Evaluation of the Relationship between the BMI and the Sequence and Chronology of Eruption in Permanent Dentition in Spanish Population
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Massler, M.; Schour, I. Studies in tooth development: Theories of eruption. Am. J. Orthod. Oral Surg. 1941, 27, 552–576. [Google Scholar] [CrossRef]
- Hernández, M.; Espasa, E.; Boj, J.R. Eruption chronology of the permanent dentition in Spanish children. J. Clin. Pediatr Dent. 2008, 32, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Suri, L.; Gagari, E.; Vastardis, H. Delayed tooth eruption: Pathogenesis, diagnosis, and treatment. A literature review. AJODO 2004, 126, 432–445. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, K.N.; Gupta, R.; Faridi, M.M.A.; Kalra, N. Permanent dentition in Delhi boys of age 5–14. Indian Pediatr. 2004, 41, 1031–1035. [Google Scholar]
- Khan, N.B.; Chohan, A.N.; AlMograbi, B.; AlDeyab, S.; Zahid, T.; AlMoutairi, M. Eruption time of permanent first molars and incisors among a sample of Saudi male schoolchildren. Saudi Dent. J. 2006, 18, 18–24. [Google Scholar]
- Kutesa, A.; Nkamba, E.B.; Muwazi, L.; William Buwembo, W.; Rwenyonyi, C.H. Weight, height and eruption times of permanent teeth of children aged 4–15 years in Kampala, Uganda. BMC Oral. Health 2013, 13, 15. [Google Scholar] [CrossRef]
- Kurosaka, H.; Itoh, S.; Morita, C.; Tsujimoto, T.; Murata, Y.; Inubushi, T.; Yamashiro, T. Development of dentition: From initiation to occlusion and related diseases. J. Oral. Biosci. 2022, 64, 159–164. [Google Scholar] [CrossRef]
- Nahajowski, M.; Hnitecka, S.; Antoszewska-Smith, J.; Rumin, K.; Dubowik, M.; Sarul, M. Factors influencing an eruption of teeth associated with a dentigerous cyst: A systematic review and meta-analysis. BMC Oral. Health 2021, 21, 180. [Google Scholar] [CrossRef]
- Paradowska-Stolarz, A. MSX1 gene in the etiology orofacial deformities. Postepy Hig. Med. Dosw. 2015, 69, 1499–1504. [Google Scholar]
- Almotairy, N.; Pegelow, M. Dental age comparison in patients born with unilateral cleft lip and palate to a control sample using Demirjian and Willems methods. Eur. J. Orthod. 2018, 40, 74–81. [Google Scholar] [CrossRef]
- Arid, J.; Vitiello, M.C.; da Silva, R.A.B.; da Silva, L.B.; Mussolino de Queiroz, A.; Küchler, E.; Nelson-Filho, P. Nutritional status is associated with permanent tooth eruption chronology. Braz. J. Oral Sci. 2017, 16, 1–7. [Google Scholar] [CrossRef]
- Reis, C.L.B.; Barbosa, M.C.F.; Henklein, S.; Madalena, I.R.; de Lima, D.; Oliveira, M.; Küchler, E.; de Oliveira, D. Nutritional Status is Associated with Permanent Tooth Eruption in a Group of Brazilian School Children. Glob. Pediatric Health 2021, 8, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ayatollahi, S.M.; Ghoreshizadeh, Z. Prevalence of obesity and overweight among adults in Iran. Obes. Rev. 2010, 11, 335–337. [Google Scholar] [CrossRef]
- Hedayati, Z.; Khalafinejad, F. Relationship between Body Mass Index, Skeletal Maturation and Dental Development in 6- to 15- Year Old Orthodontic Patients in a Sample of Iranian Population. J. Dent. Shiraz. Univ. Med. Sci. 2014, 15, 180–186. [Google Scholar]
- Heinrich-Weltzien, R.; Zorn, C.; Monse, B.; Kromeyer-Hauschild, K. Relationship between Malnutrition and the Number of Permanent Teeth in Filipino 10- to 13-Year-Olds. BioMed. Res. Int. 2013, 2013, 1–8. [Google Scholar] [CrossRef] [PubMed]
- WHO; Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index- for-Age: Methods and Development; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Sánchez-Pérez, L.; Irigoyen, M.E.; Zeped, M. Dental caries, tooth eruption timing and obesity: A longitudinal study in a group of Mexican schoolchildren. Acta Odontol. Scand. 2010, 68, 57–64. [Google Scholar] [CrossRef]
- Pecoraro, P.; Guida, B.; Caroli, M.; Trio, R.; Falconi, C.; Principato, S.; Pietrobelli, A. Body mass index and skinfold thickness versus bioimpedance analysis: Fat mass prediction in children. Acta Diabetol. 2003, 40 (Suppl. S1), S278–S281. [Google Scholar] [CrossRef]
- Sandhu, J.; Ben-Shlomo, Y.; Cole, T.J.; Holly, J.; Davey Smith, G. The impact of childhood body mass index on timing of puberty, adult stature and obesity: A follow-up study based on adolescent anthropometry recorded at Christ’s Hospital (1936–1964). Int. J. Obes. 2006, 30, 14–22. [Google Scholar] [CrossRef]
- Hilgers, K.; Akridge, M.; Scheetz, J.; Kinane, D. Childhood Obesity and Dental Development. Pediatr. Dent. 2006, 28, 18–22. [Google Scholar]
- Evangelista, S.; Felizardo, K.R.; Xavier, T.A.; Oliveira, S.; Luiz, A.; Nelson-Filho, P.; da Silva, L.; Segato, R.; de Queiroz, A.; Küchler, E. Timing of Permanent Tooth Emergence is Associated with Overweight/Obesity in Children from the Amazon Region. Braz. Dent. J. 2018, 29, 465–468. [Google Scholar] [CrossRef]
- Dietrich, T.; Joshipura, K.J.; Dawson-Hughes, B.; Bischoff-Ferrari, H.A. Association between serum concentrations of 25-hydroxyvitamin D3 and periodontal disease in the US population. Am. J. Clin. Nutr. 2004, 80, 108–113. [Google Scholar] [PubMed]
- Nishida, M.; Grossi, S.G.; Dunford, R.G.; Ho, A.W.; Trevisan, M.; Genco, R.J. Dietary vitamin C and the risk for periodontal disease. J. Periodontol. 2000, 71, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.L.; Psoter, W.J.; Jean-Charles, G.; Prophte, S.; Gebrian, B. Protein-energy malnutrition during early childhood and periodontal disease in the permanent dentition of Haitian adolescents aged 12–19 years: A Retrospective Cohort Study. Int. J. Paediatr. Dent. 2010, 20, 222–229. [Google Scholar] [CrossRef]
- Mack, K.B.; Phillips, C.; Jain, N.; Koroluk, L.D. Relationship between body mass index percentile and skeletal maturation and dental development in orthodontic patients. Am. J. Orthod. Dentofac. Orthop. 2013, 143, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Mohamedhussein, N.; Busuttil-Naudi, A.; Mohammed, H.; Uihaq, A. Association of obesity with the eruption of first and second permanent molars in children: A systematic review. Eur. Arch. Paediatr. Dent. 2020, 21, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Neto, P.G.F.; Falcão, M.C. Eruption chronology of the first deciduous teeth in children born prematurely with birth weight less than 1500 g. Rev. Paul. Pediatr. 2014, 32, 17–23. [Google Scholar] [CrossRef][Green Version]
- Paulsson, L.; Arvini, S.; Bergström, N.; Klingberg, G.; Lindh, C. The impact of premature birth on dental maturation In the permanent dentition. Clin. Oral. Investig. 2019, 23, 855–861. [Google Scholar] [CrossRef]
- Pancheva-Dimitrova, R.; Toneva, A.; Georgieva, M.; Konstantinova, D.; Petrova, S. Nutritional status, macro-and micronutrient deficiency in children with neurodevelopmental disorders. Scr. Sci. Salut. Publicae 2018, 4, 23–30. [Google Scholar] [CrossRef]
- Kiliç, M.; Gurbuz, T.; Kahraman, C.Y.; Cayir, A.; Bilgiç, A.; Kurt, Y. Relationship between the TAS2R38 and TAS1R2 polymorphisms and the dental status in obese children. Dent. Med. Probl. 2022. [Google Scholar] [CrossRef]
- Schmidt, J.; Vogel, M.; Poulain, T.; Kiess, W.; Hirsch, C.; Ziebolz, D.; Haak, R. Association of Oral Health Conditions in Adolescents with Social Factors and Obesity. Int. J. Environ. Res. Public Health 2022, 19, 2905. [Google Scholar] [CrossRef]
- Vaziri, F.; Bahrololoomi, Z.; Savabieh, Z.; Sezavar, K. The relationship between children’s body mass index and periodontal status. J. Indian Soc. Periodontol. 2022, 26, 64–68. [Google Scholar]
- Iglesias Yunes, E.A.; Costa Martins, A.; Feitoza de Jesus, S.; Juber, P.; Boabaid Loureiro, B.; Cruvinel Zuza, E. Prevalence of Periodontal Disease and Alveolar Bone Loss in Overweight/Obese Brazilian Adolescents. J. Dent. Child. (Chic). 2021, 88, 196–201. [Google Scholar]
- Zangouei-Booshehri, M.; Ezoddini-Ardakani, F.; Aghilia, H.A.; Sharifi, A. Assessment of the relationship between body mass index (BMI) and dental age. Health 2011, 3, 253–257. [Google Scholar] [CrossRef]
- Must, A.; Phillips, S.M.; Tybor, D.J.; Lividini, K.; Hayes, C. The association between childhood obesity and tooth eruption. Obesity 2012, 20, 2070–2074. [Google Scholar] [CrossRef]
- Nicholas, C.L.; Kevan Kadavy, K.; Holton, N.E.; Marshall, T.; Richter, A.; Southardb, T. Childhood body mass index is associated with early dental development and eruption in a longitudinal sample from the Iowa Facial Growth Study. Am. J. Orthod. Dentofacial. Orthop. 2018, 154, 72–81. [Google Scholar] [CrossRef]
- Sindelarova, R.; Soukup, P.; Broukal, Z. The relationship of obesity to the timing of permanent tooth emergence in Czech children. Acta Odontol. Scand. 2018, 76, 220–225. [Google Scholar] [CrossRef]
- Dimaisip-Nabuab, J.; Duijster, D.; Benzian, H. Nutritional status, dental caries and tooth eruption in children: A longitudinal study in Cambodia, Indonesia and Lao PDR. BMC Pediatr. 2018, 18, 1–11. [Google Scholar] [CrossRef]
- Anu, V.; Brindha, J.R.; Carol, P.T.; Diana, P.C.; Elsy, J.D.; Garima, S. Does body mass index affect tooth eruption sequence? A study 6-7 years old schoolchildren in Chennai, India. Int. J. Clin. Pediatr. Dent. 2020, 13, 261–263. [Google Scholar]
- Esan, T.A.; Schepartz, L.A. Does nutrition have an effect on the timing of tooth formation? Am. J. Phys. Antropol. 2019, 171, 470–480. [Google Scholar] [CrossRef]
- Crowe, M.; O’ Sullivan, M.; Cassetti, O.; O’ Sullivan, A. Weight Status and Dental Problems in Early Childhood: Classification Tree Analysis of a National Cohort. Dent. J. 2017, 5, 25. [Google Scholar] [CrossRef]
- Traver-Ferrando, C.; Barcia-González, J. Early permanent dental eruption in obese/overweigh schoolchildren. J. Clin. Exp. Dent. 2022, 14, e199–e204. [Google Scholar] [CrossRef]
- Militi, A.; Nucera, R.; Ciraolo, L.; Alibrandi, A.; Fastuca, R.; Lo Giudice, R.; Portelli, M. Correlation between Caries, Body Mass Index and Occlusion in an Italian Pediatric Patients Sample: A Transverse Observational Study. Int. J. Environ. Res. Public Health 2020, 17, 2994. [Google Scholar] [CrossRef]
- Carr, L.M. Eruption ages of permanent teeth. Aust. Dent. J. 1962, 7, 367–373. [Google Scholar] [CrossRef]
- Cole, T.J.; Lobstein, T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr. Obes. 2012, 7, 284–294. [Google Scholar] [CrossRef]
- Barbería Leache, E.; Marañes Pallardo, J.P.; Mourelle Martínez, M.R.; Moreno González, J.P. Tooth eruption in children with growth deficit. J. Int. Assoc. Dent. Child. 1988, 19, 29–35. [Google Scholar]
- Shaweesh, A.I.; Alsoleihat, F.D. Association between body mass index and timing of permanent tooth emergence in Jordanian children and adolescents. Int. J. Stomatol. Occlusion. Med. 2013, 6, 50–58. [Google Scholar] [CrossRef]
- Wong, H.M.; Peng, S.M.; Yang, Y.; King, N.M.; McGrath, C.P.J. Tooth eruption and obesity in 12-year-old children. J. Dent. Sci. 2017, 12, 126–132. [Google Scholar] [CrossRef]
- Díaz-Orahulio, G.D.; León-Manco, R.A. Nutritional status and tooth eruption sequence in children´s under 12 years of age Pachacámac SOS Children´s Village, District Pachacámac, Lima-Perú. Rev Estomatol Herediana 2014, 4, 213–219. [Google Scholar] [CrossRef]
- Gaur, R.; Boparai, G.; Saini, K. Effect of under-nutrition on permanent tooth emergence among Rajputs of Himachal Pradesh, India. Ann. Hum. Biol. 2011, 38, 84–92. [Google Scholar] [CrossRef]
- Babczynska, A.; Kawala, B.; Sarul, M. Genetic Factors That Affect Asymmetric Mandibular Growth—A Systematic Review. Symmetry 2022, 14, 490. [Google Scholar] [CrossRef]


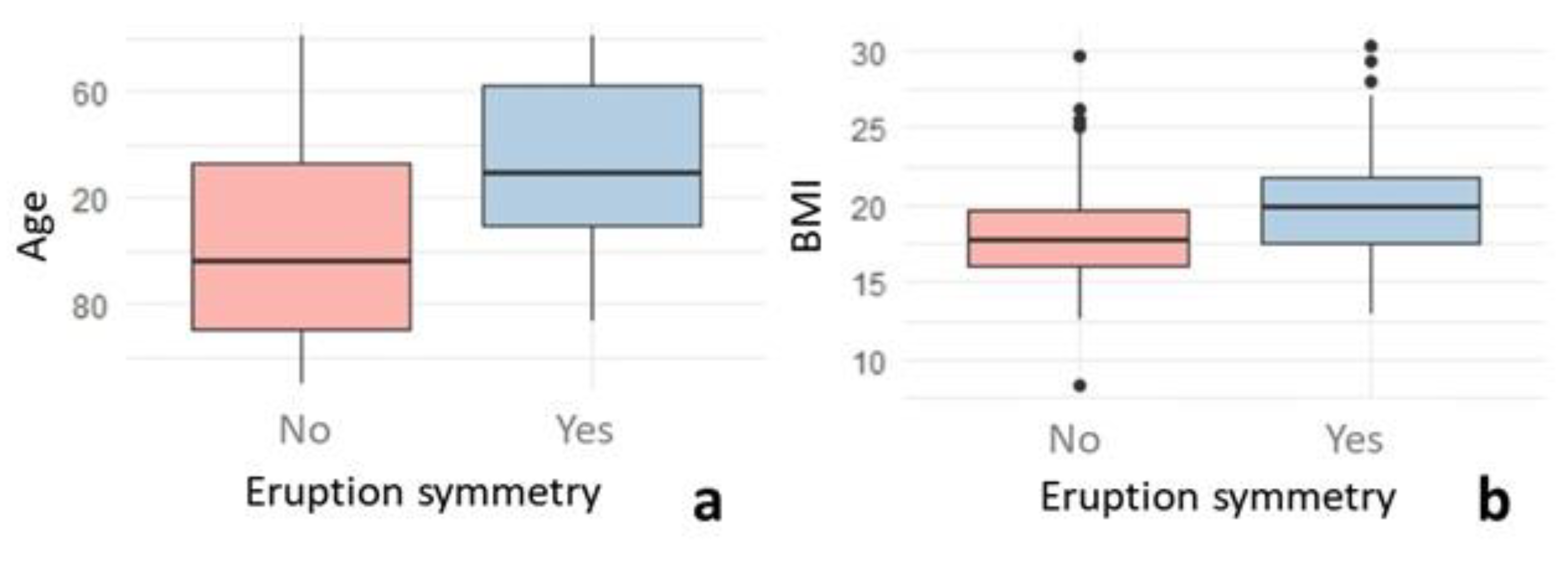
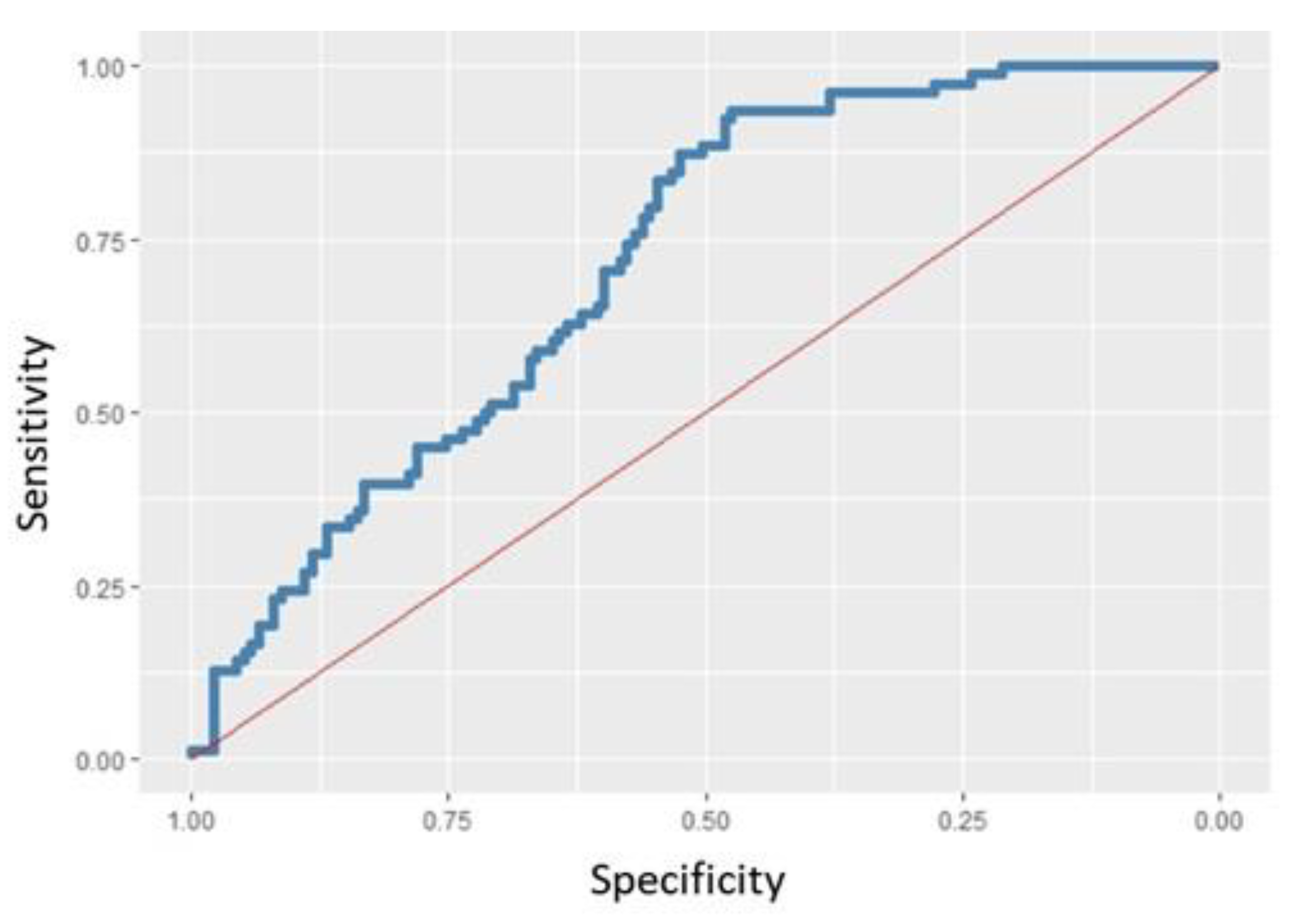

| BMICat. # | Race ¬ | Age (Months) | BMI (kg/m2) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | C | H | Mean | SD + | Mean | SD | ||
| Sex * | Sig. | p-ꭓ2 = 0.6582 | p-ꭓ2 = 1 | p-Ttest = 0.9159 | p-Ttest = 0.6262 | |||||
| F | 183 | 172 | 12 | 342 | 25 | 115 | 36.7 | 18.8 | 3.02 | |
| M | 176 | 174 | 8 | 333 | 25 | 115 | 37.2 | 18.7 | 2.95 | |
| IMC cat. | Sig. | p-ꭓ2 = 0.3511 | p-Welch < 0.001 | p-Welch < 0.001 | ||||||
| 1 | 331 | 28 | 96.4 | 31.5 | 16.3 | 1.42 | ||||
| 2 | 324 | 22 | 133 | 32.5 | 20.8 | 1.64 | ||||
| 3 | 20 | 0 | 153 | 21.6 | 26.4 | 1.64 | ||||
| Race | Sig. | p-Welch < 0.001 | p-Ttest = 0.1536 | |||||||
| C | 117 | 36.8 | 18.8 | 3.00 | ||||||
| H | 91.9 | 31.1 | 18.2 | 2.61 | ||||||
| Quadrant | Eruption Order |
|---|---|
| First quadrant | 1 ≡ 6 < 2 < 4 < 3 ≡ 5 < 7 |
| Second quadrant | |
| Third quadrant | 1 < 6 < 2 < 3 < 4 < 5 < 7 |
| Fourth quadrant | 1 ≡ 6 < 2 < 3 < 4 < 5 < 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paz-Cortés, M.M.; Muñoz-Cano, L.; Diéguez-Pérez, M. Evaluation of the Relationship between the BMI and the Sequence and Chronology of Eruption in Permanent Dentition in Spanish Population. Healthcare 2022, 10, 1046. https://doi.org/10.3390/healthcare10061046
Paz-Cortés MM, Muñoz-Cano L, Diéguez-Pérez M. Evaluation of the Relationship between the BMI and the Sequence and Chronology of Eruption in Permanent Dentition in Spanish Population. Healthcare. 2022; 10(6):1046. https://doi.org/10.3390/healthcare10061046
Chicago/Turabian StylePaz-Cortés, Marta Macarena, Laura Muñoz-Cano, and Montserrat Diéguez-Pérez. 2022. "Evaluation of the Relationship between the BMI and the Sequence and Chronology of Eruption in Permanent Dentition in Spanish Population" Healthcare 10, no. 6: 1046. https://doi.org/10.3390/healthcare10061046
APA StylePaz-Cortés, M. M., Muñoz-Cano, L., & Diéguez-Pérez, M. (2022). Evaluation of the Relationship between the BMI and the Sequence and Chronology of Eruption in Permanent Dentition in Spanish Population. Healthcare, 10(6), 1046. https://doi.org/10.3390/healthcare10061046








