Effect of Aging on Educational Differences in the Risk of Cognitive Impairment: A Gender-Specific Analysis Using Korean Longitudinal Study of Aging (2006–2016)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source and Study Sample
2.2. Measures and Variables
2.3. Statistical Analysis
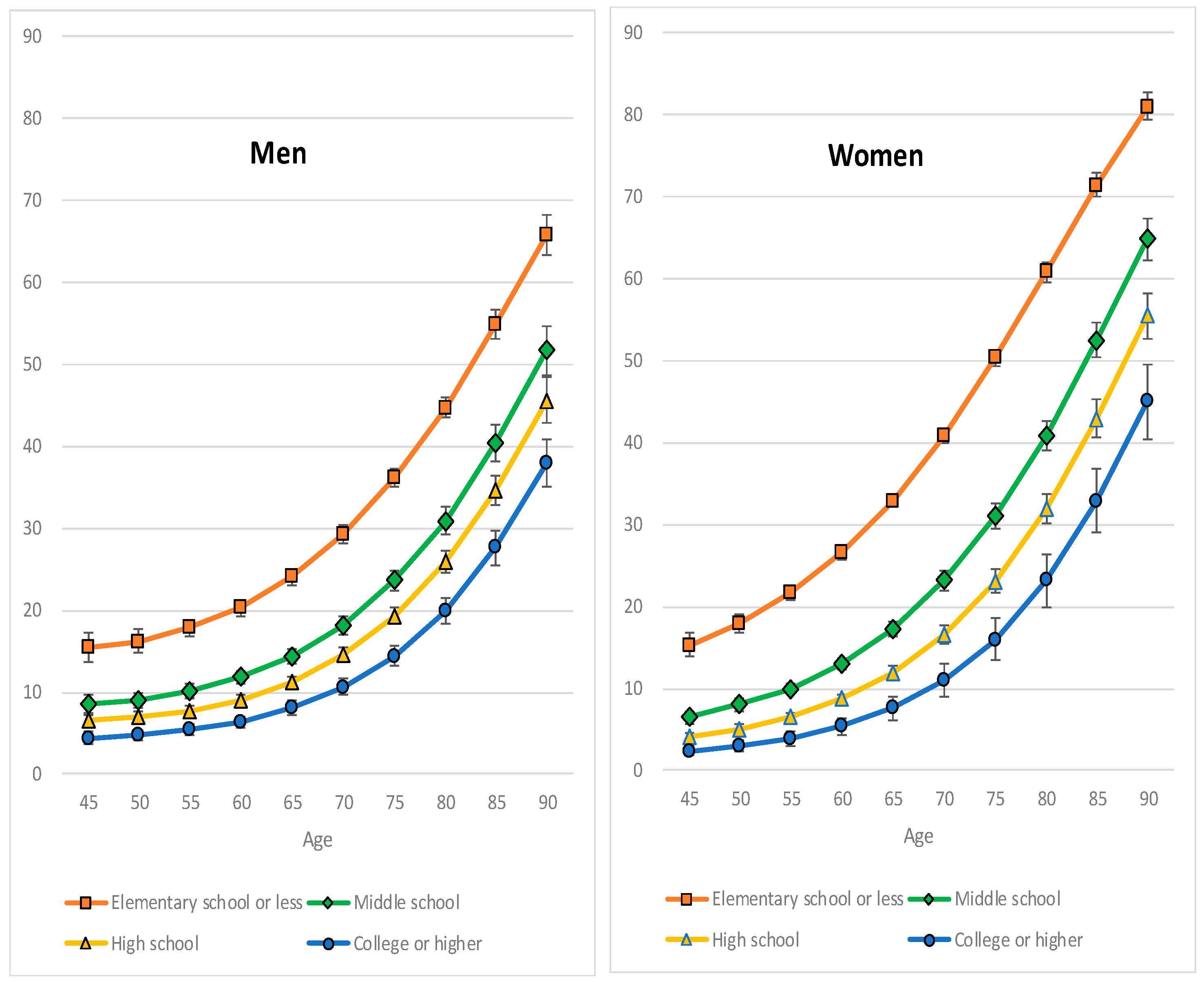
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations. Growing at a Slower Pace, World Population Is Expected to Reach 9.7 Billion in 2050 and Could Peak at Nearly 11 Billion around 2100; United Nations: New York, NY, USA, 2019. [Google Scholar]
- Crimmins, E.M.; Kim, J.K.; Langa, K.M.; Weir, D.R. Assessment of cognition using surveys and neuropsychological assessment: The Health and Retirement Study and the Aging, Demographics, and Memory Study. J. Gerontol. B Psychol. Sci. Soc. Sci. 2011, 66 (Suppl. S1), i162–i171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prince, M.; Bryce, R.; Albanese, E.; Wimo, A.; Ribeiro, W.; Ferri, C.P. The global prevalence of dementia: A systematic review and metaanalysis. Alzheimer’s Dement. 2013, 9, 63–75.e62. [Google Scholar] [CrossRef]
- Sharp, E.S.; Gatz, M. Relationship between education and dementia: An updated systematic review. Alzheimer Dis. Assoc. Disord. 2011, 25, 289–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pais, R.; Ruano, L.; Moreira, C.; Carvalho, O.P.; Barros, H. Prevalence and incidence of cognitive impairment in an elder Portuguese population (65–85 years old). BMC Geriatr. 2020, 20, 470. [Google Scholar] [CrossRef]
- Deng, Q.; Liu, W. Inequalities in cognitive impairment among older adults in China and the associated social determinants: A decomposition approach. Int. J. Equity Health 2021, 20, 82. [Google Scholar] [CrossRef] [PubMed]
- CDC. Cognitive Impairment: A Call for Action, Now! CDC: Atlanta, GA, USA, 2011. [Google Scholar]
- Lenehan, M.E.; Summers, M.J.; Saunders, N.L.; Summers, J.J.; Vickers, J.C. Relationship between education and age-related cognitive decline: A review of recent research. Psychogeriatrics 2015, 15, 154–162. [Google Scholar] [CrossRef]
- Stern, Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012, 11, 1006–1012. [Google Scholar] [CrossRef] [Green Version]
- Ren, L.; Zheng, Y.; Wu, L.; Gu, Y.; He, Y.; Jiang, B.; Zhang, J.; Zhang, L.; Li, J. Investigation of the prevalence of Cognitive Impairment and its risk factors within the elderly population in Shanghai, China. Sci. Rep. 2018, 8, 3575. [Google Scholar] [CrossRef]
- Legdeur, N.; Heymans, M.W.; Comijs, H.C.; Huisman, M.; Maier, A.B.; Visser, P.J. Age dependency of risk factors for cognitive decline. BMC Geriatr. 2018, 18, 187. [Google Scholar] [CrossRef]
- Berggren, R.; Nilsson, J.; Lövdén, M. Education Does Not Affect Cognitive Decline in Aging: A Bayesian Assessment of the Association Between Education and Change in Cognitive Performance. Front. Psychol. 2018, 9, 1138. [Google Scholar] [CrossRef] [Green Version]
- Farmer, M.E.; Kittner, S.J.; Rae, D.S.; Bartko, J.J.; Regier, D.A. Education and change in cognitive function. The Epidemiologic Catchment Area Study. Ann. Epidemiol. 1995, 5, 1–7. [Google Scholar] [CrossRef]
- Wang, L.; Tian, T. Gender Differences in Elderly With Subjective Cognitive Decline. Front. Aging Neurosci. 2018, 10, 166. [Google Scholar] [CrossRef] [PubMed]
- Barnes, L.L.; Wilson, R.S.; Schneider, J.A.; Bienias, J.L.; Evans, D.A.; Bennett, D.A. Gender, cognitive decline, and risk of AD in older persons. Neurology 2003, 60, 1777–1781. [Google Scholar] [CrossRef]
- Zarantonello, L.; Schiff, S.; Amodio, P.; Bisiacchi, P. The effect of age, educational level, gender and cognitive reserve on visuospatial working memory performance across adult life span. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 2020, 27, 302–319. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.A.; Gross, A.L.; Briceño, E.M.; Tilton, N.; Giordani, B.J.; Sussman, J.B.; Hayward, R.A.; Burke, J.F.; Hingtgen, S.; Elkind, M.S.V.; et al. Sex Differences in Cognitive Decline among US Adults. JAMA. Netw. Open 2021, 4, e210169. [Google Scholar] [CrossRef] [PubMed]
- Gerstorf, D.; Herlitz, A.; Smith, J. Stability of sex differences in cognition in advanced old age: The role of education and attrition. J. Gerontol. B Psychol. Sci. Soc. Sci. 2006, 61, P245–P249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lövdén, M.; Rönnlund, M.; Wahlin, A.; Bäckman, L.; Nyberg, L.; Nilsson, L.G. The extent of stability and change in episodic and semantic memory in old age: Demographic predictors of level and change. J. Gerontol. B Psychol. Sci. Soc. Sci. 2004, 59, P130–P134. [Google Scholar] [CrossRef] [Green Version]
- Zahodne, L.B.; Stern, Y.; Manly, J.J. Differing effects of education on cognitive decline in diverse elders with low versus high educational attainment. Neuropsychology 2015, 29, 649–657. [Google Scholar] [CrossRef] [Green Version]
- Christensen, H.; Hofer, S.M.; Mackinnon, A.J.; Korten, A.E.; Jorm, A.F.; Henderson, A.S. Age is no kinder to the better educated: Absence of an association investigated using latent growth techniques in a community sample. Psychol. Med. 2001, 31, 15–28. [Google Scholar] [CrossRef]
- Roe, C.M.; Xiong, C.; Miller, J.P.; Morris, J.C. Education and Alzheimer disease without dementia: Support for the cognitive reserve hypothesis. Neurology 2007, 68, 223–228. [Google Scholar] [CrossRef]
- Murman, D.L. The Impact of Age on Cognition. Semin. Hear. 2015, 36, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Lyketsos, C.G.; Chen, L.S.; Anthony, J.C. Cognitive decline in adulthood: An 11.5-year follow-up of the Baltimore Epidemiologic Catchment Area study. Am. J. Psychiatry 1999, 156, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Deckers, K.; Nooyens, A.; van Boxtel, M.; Verhey, F.; Verschuren, M.; Kohler, S. Gender and Educational Differences in the Association between Lifestyle and Cognitive Decline over 10 Years: The Doetinchem Cohort Study. J. Alzheimers Dis. 2019, 70, S31–S41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proust-Lima, C.; Amieva, H.; Letenneur, L. Gender and education impact on brain aging: A general cognitive factor approach. Psychol. Aging 2008, 23, 608–620. [Google Scholar] [CrossRef] [Green Version]
- Zahodne, L.B.; Glymour, M.M.; Sparks, C.; Bontempo, D.; Dixon, R.A.; MacDonald, S.W.; Manly, J.J. Education does not slow cognitive decline with aging: 12-year evidence from the victoria longitudinal study. J. Int. Neuropsychol. Soc. 2011, 17, 1039–1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, W.; Kim, R. Which Occupation is Highly Associated with Cognitive Impairment? A Gender-Specific Longitudinal Study of Paid and Unpaid Occupations in South Korea. Int. J. Environ. Res. Public Health 2020, 17, 7749. [Google Scholar] [CrossRef]
- Chung, W.; Kim, R. Differential Risk of Cognitive Impairment across Paid and Unpaid Occupations in the Middle-Age Population: Evidence from the Korean Longitudinal Study of Aging, 2006–2016. Int. J. Environ. Res. Public Health 2020, 17, 3124. [Google Scholar] [CrossRef]
- Lee, S.; Oh, J.W.; Son, N.H. Association between Handgrip Strength and Cognitive Function in Older Adults: Korean Longitudinal Study of Aging (2006–2018). Int. J. Environ. Res. Public Health 2022, 19, 1048. [Google Scholar] [CrossRef]
- Kang, Y.; Na, D.L.; Hahn, S. A validity study on the Korean Mini-Mental State Examination (K-MMSE) in dementia patients. J. Korean Neurol. Assoc. 1997, 15, 300–308. [Google Scholar]
- Han, C.; Jo, S.A.; Jo, I.; Kim, E.; Park, M.H.; Kang, Y. An adaptation of the Korean mini-mental state examination (K-MMSE) in elderly Koreans: Demographic influence and population-based norms (the AGE study). Arch. Gerontol. Geriatr. 2008, 47, 302–310. [Google Scholar] [CrossRef]
- Jeong, S.-M.; Choi, S.; Kim, K.; Kim, S.M.; Kim, S.; Park, S.M. Association among handgrip strength, body mass index and decline in cognitive function among the elderly women. BMC Geriatr. 2018, 18, 225. [Google Scholar] [CrossRef] [PubMed]
- Cacciatore, F.; Abete, P.; Ferrara, N.; Calabrese, C.; Napoli, C.; Maggi, S.; Varricchio, M.; Rengo, F.; Osservatorio Geriatrico Campano Study Group. Congestive Heart Failure and Cognitive Impairment in an Older Population. J. Am. Geriatr. Soc. 1998, 46, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- Brandolini, A.; Smeeding, T.M. Income inequality in richer and OECD countries. In The Oxford Handbook of Economic Inequality; Oxford University Press, Inc.: New York, NY, USA, 2009; pp. 71–100. [Google Scholar]
- Andresen, E.M.; Malmgren, J.A.; Carter, W.B.; Patrick, D.L. Screening for depression in well older adults: Evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). Am. J. Prev. Med. 1994, 10, 77–84. [Google Scholar] [CrossRef]
- Ko, K.D.; Cho, Y.T.; Cho, S.I.; Sung, J.H.; Cho, B.L.; Son, K.Y.; Choi, H.C. Association of health risk behaviors with mental health among elderly Koreans. Ann. Geriatr. Med. Res. 2012, 16, 66–73. [Google Scholar]
- WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004, 363, 157. [Google Scholar] [CrossRef]
- Rabe-Hesketh, S.; Skrondal, A. Multilevel modelling of complex survey data. J. R. Stat. Soc. Ser. A Stat. Soc. 2006, 169, 805–827. [Google Scholar] [CrossRef]
- Pfeffermann, D.; Skinner, C.J.; Holmes, D.J.; Goldstein, H.; Rasbash, J. Weighting for unequal selection probabilities in multilevel models. J. R. Stat. Soc. Ser. B Stat. Method. 1998, 60, 23–40. [Google Scholar] [CrossRef]
- Laws, K.R.; Irvine, K.; Gale, T.M. Sex differences in cognitive impairment in Alzheimer’s disease. World J. Psychiatry 2016, 6, 54–65. [Google Scholar] [CrossRef] [Green Version]
- Lipnicki, D.M.; Crawford, J.D.; Dutta, R.; Thalamuthu, A.; Kochan, N.A.; Andrews, G.; Lima-Costa, M.F.; Castro-Costa, E.; Brayne, C.; Matthews, F.E.; et al. Age-related cognitive decline and associations with sex, education and apolipoprotein E genotype across ethnocultural groups and geographic regions: A collaborative cohort study. PLoS Med. 2017, 14, e1002261. [Google Scholar] [CrossRef] [Green Version]
- Miller, D.I.; Halpern, D.F. The new science of cognitive sex differences. Trends Cogn. Sci. 2014, 18, 37–45. [Google Scholar] [CrossRef]
- Stern, Y. What is cognitive reserve? Theory and research application of the reserve concept. J. Int. Neuropsychol. Soc. 2002, 8, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.Y.; Han, L.; Carreno, C.A.; Zhang, Z.; Rodriguez, R.M.; LaRose, M.; Hassenstab, J.; Wig, G.S. Long-term prognosis and educational determinants of brain network decline in older adult individuals. Nat. Aging 2021, 1, 1053–1067. [Google Scholar] [CrossRef] [PubMed]
- Foubert-Samier, A.; Catheline, G.; Amieva, H.; Dilharreguy, B.; Helmer, C.; Allard, M.; Dartigues, J.F. Education, occupation, leisure activities, and brain reserve: A population-based study. Neurobiol. Aging 2012, 33, e415–e425. [Google Scholar] [CrossRef]
- Salthouse, T.A. Mental Exercise and Mental Aging: Evaluating the Validity of the “Use It or Lose It” Hypothesis. Perspect. Psychol. Sci. 2006, 1, 68–87. [Google Scholar] [CrossRef]
- Arenaza-Urquijo, E.M.; Landeau, B.; La Joie, R.; Mevel, K.; Mézenge, F.; Perrotin, A.; Desgranges, B.; Bartrés-Faz, D.; Eustache, F.; Chételat, G. Relationships between years of education and gray matter volume, metabolism and functional connectivity in healthy elders. NeuroImage 2013, 83, 450–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, S.R.; Dickie, D.A.; Ritchie, S.J.; Karama, S.; Pattie, A.; Royle, N.A.; Corley, J.; Aribisala, B.S.; Valdés Hernández, M.; Muñoz Maniega, S.; et al. Associations between education and brain structure at age 73 years, adjusted for age 11 IQ. Neurology 2016, 87, 1820–1826. [Google Scholar] [CrossRef] [Green Version]
- Alley, D.; Suthers, K.; Crimmins, E. Education and Cognitive Decline in Older Americans: Results From the AHEAD Sample. Res. Aging 2007, 29, 73–94. [Google Scholar] [CrossRef]
- Bosma, H.; van Boxtel, M.P.; Ponds, R.W.; Houx, P.J.; Burdorf, A.; Jolles, J. Mental work demands protect against cognitive impairment: MAAS prospective cohort study. Exp. Aging Res. 2003, 29, 33–45. [Google Scholar] [CrossRef]
- Gross, A.L.; Mungas, D.M.; Crane, P.K.; Gibbons, L.E.; MacKay-Brandt, A.; Manly, J.J.; Mukherjee, S.; Romero, H.; Sachs, B.; Thomas, M.; et al. Effects of education and race on cognitive decline: An integrative study of generalizability versus study-specific results. Psychol. Aging 2015, 30, 863–880. [Google Scholar] [CrossRef]
- Mielke, M.M.; Vemuri, P.; Rocca, W.A. Clinical epidemiology of Alzheimer’s disease: Assessing sex and gender differences. Clin. Epidemiol. 2014, 6, 37–48. [Google Scholar] [CrossRef] [Green Version]
- GBD 2016 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1260–1344. [Google Scholar] [CrossRef] [Green Version]
- Podcasy, J.L.; Epperson, C.N. Considering sex and gender in Alzheimer disease and other dementias. Dialogues Clin. Neurosci. 2016, 18, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Andrew, M.K.; Tierney, M.C. The puzzle of sex, gender and Alzheimer’s disease: Why are women more often affected than men? Womens Health 2018, 14, 1745506518817995. [Google Scholar] [CrossRef] [Green Version]
- Vitaliano, P.P.; Murphy, M.; Young, H.M.; Echeverria, D.; Borson, S. Does caring for a spouse with dementia promote cognitive decline? A hypothesis and proposed mechanisms. J. Am. Geriatr. Soc. 2011, 59, 900–908. [Google Scholar] [CrossRef]
- Seedat, S.; Rondon, M. Women’s wellbeing and the burden of unpaid work. BMJ Clin. Res. Ed. 2021, 374, n1972. [Google Scholar] [CrossRef]
- Lövdén, M.; Fratiglioni, L.; Glymour, M.M.; Lindenberger, U.; Tucker-Drob, E.M. Education and Cognitive Functioning Across the Life Span. Psychol. Sci. Public Interest 2020, 21, 6–41. [Google Scholar] [CrossRef]
- Bloomberg, M.; Dugravot, A.; Dumurgier, J.; Kivimaki, M.; Fayosse, A.; Steptoe, A.; Britton, A.; Singh-Manoux, A.; Sabia, S. Sex differences and the role of education in cognitive ageing: Analysis of two UK-based prospective cohort studies. Lancet Public Health 2021, 6, e106–e115. [Google Scholar] [CrossRef]
- Monsch, A.U.; Mistridis, P.; Thomann, A. Postponing Cognitive Decline. In Prevention of Chronic Diseases and Age-Related Disability; Michael, J.-P., Ed.; Practical Issues in Geriatrics; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Sattler, C.; Toro, P.; Schönknecht, P.; Schröder, J. Cognitive activity, education and socioeconomic status as preventive factors for mild cognitive impairment and Alzheimer’s disease. Psychiatry Res. 2012, 196, 90–95. [Google Scholar] [CrossRef]
- Akbaraly, T.N.; Portet, F.; Fustinoni, S.; Dartigues, J.F.; Artero, S.; Rouaud, O.; Touchon, J.; Ritchie, K.; Berr, C. Leisure activities and the risk of dementia in the elderly: Results from the Three-City Study. Neurology 2009, 73, 854–861. [Google Scholar] [CrossRef]
- Lachman, M.E.; Agrigoroaei, S.; Murphy, C.; Tun, P.A. Frequent cognitive activity compensates for education differences in episodic memory. Am. J. Geriatr. Psychiatry 2010, 18, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Reed, B.R.; Dowling, M.; Tomaszewski Farias, S.; Sonnen, J.; Strauss, M.; Schneider, J.A.; Bennett, D.A.; Mungas, D. Cognitive activities during adulthood are more important than education in building reserve. J. Int. Neuropsychol. Soc. 2011, 17, 615–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spencer, R.J.; Wendell, C.R.; Giggey, P.P.; Katzel, L.I.; Lefkowitz, D.M.; Siegel, E.L.; Waldstein, S.R. Psychometric limitations of the mini-mental state examination among nondemented older adults: An evaluation of neurocognitive and magnetic resonance imaging correlates. Exp. Aging Res. 2013, 39, 382–397. [Google Scholar] [CrossRef] [PubMed]
- Jo, K.; Jhoo, J.H.; Mun, Y.J.; Kim, Y.M.; Kim, S.K.; Kim, S.; Lee, S.H.; Jang, J.W. The Effect of Cognitive Intervention on Cognitive Improvement in Patients with Dementia. Dement. Neurocogn. Disord. 2018, 17, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Arevalo-Rodriguez, I.; Smailagic, N.; Roque, I.F.M.; Ciapponi, A.; Sanchez-Perez, E.; Giannakou, A.; Pedraza, O.L.; Bonfill Cosp, X.; Cullum, S. Mini-Mental State Examination (MMSE) for the detection of Alzheimer’s disease and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst. Rev. 2015, 2015, CD010783. [Google Scholar] [CrossRef]
- Jang, S.N.; Cho, S.I.; Chang, J.; Boo, K.; Shin, H.G.; Lee, H.; Berkman, L.F. Employment status and depressive symptoms in Koreans: Results from a baseline survey of the Korean Longitudinal Study of Aging. J. Gerontol. B Psychol. Sci. Soc. Sci. 2009, 64, 677–683. [Google Scholar] [CrossRef] [Green Version]
- Perini, G.; Cotta Ramusino, M.; Sinforiani, E.; Bernini, S.; Petrachi, R.; Costa, A. Cognitive impairment in depression: Recent advances and novel treatments. Neuropsychiatr. Dis. Treat. 2019, 15, 1249–1258. [Google Scholar] [CrossRef] [Green Version]
| Characteristics | Men | Women |
|---|---|---|
| Cognitive function score: Mean (SD) | 26.7 (4.2) | 24.6 (5.7) |
| Age, years: Mean (SD) | 61.1 (10.5) | 61.8 (11.4) |
| 45–64 | 61.2% | 59.1% |
| 65–75 | 27.2% | 25.2% |
| 75–84 | 10.2% | 13.3% |
| 85 and above | 1.4% | 2.4% |
| Non-married | 7.8% | 32.4% |
| Religion, yes | 44.5% | 63.9% |
| Resides in a rural area | 22.9% | 22.8% |
| Education level | ||
| Elementary school or less | 31.6% | 58.2% |
| Middle school | 17.0% | 15.6% |
| High school | 33.8% | 21.3% |
| College or higher | 17.6% | 4.9% |
| Occupation | ||
| No job | 43.3% | 76.0% |
| Blue collar job | 40.6% | 20.5% |
| White collar job | 16.1% | 3.5% |
| Household income | ||
| Lower half | 44.0% | 47.6% |
| Higher half | 49.1% | 43.6% |
| Unreported | 6.9% | 8.8% |
| House renter | 21.3% | 24.2% |
| Smoking, yes | 40.6% | 3.1% |
| Alcohol intake, yes | 64.0% | 18.7% |
| Routine physical exercise, active | 43.1% | 35.3% |
| Obese, yes | 21.3% | 23.1% |
| Chronic disease, yes | 37.8% | 39.1% |
| Depressive symptoms, yes | 24.1% | 35.4% |
| Number of observations | 4278 | 5495 |
| Characteristics | Prevalence (%) | Distribution of Observations (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | ||||||||||
| Rate | (95% CI) | Rate | (95% CI) | Wave 1 | Wave 2 | Wave 3 | Wave 4 | Wave 5 | Wave 6 | Overall | |
| Overall | 10.9 | (10.0–11.9) | 26.4 | (25.3–27.6) | |||||||
| Chi-square test, p-value | <0.0001 | ||||||||||
| Age, years | |||||||||||
| 45–64 | 5.7 | (4.8–6.6) | 11.5 | (10.5–12.7) | 60.0 | 54.3 | 50.0 | 45.0 | 40.4 | 31.4 | 48.6 |
| 65–75 | 21.6 | (19.2–24.3) | 47.3 | (44.5–50.1) | 26.1 | 28.6 | 29.8 | 30.8 | 31.4 | 32.8 | 29.5 |
| 75–84 | 40.2 | (35.4–45.1) | 76.9 | (73.6–79.9) | 12.0 | 14.2 | 16.7 | 19.9 | 22.8 | 27.4 | 17.9 |
| 85 and above | 64.6 | (50.4–76.7) | 88.7 | (79.8–94.0) | 1.9 | 2.9 | 3.5 | 4.3 | 5.4 | 8.4 | 4.0 |
| Chi-square test, p-value | <0.0001 | <0.0001 | |||||||||
| Linear trend test, p-value | <0.0001 | <0.0001 | |||||||||
| Education level | |||||||||||
| Elementary school or less | 26.4 | (24.0–28.9) | 46.9 | (45.0–48.7) | 46.6 | 46.9 | 46.8 | 46.1 | 44.9 | 43.6 | 46.0 |
| Middle school | 9.9 | (7.8–12.3) | 10.3 | (8.4–12.7) | 16.2 | 16.2 | 16.7 | 16.9 | 17.1 | 17.3 | 16.6 |
| High school | 6.0 | (4.8–7.4) | 3.5 | (2.6–4.7) | 26.8 | 27.0 | 26.9 | 27.2 | 27.9 | 30.1 | 27.5 |
| College or higher | 2.4 | (1.5–3.7) | 1.6 | (0.6–3.8) | 10.4 | 9.9 | 9.6 | 9.8 | 10.1 | 9.0 | 9.9 |
| Chi-squared test, p-value | <0.0001 | <0.0001 | |||||||||
| Linear trend test, p-value | <0.0001 | <0.0001 | |||||||||
| Number of observations | 4278 | 5495 | 9773 | 8131 | 7111 | 6503 | 5996 | 5523 | 43,037 | ||
| Characteristics | Model with No Covariate | Model with All Studied Covariates | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | |||||||||
| OR | (95% CI) | p | OR | (95% CI) | p | OR | (95% CI) | p | OR | (95% CI) | p | |
| Age | 1.11 | (1.10–1.13) | <0.001 | 1.13 | (1.12–1.14) | <0.001 | 1.08 | (1.06–1.09) | <0.001 | 1.11 | (1.10–0.12) | <0.001 |
| Age squared | 1.00 | (1.00–1.00) | <0.001 | 1.00 | (1.00–1.00) | <0.001 | 1.00 | (1.00–1.00) | <0.001 | 1.00 | (1.00–1.00) | <0.001 |
| Education level (Ref: Elementary school or less) | ||||||||||||
| Middle school | 0.35 | (0.27–0.44) | <0.001 | 0.21 | (0.17–0.26) | <0.001 | 0.40 | (0.32–0.50) | <0.001 | 0.27 | (0.23–0.34) | <0.001 |
| High school | 0.20 | (0.16–0.25) | <0.001 | 0.10 | (0.08–0.12) | <0.001 | 0.27 | (0.22–0.34) | <0.001 | 0.15 | (0.12–0.18) | <0.001 |
| College or higher | 0.11 | (0.08–0.15) | <0.001 | 0.04 | (0.03–0.08) | <0.001 | 0.16 | (0.12–0.22) | <0.001 | 0.08 | (0.04–0.13) | <0.001 |
| Non-married (Ref: Married) | 1.25 | (0.98–1.59) | 0.069 | 1.28 | (1.11–1.48) | 0.001 | ||||||
| Religion (Ref: No) | 0.86 | (0.75–0.99) | 0.042 | 0.75 | (0.67–0.84) | <0.001 | ||||||
| Resides in a rural area (Ref: Reside in a urban area) | 0.94 | (0.78–1.13) | 0.503 | 1.49 | (1.27–1.73) | <0.001 | ||||||
| Occupation (Ref: No job) | ||||||||||||
| Blue collar job | 0.49 | (0.41–0.58) | <0.001 | 0.61 | (0.53–0.70) | <0.001 | ||||||
| White collar job | 0.48 | (0.35–0.66) | <0.001 | 0.27 | (0.15–0.49) | <0.001 | ||||||
| Household income, higher half (Ref: Lower half and unreported) | 0.81 | (0.70–0.94) | 0.004 | 0.78 | (0.70–0.87) | <0.001 | ||||||
| House renter (Ref: House owner) | 1.07 | (0.88–1.31) | 0.493 | 1.32 | (1.13–1.54) | <0.001 | ||||||
| Smoking, yes (Ref: Non-smoking) | 0.90 | (0.76–1.06) | 0.202 | 1.12 | (0.78–1.62) | 0.545 | ||||||
| Alcohol intake, yes (Ref: Non-alcohol intake) | 0.81 | (0.69–0.94) | 0.005 | 0.79 | (0.66–0.94) | 0.007 | ||||||
| Active physical exercise (Ref: Inactive) | 0.53 | (0.46–0.61) | <0.001 | 0.63 | (0.56–0.71) | <0.001 | ||||||
| Obese, yes (Ref: Non-obese) | 0.86 | (0.71–1.04) | 0.111 | 0.80 | (0.70–0.92) | 0.002 | ||||||
| Chronic disease, yes (Ref: No) | 1.24 | (1.07–1.45) | 0.005 | 1.12 | (0.99–1.28) | 0.071 | ||||||
| Depressive symptom, yes (Ref: No) | 2.20 | (1.94–2.49) | <0.001 | 1.98 | (1.79–2.18) | <0.001 | ||||||
| Number of observations | 18,654 | 24,383 | 18,654 | 24,383 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, R.; Chung, W. Effect of Aging on Educational Differences in the Risk of Cognitive Impairment: A Gender-Specific Analysis Using Korean Longitudinal Study of Aging (2006–2016). Healthcare 2022, 10, 1062. https://doi.org/10.3390/healthcare10061062
Kim R, Chung W. Effect of Aging on Educational Differences in the Risk of Cognitive Impairment: A Gender-Specific Analysis Using Korean Longitudinal Study of Aging (2006–2016). Healthcare. 2022; 10(6):1062. https://doi.org/10.3390/healthcare10061062
Chicago/Turabian StyleKim, Roeul, and Woojin Chung. 2022. "Effect of Aging on Educational Differences in the Risk of Cognitive Impairment: A Gender-Specific Analysis Using Korean Longitudinal Study of Aging (2006–2016)" Healthcare 10, no. 6: 1062. https://doi.org/10.3390/healthcare10061062






