Augmented Rehabilitation Program for Patients 60 Years and Younger Following Total Hip Arthroplasty—Feasibility Study
Abstract
:1. Introduction
2. Methods
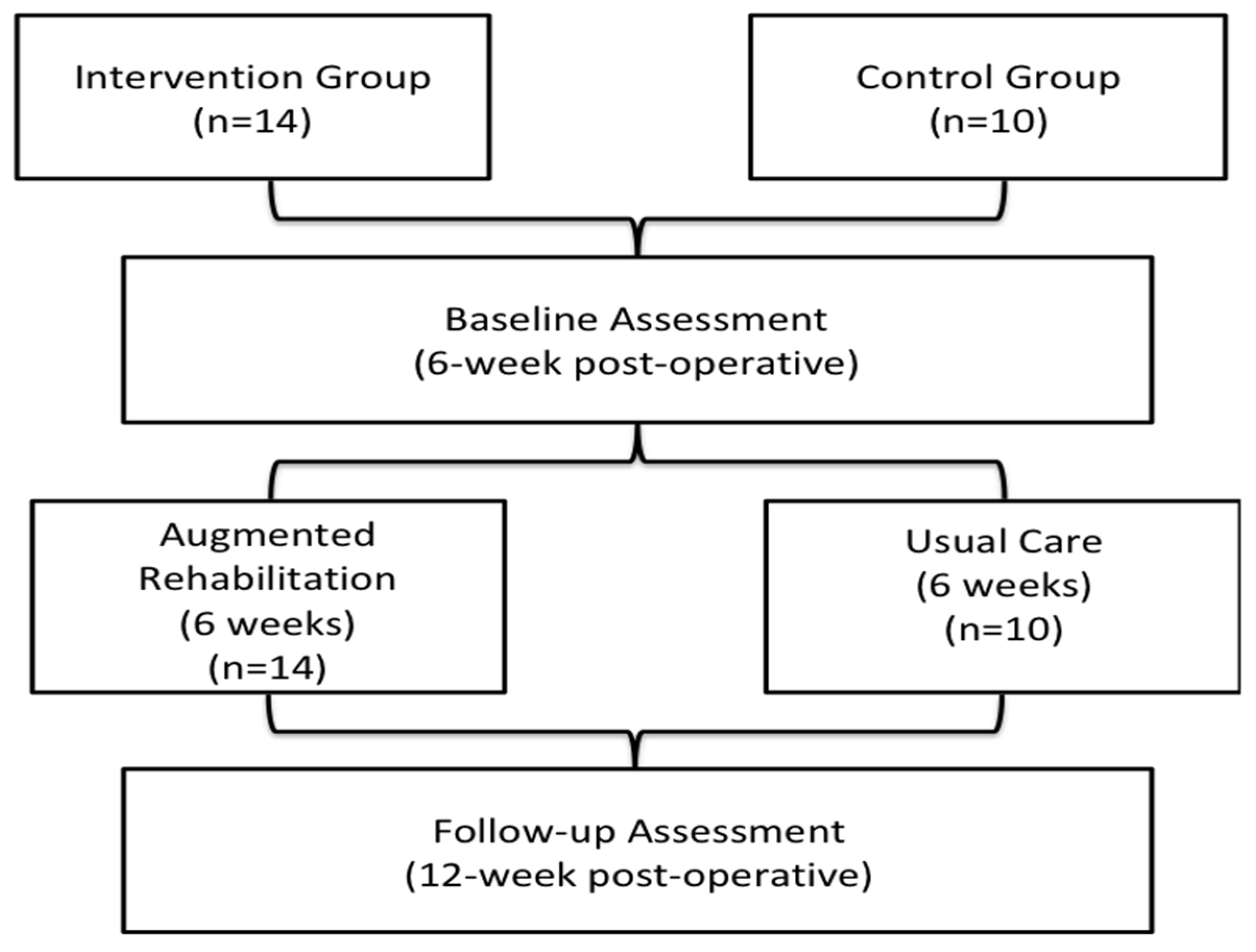
2.1. Study Design and Patients
2.2. Augmented Rehabilitation Program
2.3. Usual Post-Operative Care
2.4. Outcomes Measures
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- CIHI. Hip and Knee Replacements in Canada, 2016–2017: Canadian Joint Replacement Registry Annual Report; CIHI: Ottawa, ON, Canada, 2018. [Google Scholar]
- Kurtz, S.M.; Lau, E.; Ong, K.; Zhao, K.; Kelly, M.; Bozic, K.J. Future young patient demand for primary and revision joint replacement: National projections from 2010 to 2030. Clin. Orthop. Relat. Res. 2009, 467, 2606–2612. [Google Scholar] [CrossRef] [Green Version]
- Kurtz, S.; Mowat, F.; Ong, K.; Chan, N.; Lau, E.; Halpern, M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J. Bone Joint Surg. Am. 2005, 87, 1487–1497. [Google Scholar] [CrossRef] [PubMed]
- Kuijpers, M.F.L.; Hannink, G.; van Steenbergen, L.N.; Schreurs, B.W. Total hip arthroplasty in young patients in the Netherlands: Trend analysis of >19,000 primary hip replacements in the Dutch Arthroplasty Register. J. Arthroplast. 2018, 33, 3704–3711. [Google Scholar] [CrossRef] [PubMed]
- Lespasio, M.J.; Sultan, A.A.; Piuzzi, N.S.; Khlopas, A.; Husni, M.E.; Muschler, G.F.; Mont, M.A. Hip Osteoarthritis: A Primer. Perm. J. 2018, 22, 17–084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sedrakyan, A.; Romero, L.; Graves, S.; Davidson, D.; de Steiger, R.; Lewis, P.; Solomon, M.; Vial, R.; Lorimer, M. Survivorship of hip and knee implants in pediatric and young adult populations: Analysis of registry and published data. J. Bone Joint Surg. Am. 2014, 96 (Suppl. S1), 73–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Studers, P.; Belajevs, D.; Jurkevics, V.; Likums, P. Ten to fifteen-year clinical and radiographic follow-up with a third-generation cementless stem in a young patient population. Int. Orthop. 2016, 40, 465–471. [Google Scholar] [CrossRef]
- Mancuso, C.A.; Ranawat, C.S.; Esdaile, J.M.; Johanson, N.A.; Charlson, M.E. Indications for total hip and total knee arthroplasties. Results of orthopaedic surveys. J. Arthroplast. 1996, 11, 34–46. [Google Scholar] [CrossRef]
- Vogel, L.A.; Carotenuto, G.; Basti, J.J.; Levine, W.N. Physical activity after total joint arthroplasty. Sports Health 2011, 3, 441–450. [Google Scholar] [CrossRef] [Green Version]
- Mancuso, C.A.; Jout, J.; Salvati, E.A.; Sculco, T.P. Fulfillment of patients’ expectations for total hip arthroplasty. J. Bone Joint Surg. Am. 2009, 91, 2073–2078. [Google Scholar] [CrossRef]
- Coulter, C.L.; Scarvell, J.M.; Neeman, T.M.; Smith, P.N. Physiotherapist-directed rehabilitation exercises in the outpatient or home setting improve strength, gait speed and cadence after elective total hip replacement: A systematic review. J. Physiother. 2013, 59, 219–226. [Google Scholar] [CrossRef] [Green Version]
- Wang, A.W.; Gilbey, H.J.; Ackland, T.R. Perioperative exercise programs improve early return of ambulatory function after total hip arthroplasty: A randomized, controlled trial. Am. J. Phys. Med. Rehabil. 2002, 81, 801–806. [Google Scholar] [CrossRef]
- Trudelle-Jackson, E.; Emerson, R.; Smith, S. Outcomes of total hip arthroplasty: A study of patients one year postsurgery. J. Orthop. Sports Phys. Ther. 2002, 32, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Husby, V.S.; Helgerud, J.; Bjørgen, S.; Husby, O.S.; Benum, P.; Hoff, J. Early postoperative maximal strength training improves work efficiency 6–12 months after osteoarthritis-induced total hip arthroplasty in patients younger than 60 years. Am. J. Phys. Med. Rehabil. 2010, 89, 304–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, C.A.; Pohar, S. Health-related quality of life after total joint arthroplasty: A scoping review. Clin. Geriatr. Med. 2012, 28, 395–429. [Google Scholar] [CrossRef] [PubMed]
- Nilsdotter, A.K.; Toksvig-Larsen, S.; Roos, E.M. Knee arthroplasty: Are patients’ expectations fulfilled? A prospective study of pain and function in 102 patients with 5-year follow-up. Acta Orthop. 2009, 80, 55–61. [Google Scholar] [CrossRef]
- Harding, P.; Holland, A.E.; Delany, C.; Hinman, R.S. Do activity levels increase after total hip and knee arthroplasty? Clin. Orthop. Relat. Res. 2014, 472, 1502–1511. [Google Scholar] [CrossRef] [Green Version]
- de Groot, I.B.; Bussmann, H.J.; Stam, H.J.; Verhaar, J.A. Small increase of actual physical activity 6 months after total hip or knee arthroplasty. Clin. Orthop. Relat. Res. 2008, 466, 2201–2208. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Doherty, M.; Peat, G.; Bierma-Zeinstra, M.A.; Arden, N.K.; Bresnihan, B.; Herrero-Beaumont, G.; Kirschner, S.; Leeb, B.F.; Lohmander, L.S.; et al. EULAR evidence-based recommendations for the diagnosis of knee osteoarthritis. Ann. Rheum. Dis. 2010, 69, 483–489. [Google Scholar] [CrossRef] [Green Version]
- Nilsdotter, A.K.; Lohmander, L.S.; Klassbo, M.; Roos, E.M. Hip disability and osteoarthritis outcome score (HOOS)—Validity and responsiveness in total hip replacement. BMC Musculoskelet. Disord. 2003, 4, 10. [Google Scholar] [CrossRef] [Green Version]
- Thabane, L.; Ma, J.; Chu, R.; Cheng, J.; Ismaila, A.; Rios, L.P.; Robson, R.; Thabane, M.; Giangregorio, L.; Goldsmith, C.H. A tutorial on pilot studies: The what, why and how. BMC Med. Res. Methodol. 2010, 10, 1. [Google Scholar] [CrossRef] [Green Version]
- Jakicic, J.M.; Marcus, M.; Gallagher, K.I.; Randall, C.; Thomas, E.; Goss, F.L.; Robertson, R.J. Evaluation of the SenseWear Pro Armband to assess energy expenditure during exercise. Med. Sci. Sports Exerc. 2004, 36, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Wetten, A.A.; Batterham, M.; Tan, S.Y.; Tapsell, L. Relative validity of 3 accelerometer models for estimating energy expenditure during light activity. J. Phys. Act. Health 2014, 11, 638–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malavolti, M.; Pietrobelli, A.; Dugoni, M.; Poli, M.; Romagnoli, E.; De Cristofaro, P.; Battistini, N.C. A new device for measuring resting energy expenditure (REE) in healthy subjects. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 338–343. [Google Scholar] [CrossRef] [Green Version]
- Cole, P.J.; LeMura, L.M.; Klinger, T.A.; Strohecker, K.; McConnell, T.R. Measuring energy expenditure in cardiac patients using the Body Media Armband versus indirect calorimetry. A validation study. J. Sports Med. Phys. Fit. 2004, 44, 262–271. [Google Scholar]
- Fruin, M.L.; Rankin, J.W. Validity of a multi-sensor armband in estimating rest and exercise energy expenditure. Med. Sci. Sports Exerc. 2004, 36, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- King, G.A.; Torres, N.; Potter, C.; Brooks, T.J.; Coleman, K.J. Comparison of activity monitors to estimate energy cost of treadmill exercise. Med. Sci. Sports Exerc. 2004, 36, 1244–1251. [Google Scholar] [CrossRef]
- St-Onge, M.; Mignault, D.; Allison, D.B.; Rabasa-Lhoret, R. Evaluation of a portable device to measure daily energy expenditure in free-living adults. Am. J. Clin. Nutr. 2007, 85, 742–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johannsen, D.L.; Calabro, M.A.; Stewart, J.; Franke, W.; Rood, J.C.; Welk, G.J. Accuracy of armband monitors for measuring daily energy expenditure in healthy adults. Med. Sci. Sports Exerc. 2010, 42, 2134–2140. [Google Scholar] [CrossRef] [PubMed]
- Matthews, C.E.; Ainsworth, B.E.; Thompson, R.W.; Bassett, D.R. Sources of variance in daily physical activity levels as measured by an accelerometer. Med. Sci. Sports Exerc. 2002, 34, 1376–1381. [Google Scholar] [CrossRef]
- Matthews, C.E.; Chen, K.Y.; Freedson, P.S.; Buchowski, M.S.; Beech, B.M.; Pate, R.R.; Troiano, R.P. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am. J. Epidemiol. 2008, 167, 875–881. [Google Scholar] [CrossRef] [Green Version]
- Troiano, R.P.; Berrigan, D.; Dodd, K.W.; Masse, L.C.; Tilert, T.; McDowell, M. Physical activity in the United States measured by accelerometer. Med. Sci. Sports Exerc. 2008, 40, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Dogra, S.; Stathokostas, L. Sedentary behavior and physical activity are independent predictors of successful aging in middle-aged and older adults. J. Aging Res. 2012, 2012, 190654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pate, R.R.; O’Neill, J.R.; Lobelo, F. The evolving definition of “sedentary”. Exerc. Sport Sci. Rev. 2008, 36, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, M.S.; Aubert, S.; Barnes, J.D.; Saunders, T.J.; Carson, V.; Latimer-Cheung, A.E.; Chastin, S.F.M.; Altenburg, T.M.; Chinapaw, M.J.M.; Participants, S.T.C.P. Sedentary Behavior Research Network (SBRN)—Terminology Consensus Project process and outcome. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 75. [Google Scholar] [CrossRef] [Green Version]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef] [PubMed]
- Haskell, W.L.; Lee, I.M.; Pate, R.R.; Powell, K.E.; Blair, S.N.; Franklin, B.A.; Macera, C.A.; Heath, G.W.; Thompson, P.D.; Bauman, A. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation 2007, 116, 1081–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colley, R.C.; Garriguet, D.; Janssen, I.; Craig, C.L.; Clarke, J.; Tremblay, M.S. Physical activity of Canadian adults: Accelerometer results from the 2007 to 2009 Canadian Health Measures Survey. Health Rep. 2011, 22, 7–14. [Google Scholar] [PubMed]
- Klässbo, M.; Larsson, E.; Mannevik, E. Hip disability and osteoarthritis outcome scoreAn extension of the Western Ontario and McMaster Universities Osteoarthritis Index. Scand. J. Rheumatol. 2003, 32, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Thorborg, K.; Roos, E.M.; Bartels, E.M.; Petersen, J.; Holmich, P. Validity, reliability and responsiveness of patient-reported outcome questionnaires when assessing hip and groin disability: A systematic review. Br. J. Sport Med. 2010, 44, 1186–1196. [Google Scholar] [CrossRef]
- Goh, S.-L.; Persson, M.S.M.; Stocks, J.; Hou, Y.; Welton, N.J.; Lin, J.; Hall, M.C.; Doherty, M.; Zhang, W. Relative efficacy of different exercises for pain, function, performance and quality of life in knee and hip osteoarthritis: Systematic review and network meta-analysis. Sports Med. 2019, 49, 743–761. [Google Scholar] [CrossRef] [Green Version]
- Paulsen, A.; Roos, E.M.; Pedersen, A.B.; Overgaard, S. Minimal clinically important improvement (MCII) and patient-acceptable symptom state (PASS) in total hip arthroplasty (THA) patients 1 year postoperatively. Acta Orthop. 2014, 85, 39–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suarez-Almazor, M.E.; Looney, C.; Liu, Y.; Cox, V.; Pietz, K.; Marcus, D.M.; Street, R.L. A randomized controlled trial of acupuncture for osteoarthritis of the knee: Effects of patient-provider communication. Arthritis Care Res. 2010, 62, 1229–1236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation coefficients: Appropriate use and interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Tudor-Locke, C.; Craig, C.L.; Aoyagi, Y.; Bell, R.C.; Croteau, K.A.; De Bourdeaudhuij, I.; Ewald, B.; Gardner, A.W.; Hatano, Y.; Lutes, L.D.; et al. How many steps/day are enough? For older adults and special populations. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 80. [Google Scholar] [CrossRef] [Green Version]
- Lyman, S.; Lee, Y.Y.; McLawhorn, A.S.; Islam, W.; MacLean, C.H. What are the minimal and substantial improvements in the HOOS and KOOS and JR versions after total joint replacement? Clin. Orthop. Relat. Res. 2018, 476, 2432–2441. [Google Scholar] [CrossRef]
- Snell, D.L.; Hipango, J.; Sinnott, K.A.; Dunn, J.A.; Rothwell, A.; Hsieh, C.J.; Dejong, G.; Hooper, G. Rehabilitation after total joint replacement: A scoping study. Disabil. Rehabil. 2018, 40, 1718–1731. [Google Scholar] [CrossRef]
- Beaupre, L.A.; Masson, E.C.; Luckhurst, B.J.; Arafah, O.; O’Connor, G.J. A randomized pilot study of a comprehensive postoperative exercise program compared with usual care following primary total hip arthroplasty in subjects less than 65 years of age: Feasibility, selection of outcome measures and timing of assessment. BMC Musculoskelet. Disord. 2014, 15, 192. [Google Scholar] [CrossRef] [Green Version]
- Crizer, M.P.; Kazarian, G.S.; Fleischman, A.N.; Lonner, J.H.; Maltenfort, M.G.; Chen, A.F. Stepping toward objective outcomes: A prospective analysis of step count after total joint arthroplasty. J. Arthroplast. 2017, 32, S162–S165. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Makimoto, K.; Tanaka, R.; Mawatari, M.; Hotokebuchi, T. Prospective study of physical activity and quality of life in Japanese women undergoing total hip arthroplasty. J. Orthop. Sci. 2013, 18, 45–53. [Google Scholar] [CrossRef]
- Canadian Society for Exercise Physiology (CESP). Canadian Physical Activity Guidelines For Older Adults; 2014; Available online: https://csepguidelines.ca/ (accessed on 20 June 2022).
- Office of Disease Prevention and Health Promotion. Physical Activity Guidelines for Americans—Current Guidelines. Available online: https://health.gov/our-work/nutrition-physical-activity/physical-activity-guidelines/current-guidelines (accessed on 20 June 2022).
- World Health Organization. WHO Guidelines on Physical activity and Sedentary Behaviour: Web Annex: Evidence Profiles; 9240015116; World Health Organization: Geneva, Switzerland, 2020; p. 535. [Google Scholar]
- Lemmey, A.; Okoro, T. The efficacy of exercise rehabilitation in restoring physical function following total hip replacement for osteoarthritis: A review. OA Musculoskelet. Med. 2013, 1, 2–13. [Google Scholar] [CrossRef]
- Bartels, E.M.; Juhl, C.B.; Christensen, R.; Hagen, K.B.; Danneskiold-Samsøe, B.; Dagfinrud, H.; Lund, H. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst. Rev. 2016, 3, CD005523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barker, A.L.; Talevski, J.; Morello, R.T.; Brand, C.A.; Rahmann, A.E.; Urquhart, D.M. Effectiveness of aquatic exercise for musculoskeletal conditions: A meta-analysis. Arch. Phys. Med. Rehabil. 2014, 95, 1776–1786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, B.E. Aquatic therapy: Scientific foundations and clinical rehabilitation applications. Phys. Med. Rehabil. 2009, 1, 859–872. [Google Scholar] [CrossRef] [PubMed]
- Batterham, S.I.; Heywood, S.; Keating, J.L. Systematic review and meta-analysis comparing land and aquatic exercise for people with hip or knee arthritis on function, mobility and other health outcomes. BMC Musculoskelet. Disord. 2011, 12, 123. [Google Scholar] [CrossRef] [Green Version]
- Blikman, T.; Stevens, M.; Bulstra, S.K.; van den Akker-Scheek, I.; Reininga, I.H. Reliability and validity of the Dutch version of the International Physical Activity Questionnaire in patients after total hip arthroplasty or total knee arthroplasty. J. Orthop. Sports Phys. Ther. 2013, 43, 650–659. [Google Scholar] [CrossRef] [Green Version]
- Loprinzi, P.D.; Davis, R.E. Bouted and non-bouted moderate-to-vigorous physical activity with health-related quality of life. Prev. Med. Rep. 2016, 3, 46–48. [Google Scholar] [CrossRef] [Green Version]
- Anokye, N.K.; Trueman, P.; Green, C.; Pavey, T.G.; Taylor, R.S. Physical activity and health related quality of life. BMC Public Health 2012, 12, 624. [Google Scholar] [CrossRef] [Green Version]
- Ravalli, S.; Roggio, F.; Lauretta, G.; Di Rosa, M.; D’Amico, A.G.; D’Agata, V.; Maugeri, G.; Musumeci, G. Exploiting real-world data to monitor physical activity in patients with osteoarthritis: The opportunity of digital epidemiology. Heliyon 2022, 8, e08991. [Google Scholar] [CrossRef]
- Fortina, M.; Carta, S.; Gambera, D.; Crainz, E.; Ferrata, P.; Maniscalco, P. Recovery of physical function and patient’s satisfaction after total hip replacement (THR) surgery supported by a tailored guide-book. Acta Biomed. 2005, 76, 152–156. [Google Scholar]
| Intervention (n = 14) | Control (n = 10) | p Value | |
|---|---|---|---|
| Demographics | |||
| Age (years), mean ± SD | 51.9 ± 4.1 | 54.5 ± 5.2 | 0.184 |
| Female, n (%) | 5 (35) | 3 (30) | 0.836 |
| Employment Status, n (%) | |||
| Employed | 10 (71%) | 6 (60) | 0.752 |
| Unemployed | 1 (7%) | 0 (0) | 0.398 |
| Disability | 2 (14%) | 1 (10) | 0.822 |
| Retired | 1 (7%) | 3 (30) | 0.229 |
| Marital Status, n (%) | |||
| Married/ common-law | 12 (86) | 9 (90) | 0.999 |
| Single/widowed/divorced | 2 (14) | 1 (10) | 0.822 |
| Education (years of school), mean ± SD | 14 ± 2.3 | 14.1 ± 2.5 | 0.920 |
| Living Status, living with, n (%) | |||
| Alone | 1 (7) | 0 | 0.398 |
| Spouse and/or others | 13 (93) | 10 (100) | 0.999 |
| Medical | |||
| BMI (kg/m2) mean ± SD | 27 ± 4.1 | 31.2 ± 5 | 0.034 |
| Number of co-morbidity/person, median (25%ile, 75%ile) | 2 (1, 2) | 4 (3, 5) | 0.010 |
| 3 Most Prevalent Comorbidities | |||
| Chronic Pain, n (%) | 8 (57) | 6 (60) | 0.999 |
| Mental Health Problem, n (%) | 4 (29) | 5 (50) | 0.421 |
| High Blood Pressure, n (%) | 4 (29) | 3 (30) | 0.999 |
| Ambulation Status, n (%) | |||
| Distance walk <1 block | 1 (7) | 0 (0) | 0.398 |
| Distance walk 1–5 blocks | 4 (29) | 6 (60) | 0.263 |
| Distance walk 6–10 blocks | 4 (29) | 1 (10) | 0.376 |
| Distance walk unlimited | 5 (36) | 3 (30) | 0.836 |
| Surgical characteristics | |||
| Type of Arthroplasty, n (%) | |||
| Non-cemented | 11 (79%) | 8 (80) | 0.999 |
| Hybrid | 2 (14%) | 2 (20) | 0.999 |
| Cemented | 1 (7%) | 0 (0) | 0.398 |
| Surgical Approach, n (%) | |||
| Antero-lateral hip | 2 (14) | 1 (10) | 0.822 |
| Posterior-lateral hip | 9 (65) | 4 (40) | 0.448 |
| Unknown | 3 (21) | 5 (50) | 0.261 |
| Intervention (n = 14) Mean ± SD | Control (n = 10) Mean ± SD | |||||
|---|---|---|---|---|---|---|
| Physical activity Measures | Baseline | Follow-up | p-value | Baseline | Follow-up | p-value |
| Waking wearing time (Hrs/day) | 14.9± 1.5 | 14.7± 1.4 | 0.658 | 15.6 ± 1.4 | 15.3 ± 1.2 | 0.782 |
| Sleeping time (Hrs/day) | 5.8 ± 1.9 | 4.7 ± 1.1 | 0.058 | 6.0 ± 1.0 | 5.2 ± 1.0 | 0.050 |
| Step (counts/day) | 4156 ± 2460 | 6596 ± 3325 | 0.005 | 5282 ± 2720 | 5855 ± 2031 | 0.439 |
| Stationary time (Hrs/day) | 10.8 ± 2.1 | 10.1 ± 2.1 | 0.120 | 10.5 ± 2.3 | 10.5 ± 1.8 | 0.906 |
| Mild Activity (Hrs/day) | 2.9 ± 1.2 | 3.1 ± 0.9 | 0.496 | 3.6 ± 1 | 3.6 ± 1.4 | 0.889 |
| MVPA (Hrs/day) | 1.1 ± 1 | 1.6 ± 1.4 | 0.085 | 1.5 ± 1.1 | 1.3 ± 0.7 | 0.463 |
| Weekly Number of bouts ≥10 min at MVPA | 14.8 ± 14.6 | 21.0 ± 29.2 | 0.286 | 20.4 ± 16.5 | 11.3 ± 8.6 | 0.110 |
| Duration of MVPA bout | 13.0 ± 4.1 | 16.9 ± 13.4 | 0.402 | 15.1 ± 3.9 | 18.4 ± 10.6 | 0.100 |
| DEE (kcal/day) | 2184 ± 470 | 2227 ± 446 | 0.531 | 2686 ± 768 | 2546 ± 714 | 0.316 |
| All PAEE > 3 METs (kcal/day) | 322 ± 252 | 445 ± 261 | 0.073 | 557 ± 474 | 477 ± 285 | 0.506 |
| Intervention (n = 14) Mean ± SD | Control (n = 10) Mean ± SD | p-Value for Group Effect * | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HOOS Scores | Baseline | Follow-Up | p-Value | Baseline | Follow-Up | p-Value | Baseline | Follow-Up | Mean Change |
| Pain | 74.2 ± 20.2 | 91.7 ± 8.6 | 0.005 | 80.6 ± 18 | 83.6 ± 14.5 | 0.387 | 0.436 | 0.141 | 0.029 |
| Symptom | 63.9 ± 14.3 | 82.9 ± 9.7 | <0.001 | 78.5 ± 18.4 | 84.5 ± 10.9 | 0.250 | 0.040 | 0.305 | 0.037 |
| ADL | 73.1 ± 16.3 | 89.1 ± 7.7 | 0.003 | 74.3 ± 18.5 | 83.1 ± 13.1 | 0.022 | 0.873 | 0.172 | 0.207 |
| Sport | 42.0 ± 26.1 | 79.9 ± 16.7 | <0.001 | 43.8 ± 29.2 | 57.5 ± 26.0 | 0.312 | 0.876 | 0.017 | 0.117 |
| Hip QoL | 42.9 ± 19.0 | 63.4 ± 17.7 | 0.007 | 53.8 ± 22.5 | 61.3 ± 18.6 | 0.161 | 0.212 | 0.777 | 0.122 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negm, A.M.; Yavarai, M.; Jhangri, G.S.; Haennel, R.; Jones, C.A. Augmented Rehabilitation Program for Patients 60 Years and Younger Following Total Hip Arthroplasty—Feasibility Study. Healthcare 2022, 10, 1274. https://doi.org/10.3390/healthcare10071274
Negm AM, Yavarai M, Jhangri GS, Haennel R, Jones CA. Augmented Rehabilitation Program for Patients 60 Years and Younger Following Total Hip Arthroplasty—Feasibility Study. Healthcare. 2022; 10(7):1274. https://doi.org/10.3390/healthcare10071274
Chicago/Turabian StyleNegm, Ahmed M., Milad Yavarai, Gian S. Jhangri, Robert Haennel, and C. Allyson Jones. 2022. "Augmented Rehabilitation Program for Patients 60 Years and Younger Following Total Hip Arthroplasty—Feasibility Study" Healthcare 10, no. 7: 1274. https://doi.org/10.3390/healthcare10071274






