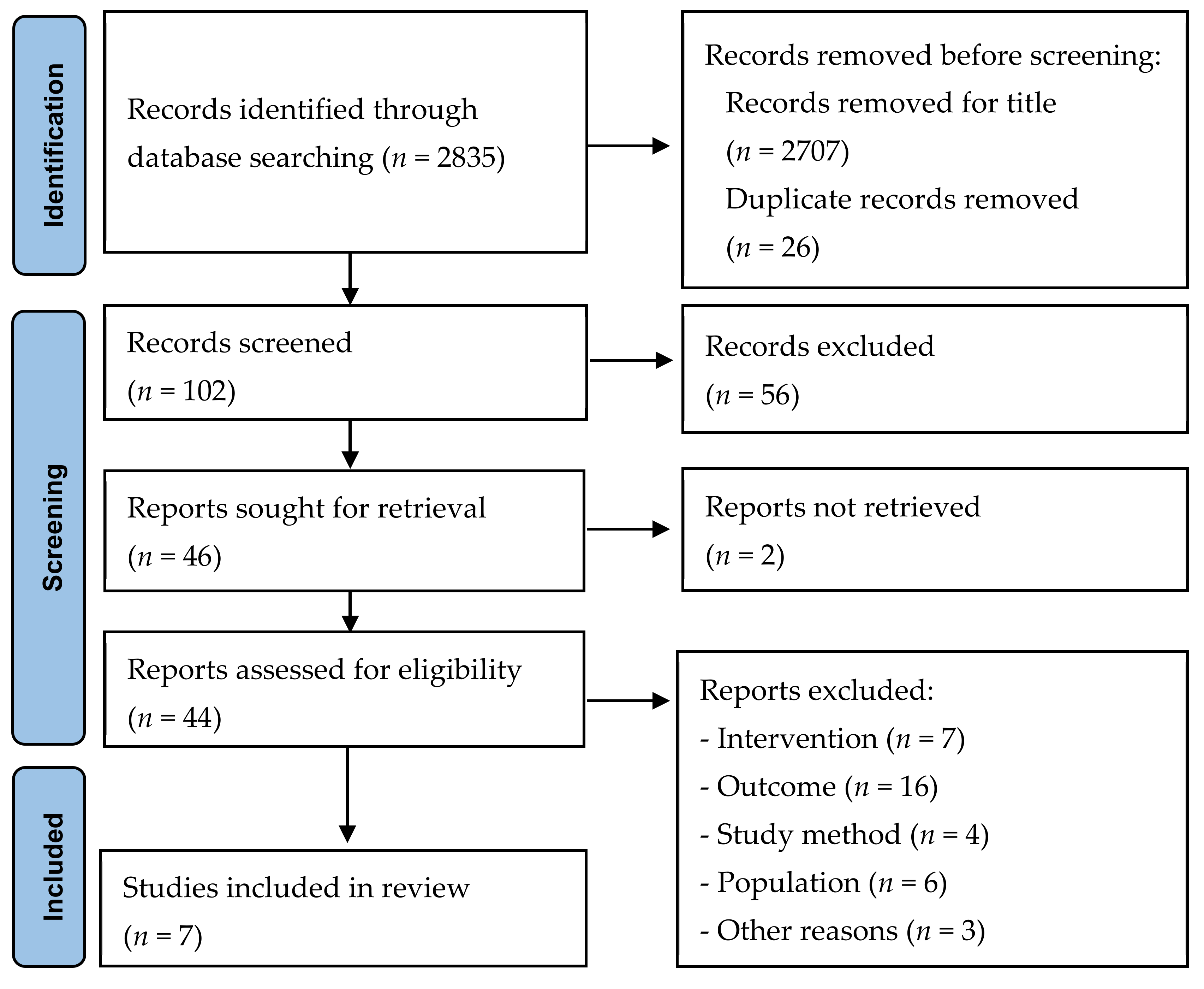
3.1. Characteristics of Included Studies
3.2. Methodological Quality
3.3. Effectiveness of PCT in Preventing Falls and Risks of Falls in the Elderly with MCI
3.3.1. Direct Fall Outcomes
3.3.2. Indirect Fall Outcomes/Risks of Fall
3.3.3. Cognitive Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shirooka, H.; Nishiguchi, S.; Fukutani, N.; Tashiro, Y.; Nozaki, Y.; Aoyama, T. Subjective cognitive decline and fall risk in community-dwelling older adults with or without objective cognitive decline. Aging Clin. Exp. Res. 2018, 30, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Tieri, G.; Morone, G.; Paolucci, S.; Iosa, M. Virtual reality in cognitive and motor rehabilitation: Facts, fiction and fallacies. Expert Rev. Med. Devices 2018, 15, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Liu-Ambrose, T.Y.; Ashe, M.C.; Graf, P.; Beattie, B.L.; Khan, K.M. Increased risk of falling in older community-dwelling women with mild cognitive impairment. Phys. Ther. 2008, 88, 1482–1491. [Google Scholar] [CrossRef] [PubMed]
- Montero-Odasso, M.; Speechley, M. Falls in cognitively impaired older adults: Implications for risk assessment and prevention. J. Am. Geriatr. Soc. 2018, 66, 367–375. [Google Scholar] [CrossRef]
- Muir, S.W.; Gopaul, K.; Montero Odasso, M.M. The role of cognitive impairment in fall risk among older adults: A systematic review and meta-analysis. Age Ageing 2012, 41, 299–308. [Google Scholar] [CrossRef]
- Camicioli, R.; Majumdar, S.R. Relationship between mild cognitive impairment and falls in older people with and without Parkinson’s disease: 1-Year prospective cohort study. Gait Posture 2010, 32, 87–91. [Google Scholar] [CrossRef]
- Delbaere, K.; Kochan, N.A.; Close, J.C.; Menant, J.C.; Sturnieks, D.L.; Brodaty, H.; Sachdev, P.S.; Lord, D.S. Mild cognitive impairment as a predictor of falls in community-dwelling older people. Am. J. Geriatr. Psychiatry 2012, 20, 845–853. [Google Scholar] [CrossRef]
- Doi, T.; Shimada, H.; Park, H.; Makizako, H.; Tsutsumimoto, K.; Uemura, K.; Nakakubo, S.; Hotta, R.; Suzuki, T. Cognitive function and falling among older adults with mild cognitive impairment and slow gait. Geriatr. Gerontol. Int. 2015, 15, 1073–1078. [Google Scholar] [CrossRef]
- Stevens, J.A.; Burns, E. A CDC Compendium of Effective Fall Interventions: What Works for Community-Dwelling Older Adults, 3rd ed.; Centers for Disease Control and Prevention, National Center for Injury Prevention and Control: Atlanta, GA, USA, 2015; pp. 103–146. [Google Scholar]
- Li, H.; Li, J.; Li, N.; Li, B.; Wang, P.; Zhou, T. Cognitive intervention for persons with mild cognitive impairment: A meta-analysis. Ageing Res. Rev. 2011, 10, 285–296. [Google Scholar] [CrossRef]
- Booth, V.; Hood, V.; Kearney, F. Interventions incorporating physical and cognitive elements to reduce falls risk in cognitively impaired older adults: A systematic review. JBI Database Syst. Rev. Implement. Rep. 2016, 14, 110–135. [Google Scholar] [CrossRef]
- Lipardo, D.S.; Aseron, A.M.C.; Kwan, M.M.; Tsang, W.W. Effect of Exercise and Cognitive Training on Falls and Fall-Related Factors in Older Adults with Mild Cognitive Impairment: A Systematic Review. Arch. Phys. Med. Rehabil. 2017, 98, 2079–2096. [Google Scholar] [CrossRef] [PubMed]
- Segev-Jacubovski, O.; Herman, T.; Yogev-Seligmann, G.; Mirelman, A.; Giladi, N.; Hausdorff, J.M. The interplay between gait, falls and cognition: Can cognitive therapy reduce fall risk? Expert Rev. Neurother. 2011, 11, 1057–1075. [Google Scholar] [CrossRef] [PubMed]
- The Joanna Briggs Institute. Joanna Briggs Institute Reviewers’ Manual, 2014th ed.; Joanna Briggs Institute: Adelaide, Australia, 2014. [Google Scholar]
- Tufanaru, C.; Munn, Z.; Aromataris, E.; Campbell, J.; Hopp, L. Chapter 3: Systematic reviews of effectiveness. In JBI Manual for Evidence Synthesis; Aromataris, E., Munn, Z., Eds.; Joanna Briggs Institute: Adelaide, Australia, 2020. [Google Scholar]
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Inform. Decis. Mak. 2007, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Makizako, H.; Doi, T.; Park, H.; Tsutsumimoto, K.; Verghese, J.; Suzuki, T. Effects of combined physical and cognitive exercises on cognition and mobility in patients with mild cognitive impairment: A randomized clinical trial. J. Am. Med. Dir. Assoc. 2018, 19, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Park, J.H.; Na, H.R.; Hiroyuki, S.; Kim, G.M.; Jung, M.K.; Kim, W.K.; Park, K.W. Combined intervention of physical activity, aerobic exercise, and cognitive exercise intervention to prevent cognitive decline for patients with mild cognitive impairment: A randomized controlled clinical study. J. Clin. Med. 2019, 8, 940. [Google Scholar] [CrossRef] [PubMed]
- Combourieu Donnezan, L.; Perrot, A.; Belleville, S.; Bloch, F.; Kemoun, G. Effects of simultaneous aerobic and cognitive training on executive functions, cardiovascular fitness and functional abilities in older adults with mild cognitive impairment. Ment. Health Phys. Act. 2018, 15, 78–87. [Google Scholar] [CrossRef]
- Lipardo, D.S.; Tsang, W.W. Effects of combined physical and cognitive training on fall prevention and risk reduction in older persons with mild cognitive impairment: A randomized controlled study. Clin. Rehabil. 2020, 34, 773–782. [Google Scholar] [CrossRef]
- Liao, Y.Y.; Chen, I.H.; Lin, Y.J.; Chen, Y.; Hsu, W.C. Effects of virtual reality-based physical and cognitive training on executive function and dual-task gait performance in older adults with mild cognitive impairment: A randomized control trial. Front. Aging Neurosci. 2019, 11, 162. [Google Scholar] [CrossRef]
- Anderson-Hanley, C.; Barcelos, N.M.; Zimmerman, E.A.; Gillen, R.W.; Dunnam, M.; Cohen, B.D.; Yerokhin, V.; Miller, K.E.; Hayes, D.J.; Arciero, P.J.; et al. The aerobic and cognitive exercise study (aces) for community-dwelling older adults with or at-risk for mild cognitive impairment (MCI): Neuropsychological, neurobiological and neuroimaging outcomes of a randomized clinical trial. Front. Aging Neurosci. 2018, 10, 76. [Google Scholar] [CrossRef]
- Schwenk, M.; Sabbagh, M.; Lin, I.; Morgan, P.; Grewal, G.S.; Mohler, J.; Coon, D.W.; Najafi, B. Sensor-based balance training with motion feedback in people with mild cognitive impairment. J. Rehabil. Res. Dev. 2016, 53, 945–958. [Google Scholar] [CrossRef]
- Delbroek, T.; Vermeylen, W.J.S. The effect of cognitive-motor dual task training with the biorescue force platform on cognition, balance and dual task performance in institutionalized older adults: A randomized controlled trial. J. Phys. Ther. Sci. 2017, 29, 1137–1143. [Google Scholar] [CrossRef]
- Gavelin, H.M.; Dong, C.; Minkov, R.; Bahar-Fuchs, A.; Ellis, A.K.; Lautenschlager, N.T.; Mellow, M.L.; Wade, A.T.; Smith, A.E.; Finke, C.; et al. Combined physical and cognitive training for older adults with and without cognitive impairment: A systematic review and network meta-analysis of randomized controlled trials. Ageing Res. Rev. 2021, 66, 101232. [Google Scholar] [CrossRef] [PubMed]
- Thapa, N.; Park, H.J.; Yang, J.G.; Son, H.; Jang, M.; Lee, J.; Kang, S.W.; Park, K.W.; Park, H. The effect of a virtual reality-based intervention program on cognition in older adults with mild cognitive impairment: A randomized control trial. J. Clin. Med. 2020, 9, 1283. [Google Scholar] [CrossRef]
- Sakaki, K.; Nouchi, R.; Matsuzaki, Y.; Saito, T.; Dinet, J.; Kawashima, R. Benefits of VR physical exercise on cognition in older adults with and without mild cognitive decline: A systematic review of randomized controlled trials. Healthcare 2021, 9, 883. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ma, Y.; Ren, Z. Rehabilitative effects of virtual reality technology for mild cognitive impairment: A systematic review with meta-analysis. Front. Psychol. 2020, 11, 1811. [Google Scholar] [CrossRef] [PubMed]
- Law, L.L.; Barnett, F.; Yau, M.K.; Gray, M.A. Effects of combined cognitive and exercise interventions on cognition in older adults with and without cognitive impairment: A systematic review. Ageing Res. Rev. 2014, 15, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Karssemeijer, E.G.A.; Aaronson, J.A.; Bossers, W.J.; Smits, T.; Olde Rikkert, M.G.M.; Kessels, R.P.C. Positive effects of combined cognitive and physical exercise training on cognitive function in older adults with mild cognitive impairment or dementia: A meta-analysis. Ageing Res. Rev. 2017, 40, 75–83. [Google Scholar] [CrossRef]
- Smith, L.; Argentina, V. The usability of physical activity and cognitive training applications in people with mild cognitive impairment. Res. Gerontol. Nurs. 2020, 13, 64–72. [Google Scholar] [CrossRef]
- Suzuki, T.; Shimada, H.; Makizako, H.; Doi, T.; Yoshida, D.; Ito, K.; Shimokata, H.; Washimi, Y.; Endo, H.; Kato, T. A randomized controlled trial of multicomponent exercise in older adults with mild cognitive impairment. PLoS ONE 2013, 8, e61483. [Google Scholar] [CrossRef]
- Klados, M.A.; Styliadis, C.; Frantzidis, C.A.; Paraskevopoulos, E.; Bamidis, P.D. Beta-band functional connectivity is reorganized in mild cognitive impairment after combined computerized physical and cognitive training. Front. Neurosci. 2016, 10, 55. [Google Scholar] [CrossRef]
- Mirza, R.A.; Yaqoob, I. Effects of combined aerobic and virtual reality-based cognitive training on 76 years old diabetic male with mild cognitive impairment. J. Coll. Physicians Surg. Pak. JCPSP 2018, 28, S210–S212. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.G.; Kim, J.H.; Jun, T.W. Effects of 12-week resistance exercise on electroencephalogram patterns and cognitive function in the elderly with mild cognitive impairment: A randomized controlled trial. Clin. J. Sport Med. 2018, 28, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, O. The effects of exercise-cognitive combined dual-task program on cognitive function and depression in elderly with mild cognitive impairment. Korean J. Adult Nurs. 2015, 27, 707–717. [Google Scholar] [CrossRef]
- Anderson-Hanley, C.; Stark, J.; Wall, K.M.; VanBrackle, M.; Michel, M.; Malloney, M.; Barcelos, N.; Striegnitz, K.; Cohen, B.D.; Kramer, A.F. The interactive Physical and Cognitive Exercise System (iPACES): Effects of a 3-month in-home pilot clinical trial for mild cognitive impairment and caregivers. Clin. Interv. Aging 2018, 13, 1565–1577. [Google Scholar] [CrossRef] [PubMed]
- Kovács, E.; Sztruhár Jónásné, I.; Karóczi, C.K.; Korpos, A.; Gondos, T. Effects of a multimodal exercise program on balance, functional mobility and fall risk in older adults with cognitive impairment: A randomized controlled single-blind study. Eur. J. Phys. Rehabil. Med. 2013, 49, 639–648. [Google Scholar] [PubMed]
- Boa Sorte Silva, N.C.; Gill, D.P.; Owen, A.M.; Liu-Ambrose, T.; Hachinski, V.; Shigematsu, R.; Petrella, R.J. Cognitive changes following multiple-modality exercise and mind-motor training in older adults with subjective cognitive complaints: The M4 study. PLoS ONE 2018, 13, e0196356. [Google Scholar] [CrossRef]
- Konstantinidisa, E.I.; Billisa, A.; Hlauschekb, W.; Panekb, P.; Bamidisa, P.D. Integration of cognitive and physical training in a smart home environment for the elderly people. Medinfo 2010, 2010, 58–60. [Google Scholar] [CrossRef]
- Thaiyanto, J.; Sittichoke, C.; Phirom, K.; Sungkarat, S. Effects of multicomponent exercise on cognitive performance and fall risk in older women with mild cognitive impairment. J. Nutr. Health Aging 2021, 25, 160–164. [Google Scholar] [CrossRef]
- Park, H. Does a cognitive-exercise combined dual-task training have better clinical outcomes for the elderly people with mild cognitive impairment than a single-task training? Ther. Sci. Neurorehabilit. 2017, 6, 71–83. [Google Scholar]
- Whitney, J.; Jackson, S.H.D.; Martin, F.C. Feasibility and efficacy of a multi-factorial intervention to prevent falls in older adults with cognitive impairment living in residential care (ProF-Cog). A feasibility and pilot cluster randomised controlled trial. BMC Geriatr. 2017, 17, 115. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Di Santo, S.G.; Franchini, F.; Arlati, S.; Zangiacomi, A.; Greci, L.; Moretti, S.; Jesuthasan, N.; Marzorati, M.; Rizzo, G.; et al. Effects of combined physical and cognitive virtual reality-based training on cognitive impairment and oxidative stress in mci patients: A pilot study. Front. Aging Neurosci. 2018, 10, 282. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Kanemaru, A.; Sugawara, Y.; Kawaji, Y.; Hiraoka, T.; Honda, T.; Nakajima, R.; Makita, A.; Itakura, A.; Yamazaki, R.; et al. A combination intervention (cognitive training and physical exercise) could improve or maintain cognitive functioning in MCI subjects. J. Neurol. Sci. 2017, 381, 668. [Google Scholar] [CrossRef]
- Sugano, K.; Yokogawa, M.; Yuki, S.; Dohmoto, C.; Yoshita, M.; Hamaguchi, T.; Yanase, D.; Iwasa, K.; Komai, K.; Yamada, M. Effect of cognitive and aerobic training intervention on older adults with mild or no cognitive impairment: A derivative study of the nakajima project. Dement. Geriatr. Cogn. Dis. Extra 2012, 2, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.-K.; Jung, H.-H.; Park, S.-K. Effects of exercise and cognitive intervention program on cognitive function, sedentary behavior and health-related quality of life in elderly women with mild cognitive impairment. Exerc. Sci. 2019, 28, 198–204. [Google Scholar] [CrossRef]
- Sacco, G.; Caillaud, C.; Ben Sadoun, G.; Robert, P.; David, R.; Brisswalter, J. Exercise plus cognitive performance over and above exercise alone in subjects with mild cognitive impairment. J. Alzheimers Dis. 2016, 50, 19–25. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Silva, F.; Ferreira, J.V.; Placido, J.; Sant’Anna, P.; Araujo, J.; Marinho, V.; Laks, J.; Deslandez, A.C. Three months of multimodal training contributes to mobility and executive function in elderly individuals with mild cognitive impairment, but not in those with Alzheimer’s disease: A randomized controlled trial. Maturitas 2019, 126, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Maffei, L.; Picano, E.; Andreassi, M.G.; Angelucci, A.; Baldacci, F.; Baroncelli, L.; Begenisic, T.; Bellinvia, P.F.; Berardi, N.; Biagi, L.; et al. Randomized trial on the effects of a combined physical/cognitive training in aged MCI subjects: The Train the Brain study. Sci. Rep. 2017, 7, 39471. [Google Scholar] [CrossRef]
- Gonzalez-Palau, F.; Franco, M.; Bamidis, P.; Losada, R.; Parra, E.; Papageorgiou, S.K.; Vivas, A.B. The effects of a computer-based cognitive and physical training program in a healthy and mildly cognitive impaired aging sample. Aging Ment. Health 2014, 18, 838–846. [Google Scholar] [CrossRef]
- Klotzbier, T.J.; Schott, N. Cognitive-motor interference during walking in older adults with probable mild cognitive impairment. Front. Aging Neurosci. 2017, 9, 350. [Google Scholar] [CrossRef]
- Hagovska, M.; Olekszyova, Z. Impact of the combination of cognitive and balance training on gait, fear and risk of falling and quality of life in seniors with mild cognitive impairment. Geriatr. Gerontol. Int. 2016, 16, 1043–1050. [Google Scholar] [CrossRef]
- Wall, K.; Stark, J.; Schillaci, A.; VanBrackle, M.; Michel, M.; Malloney, M.; Barcelos, N.; Striegnitz, K.; Cohen, B.D.; Kramer, A.F. The enhanced interactive physical and cognitive exercise system (IPACES(tm) v2.0): Pilot clinical trial of an in-home ipad-based neuro-exergame for mild cognitive impairment (MCI). J. Clin. Med. 2018, 7, 1565. [Google Scholar] [CrossRef] [PubMed]
- Barcelos, N.; Shah, N.; Cohen, K.; Hogan, M.J.; Mulkerrin, E.; Arciero, P.J.; Cohen, B.D.; Kramer, A.F.; Anderson-Hanley, C. Aerobic and cognitive exercise (ACE) pilot study for older adults: Executive function improves with cognitive challenge while exergaming. J. Int. Neuropsychol. Soc. 2015, 21, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Hagovska, M.; Nagyova, I. The transfer of skills from cognitive and physical training to activities of daily living: A randomised controlled study. Eur. J. Ageing 2017, 14, 133–142. [Google Scholar] [CrossRef] [PubMed]
- De Boer, C.; Echlin, H.V.; Rogojin, A.; Baltaretu, B.R.; Sergio, L.E. Thinking-while-moving exercises may improve cognition in elderly with mild cognitive deficits: A proof-of-principle study. Dement. Geriatr. Cogn. Dis. Extra 2018, 8, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Shatil, E. Does combined cognitive training and physical activity training enhance cognitive abilities more than either alone? A four-condition randomized controlled trial among healthy older adults. Front. Aging Neurosci. 2013, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Hagovská, M.; Takáč, P.; Dzvoník, O. Effect of a combining cognitive and balanced training on the cognitive, postural and functional status of seniors with a mild cognitive deficit in a randomized, controlled trial. Eur. J. Phys. Rehabil. Med. 2016, 52, 101–109. [Google Scholar]
- Nishiguchi, S.; Yamada, M.; Tanigawa, T.; Sekiyama, K.; Kawagoe, T.; Suzuki, M.; Yoshikawa, S.; Abe, N.; Otsuka, Y.; Nakai, R.; et al. A 12-week physical and cognitive exercise program can improve cognitive function and neural efficiency in community-dwelling older adults: A randomized controlled trial. J. Am. Geriatr. Soc. 2015, 63, 1355–1363. [Google Scholar] [CrossRef]
- Desjardins-Crepeau, L.; Berryman, N.; Fraser, S.A.; Vu, T.T.M.; Kergoat, M.-J.; Li, K.Z.; Bosquet, L.; Bherer, L. Effects of combined physical and cognitive training on fitness and neuropsychological outcomes in healthy older adults. Clin. Interv. Aging 2016, 11, 1287–1299. [Google Scholar] [CrossRef]
- Damirchi, A.; Hosseini, F.; Babaei, P. Mental training enhances cognitive function and bdnf more than either physical or combined training in elderly women with mci: A small-scale study. Am. J. Alzheimers. Dis. Other Demen. 2018, 33, 20–29. [Google Scholar] [CrossRef]
- Adcock, M.; Thalmann, M.; Schattin, A.; Gennaro, F.; de Bruin, E.D. A pilot study of an in-home multicomponent exergame training for older adults: Feasibility, usability and pre-post evaluation. Front. Aging Neurosci. 2019, 11, 304. [Google Scholar] [CrossRef]
- Lisanne, T.B.; Best, J.; Chan, J.; Ghag, C.; Erickson, K.; Handy, T.C.; Liu-Ambrose, T. The effects of computerized cognitive training with and without physical exercise on cognitive function in older adults: An 8-week randomized controlled trial. J. Gerontol. Ser. A 2019, 75, 755–763. [Google Scholar] [CrossRef]
- Titheridge, S. The Effect of a Combined Multiple-Modality Exercise Intervention on Sensorimotor Function in Community Dwelling Older Adults, with a Subjective Cognitive Complaint: The M4 Study (Multimodal; Mind Motor). Master’s Thesis Degree, Western University, London, ON, Canada, 29 October 2015. [Google Scholar]
- Makizako, H.; Doi, T.; Shimada, H.; Yoshida, D.; Tsutsumimoto, K.; Uemura, K.; Suzuki, T. Does a multicomponent exercise program improve dual-task performance in amnestic mild cognitive impairment? A randomized controlled trial. Aging Clin. Exp. Res. 2012, 24, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Fiatarone Singh, M.A.; Gates, N.; Saigal, N.; Wilson, G.C.; Meiklejohn, J.; Brodaty, H.; Wen, W.; Singh, N.; Baune, B.T.; Suo, C.; et al. The Study of Mental and Resistance Training (SMART) study-resistance training and/or cognitive training in mild cognitive impairment: A randomized, double-blind, double-sham controlled trial. J. Am. Med. Dir. Assoc. 2014, 15, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Kobe, T.; Witte, A.V.; Schnelle, A.; Lesemann, A.; Fabian, S.; Tesky, V.A.; Pantel, J.; Floel, A. Combined omega-3 fatty acids, aerobic exercise and cognitive stimulation prevents decline in gray matter volume of the frontal, parietal and cingulate cortex in patients with mild cognitive impairment. Neuroimage 2016, 131, 226–238. [Google Scholar] [CrossRef]
| Study | Setting | Participants | Outcomes of Interest | Length of Intervention | Intervention Setting | Intervention |
|---|---|---|---|---|---|---|
| Lipardo et al. (2020) [20] | Philippines: Community-based program | • Gr1: n = 23 • Gr2: n = 23 • Gr3: n = 23 • Gr4: n = 23 Age 69.5 y, MMSE score N/A, MoCA score 18.3 (4.6) | • Fall outcomes: (1) Information on fall incidence • Risk of falls: (1) Physiological profile assessment—short form • Dynamic balance and functional mobility: (1) TUG test • Walking speed: (1) The 10-meter walk test • Lower extremity muscular strength: (1) 30-second chair-stand test | 12 weeks | Group | Gr1: PT • Duration: 60–90 min/session; 3 times/week; 12 weeks Gr2: PT and PCT • Duration: 60–90 min/session; 3 times/week; 12 weeks Gr3: CT • Duration: 60–90 min/session; 1 time/week; 12 weeks Gr4: Usual daily routine |
| Liao et al. (2019) [21] | Taiwan: Community | • Gr1: n = 21, age 75.5 (5.2) y, MMSE score 27.2 (1.9) • Gr2: n = 21, age 73.1 (6.8) y, MMSE score 27.2 (1.6) | • Executive function: (1) TMT (2) SCWT • Gait performance: (1) Single-task gait (2) Cognitive dual-task gait (3) Motor dual-task gait | 12 weeks | Group | Gr1: VR-based PCT. Gr2: PCT • Duration: 60 min/session, 3 times/week, 36 sessions over 12 weeks |
| Park (2019) [18] | South Korea: Community | • IG: n = 25, age 70.6 (6.46) y, MMSE score 24.6 (2.6) • CG: n = 24, age 72.8 (5.37), MMSE score 24.4 (3.1) | Primary outcomes: (1) K-MMSE, (2) ADAS-Cog Secondary outcome: • Cognitive assessment: (1) DST, (2) TMT, (3) SDST. • Physical function assessment: (1) Grip strength, (2) TUG, (3) sit-to-stand time • Physical activity measurements (1) MVPA, (2) step count | 24 weeks | Group 1:1 | IG: Dual-task exercise program. • Duration: 110 min sessions/week over 24 weeks CG: No intervention |
| Donnezan et al. (2018) [19] | France: Urban elderly clubs | • Gr1: n = 21, age 77.1 (1.44) y, MMSE score 28.2 (0.43) • Gr2: n = 19, age 76.3 (1.5) y, MMSE score 27.3 (0.42). • Gr3: n = 21, age 75.2 (1.3) y, MMSE score 28.1 (0.36) • Gr4: n = 15, age 79.2 (4.0) y, MMSE score 27.3 (0.5) | • Executive measures: (1) Matrix Reasoning Test, (2) SCWT, (3) DSF, (4) DSB • Motor measures: (1) Rockport test (VO2max), (2) TUG test, (3) single-task walking, (4) complex walking (gait speed WSC test: bw, w, c), (5) dual-task walking | 12 weeks | Group | • Gr1: PT • Gr2: CT • Gr3: PCT • Duration: two 1-hour sessions/week over 12 weeks; same to all groups. Gr4: No intervention (usual lifestyle) |
| Shimada et al. (2018) [17] | Japan: Residential suburb of Nagoya | • IG: n = 154, age 71.6 (5.0) y, MMSE score 26.6 (1.8) • CG: n = 154, age 71.6 (4.9) y, MMSE score 26.8 (1.8) | • Functional outcomes: (1) MMSE, (2) WMS-LM II, (3) RAVLT • Cognitive outcomes: (1) VFT—letters, (2) VFT—category, (3) TMT • Mobility: (1) Total daily steps, (2) MVPA | 40 weeks | Group 1:1 | IG: Combined activity program: physical and cognitive activities. • Duration: 90 min/ sessions/ 40 weeks CG: Health education • 90 min health promotion classes thrice during the 40-weeks trial period |
| Anderson-Hanley et al. (2018) [22] | USA: Community | • Gr1: n = 46 • Gr2: n = 45 • Gr3: n = 20 Age 78.1 (9.9) y, MMSE score N/A, MoCA score 23.7 (3.1) (screened as MCI” based on MoCA score < 26) | Primary measures: • Executive function: (1) Stroop test, (2) color-trails test, (3) DST Secondary measures: (1) Ecological validity, (2) verbal memory immediate and delayed recall, (3) get-up-and-go test | 6 months | Group | • Gr1: exer-tour: physical exercise interactive with relatively passive, low cognitive load, virtual scenic bike tour. • Gr2: exer-score: interactive physical exercise a relatively high effort, high cognitive demand, video game • Gr3: game-only: the same videogame operated by a joystick or keyboard • Duration: 20 min/twice/week and increase 45 min/3–5 times/week for 6 months. |
| Schwenk et al.(2016) [23] | USA: Cleo Roberts Memory and Movement Disorders Center | • IG: n = 12, MoCA score 23.3 (3.1) • CG: n = 10, MoCA score 22.4 (3.0). Age 78.2 (8.7) y. | Balance (EC, EO): (1) CoM sway, (2) ML CoM sway, (3) AP CoM sway Gait: (1) Habitual walking, (2) fast walking Fear of falling: (1) FES-I Cognitive performance: (1) MoCA, (2) TMT | 4 weeks | Group 1:1 | IG: Sensor-based balance training with motion feedback • Duration: 45 min/session; 2 training sessions/ week for 4 weeks. CG: No training. |
| Study | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 | Total Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Park et al. (2019) [18] | Y | U | Y | N | N | U | N | Y | Y | Y | Y | Y | Y | 8 |
| Shimada et al. (2018) [17] | Y | Y | Y | N | N | Y | Y | Y | Y | Y | Y | Y | Y | 11 |
| Lipardo et al. (2020) [20] | Y | Y | Y | Y | U | Y | Y | Y | Y | Y | Y | Y | Y | 12 |
| Donnezan et al. (2018) [19] | Y | Y | Y | N | N | N | N | Y | N | Y | Y | Y | Y | 8 |
| Liao et al. (2019) [21] | Y | Y | Y | N | N | Y | N | Y | U | Y | Y | N | Y | 8 |
| Anderson-Hanley et al. (2018) [22] | Y | U | Y | N | N | N | Y | Y | N | Y | Y | Y | Y | 8 |
| Schwenk et al. (2016) [23] | Y | Y | Y | N | N | N | Y | Y | Y | Y | Y | Y | Y | 10 |
| Delbroek et al. (2017) [24] | Y | U | Y | N | N | Y | N | N | N | N | Y | Y | Y | 6 |
| Total (%) | 100 | 70 | 100 | 30 | 0 | 60 | 40 | 90 | 60 | 90 | 100 | 90 | 100 | Mean score: 8.87 |
| Study | Measurement | Results | Significant Difference between Groups | Effective |
|---|---|---|---|---|
| FALLS OUTCOME | ||||
| Lipardo et al. (2020) [17] | Fall incidence rate in: previous 12 mo; post intervention (12 and 36 weeks) | Gr1: pre. n = 6; post. n = 0 and 4 Gr2: pre. n = 6; post. n = 5 and 6 Gr3: pre. n = 7; post. n = 3 and 5 Gr4: pre. n = 6; post. n = 5 and 5 | At 12 weeks (p = 0.152) or 36 weeks (p = 0.954), there were no significant differences in fall incidence rates according to time or group. | No |
| Shimada et al. (2018) [18] | Total number of falls in: post intervention (40 weeks) | IG: post. n = 11 CG: post. n = 13 | Fall in daily life was not significant different between-group (p = 0.811) | No |
| FALLS-RELATED OUTCOMES | ||||
| Balance ability: Timed-up-and-go test (TUG); get-up-and-go test (GUG) | ||||
| Lipardo et al. (2020) [20] | Mean TUG (s) at: baseline; 12th week, 36th week follow up | Gr1: 10.7 (2.8); 9.34 (2.0); 9.0 (1.3) Gr2: 10.6 (3.0); 10.45 (2.6); 9.6 (1.4) Gr3: 9.1 (3.0); 8.91 (2.6); 8.6 (2.0) Gr4: 10.6 (4.0); 11.2 (3.5); 11.1 (2.6) | Significant improvement in dynamic balance based on timed-up-and-go test in the combined physical and cognitive training group (9.0 s with p = 0.001) and in the cognitive training alone group (8.6 s with p = 0.012) compared to waitlist group (11.1 s) at 36 weeks. | Yes |
| Park (2019) [18] | Mean TUG at: baseline; 24 weeks follow up | IG: 10.1 (3.1); 8.9 (3.4) CG: 9.7 (4.1); 9.5 (3.9) | Compared with the control group, timed-up-and-go test showed significant improvement after the intervention (p < 0.01) | Yes |
| Donnezan et al. (2018) [19] | Mean TUG at: baseline; 6 mo follow up | Gr1: 9.96 (1.75); 8.90 (1.21) Gr2: 10.24 (2.68); 9.97 (3.44) Gr3: 12.79 (2.17); 9.84 (1.18) Gr4: 11.65 (2.04); 1.58 (2.18) | The TUG test score in the PCT group decreased significantly after 12 weeks and 6 months both compared with the control group and within the group. | Yes |
| Anderson-Hanley et al. (2018) [22] | Mean GUG at: baseline; 6 mo follow up. | Gr1: 12.7 (2.2); 14.0 (1.5) Gr2: 10.3 (1.7); 11.0 (2.0) | The GUG test in the exer-tour group increased significantly more than in the exer-score group (p = 0.001) | Yes |
| Gait performance | ||||
| Lipardo et al. (2020) [20] | Mean 10-meter walk test (m/s) at: baseline; 12th week and 36th week. | Gr1: Preferred speed: 1.08 (0.17); 1.11 (0.18); 1.13 (0.16) Fastest speed: 1.38 (0.23); 1.41 (0.25); 1.42 (0.24) Gr2: Preferred speed: 0.99 (0.18); 1.09 (0.18); 1.09 (0.18). Fastest speed: 1.24 (0.23); 1.39 (0.24); 1.38 (0.23). Gr3: Preferred speed: 1.13 (0.20); 1.20 (0.24); 1.12 (0.21) Fastest speed: 1.51 (0.30); 1.54 (0.31); 1.47 (0.29). Gr4: Preferred speed: 1.02 (0.23); 1.02 (0.21); 1.01 (0.17). Fastest speed: 1.32 (0.32); 1.33 (0.30); 1.31 (0.28). | The PCT group did not improve significantly compared with the control group following the intervention | No |
| Liao et al. (2019) [21] | Gait speed (cm/s) in single-task gait, cognitive dual-task gait, and motor dual-task gait at: baseline; 12-week follow up | Gr1: (VR group) • Single-task walking: 82.3 (29.1); 92.9 (28.5) • Complex walking: 68.1 (26.9); 82.5 (30.6) • Dual-task walking: 79.9 (29.9); 92.3 (32.8) Gr2: (CPC group) • Single-task walking: 89.3 (23.3); 100.19 (25.7) • Complex walking: 72.8 (25.9); 78.1 (33.2) • Dual-task walking: 86.5 (25.0); 96.1 (27.3) | Gait speed in three conditions significantly improved within VR group and CPC group except for cognitive dual tasks in CPC group. This gait speed did not significantly change between groups. | Yes within group No between groups |
| Donnezan et al. (2018) [19] | Gait speed in single task (cm/s) at: baseline; 6 month follow up | Gr1: 115.93 (18.6); 119.42 (17.89) Gr2: 111.34 (19.91); 112.58 (26.13) Gr3: 102.43 (12.60); 114.44 (16.03) Gr4: 99.64 (14.29); 90.56 (15.23) | Gait speed in the PCT group significantly improved after 6 months of intervention (p = 0.001) but did not change between groups. | No |
| Schwenk et al.(2016) [23] | Gait speed (m/s) in habitual walking and fast walking | IG: • Habitual walking: 0.98 (0.22); 1.05 (0.22) • Fast walking: 1.39 (0.35); 1.43 (0.34) CG: • Habitual walking: 1.06 (0.17); 1.10 (0.20) • Fast walking: 1.44 (0.22); 1.34 (0.37) | Gait speeds were nonsignificant between groups (p > 0.05) | No |
| Muscular strength | ||||
| Park (2019) [18] | Mean sit-to-stand time: at baseline; 24 weeks follow up | Gr1: 18.0 (5.2); 16.9 (4.5) Gr2: 17.3 (4.7); 17.7 (4.8) | Compared with the control group, the sit-to-stand time showed significant improvement after the intervention (p < 0.01) | Yes |
| Lipardo et al. (2020) [20] | Median 30-second chair-stand test: at baseline, 12th week, 36th week | Gr1: 13 (3); 13 (2); 14 (2) Gr2: 13 (3); 15 (4.5); 15 (3) Gr3: 15 (5.5); 15 (5.5); 16 (5) Gr4: 14 (6); 15 (5); 13 (4) | No significant group effect was observed in the 30-second chair-stand test for lower limb muscle strength at 12 weeks (p = 0.186) and at 36 weeks (p = 0.110), but there was a significant time effect (p < 0.001) | No |
| Global cognitive functions | ||||
| Shimada et al. (2018) [17] | Mean difference MMSE after 40 weeks follow up (OR, 95%CI) | IG: 0.0 (−0.4 to 0.4) CG: −0.8 (−1.2 to −0.4) | Compared with the controls, the combined activity group exhibited significantly greater score changes on the MMSE (difference = 0.8, p = 0.012) | Yes |
| Park (2019) [18] | Mean MMSE at: baseline, 24th week follow up Mean Modified ADAS-Cog at: baseline; 24th week follow up | IG: 24.6 (2.6); 24.8 (3.7) CG: 24.4 (3.1); 24.2 (3.0) IG: 26.2 (2.9); 24.6 (3.3) CG: 25.7 (3.1); 26.1 (2.7) | The combined intervention group showed significantly greater differences between scores on the ADAS-Cog (p < 0.01) compared with the control group after the intervention but did not change on the MMSE score (p = 0.06) | Yes for the ADAS-Cog test No for MMSE test. |
| Schwenk et al.(2016) [23] | Mean MOCA score at: baseline; 4th week follow up | IG: 23.3 (3.1); 23.7 (3.9) CG: 22.4 (3.0); 25.3 (1.9) | The MoCA scores did not change after the intervention. | No |
| Executive functions: | ||||
| The trail making test (TMT) | ||||
| Shimada et al. (2018) [17] | Mean difference TMT at: baseline; 40th week follow up (OR, 95%CI) | IG: −0.3 (−0.7 to 0.1) CG: 0.0 (−0.2 to 0.3) | The control and combined activity groups did not significantly differ in TMT score after intervention (p = 0.35) | No |
| Park (2019) [18] | Mean TMT-A at: baseline; 24th week follow up | Gr1: 25.3 (7.1); 23.1 (6.3) Gr2: 24.7 (6.2); 24.1 (6.7) | There were no significant differences in TMT-A between groups after 24 weeks (p = 0.1) | No |
| Liao et al. (2019) [21] | Mean TMT-B at: baseline; 12th week follow up. | Gr1: 179.22 (58.06); 134.21 (48.23) Gr2: 154.50 (63.50); 136.37 (48.58) | There were significant differences in TMT-B between groups after 12 weeks of intervention (p = 0.032) | Yes |
| Schwenk et al.(2016) [23] | Mean TMT-A, TMT-B at: baseline; 4th week follow up | IG: TMT-A: 51.8 (24.3); 46.0 (14.1) TMT-B: 149.2 (89.5); 155.6 (101.3) CG: TMT-A: 42.4 (20.0); 45.1 (21.0) TMT-B: 98.9 (43.0); 99.8 (39.5) | There were significant differences in TMT-A and TMT-B between groups after 4 weeks of intervention (p = 0.009, p = 0.006, respectively) | Yes |
| Executive functions: The digit span test (DST) | ||||
| Park (2019) [18] | Mean DST at: baseline; 24th week follow up | Gr1: 2.7 (0.2); 2.4 (0.2) Gr2: 2.6 (0.3); 2.9 (0.2) | There were significant differences in DST between groups after 24 weeks of intervention (p = 0.02) | Yes |
| Donnezan et al. (2018) [19] | DSF and DSB at: baseline; 6 months follow up. | Gr1: 5.38 (1.14); 5.94 (0.87) Gr2: 5.18 (0.91); 6.18 (1.11) Gr3: 5.48 (0.88); 6.15 (1.06) Gr4: 5.21 (1.12); 5.36 (0.84) | DSF and DSB tests showed significant differences after PT, CT, and PCT interventions. | Yes |
| Executive functions: Stroop test | ||||
| Liao et al. (2019) [21] | Stroop color and word test (number; time) at: baseline; 12 week follow up | Gr1: Number: 15.05 (6.59); 19.44 (9.05); Time: 126.83 (41.03); 100.66 (33.93) Gr2: Number: 126.83 (41.03); 100.66 (33.93); Time: 119.87 (54.35); 100.18 (41.89) | VR and PCT groups exhibited significant improvements in Stroop test scores, but there was no significant difference between the groups. | Yes within group No deferent group |
| Anderson-Hanley et al. (2018) [22] | Stroop A/C at: baseline; 6th month follow up | Gr1: 0.40 (0.12); 0.48 (0.11) Gr2: 0.40 (0.14); 0.47 (0.15) | The results showed that StroopA/C improved significantly in both the exer-tour (p = 0.049) and exer-score conditions (p = 0.001) and between groups (p = 0.002) | Yes within group No deferent group |
| Donnezan et al. (2018) [19] | Stroop test: task switching (number) at: baseline; 6 month follow up. | Gr1: 28.89 (6.45); 30.94 (6.24) Gr2: 27.19 (8.82); 25.5 (8.37) Gr3: 26.52 (6.8); 29.05 (7.19) Gr4: 24.71 (10.16); 26.42 (6.53) | The finding indicated an improvement in Stroop test scores after combined PCT, but the difference was not statistically significant between groups (p = 0.06) | Yes within group No deferent group |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mai Ba, H.; Kim, J. The Effects of Combined Physical and Cognitive Interventions on Direct and Indirect Fall Outcomes for the Elderly with Mild Cognitive Impairment: A Systematic Review. Healthcare 2022, 10, 862. https://doi.org/10.3390/healthcare10050862
Mai Ba H, Kim J. The Effects of Combined Physical and Cognitive Interventions on Direct and Indirect Fall Outcomes for the Elderly with Mild Cognitive Impairment: A Systematic Review. Healthcare. 2022; 10(5):862. https://doi.org/10.3390/healthcare10050862
Chicago/Turabian StyleMai Ba, Hai, and Jiyun Kim. 2022. "The Effects of Combined Physical and Cognitive Interventions on Direct and Indirect Fall Outcomes for the Elderly with Mild Cognitive Impairment: A Systematic Review" Healthcare 10, no. 5: 862. https://doi.org/10.3390/healthcare10050862
APA StyleMai Ba, H., & Kim, J. (2022). The Effects of Combined Physical and Cognitive Interventions on Direct and Indirect Fall Outcomes for the Elderly with Mild Cognitive Impairment: A Systematic Review. Healthcare, 10(5), 862. https://doi.org/10.3390/healthcare10050862







