Should We Use Hyperbaric Oxygen for Carbon Monoxide Poisoning Management? A Network Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Methods
2.1. Eligibility Criteria and Evidence Selection
2.2. Data Extraction and Quality Assessment
2.3. Data Synthesis and Analysis
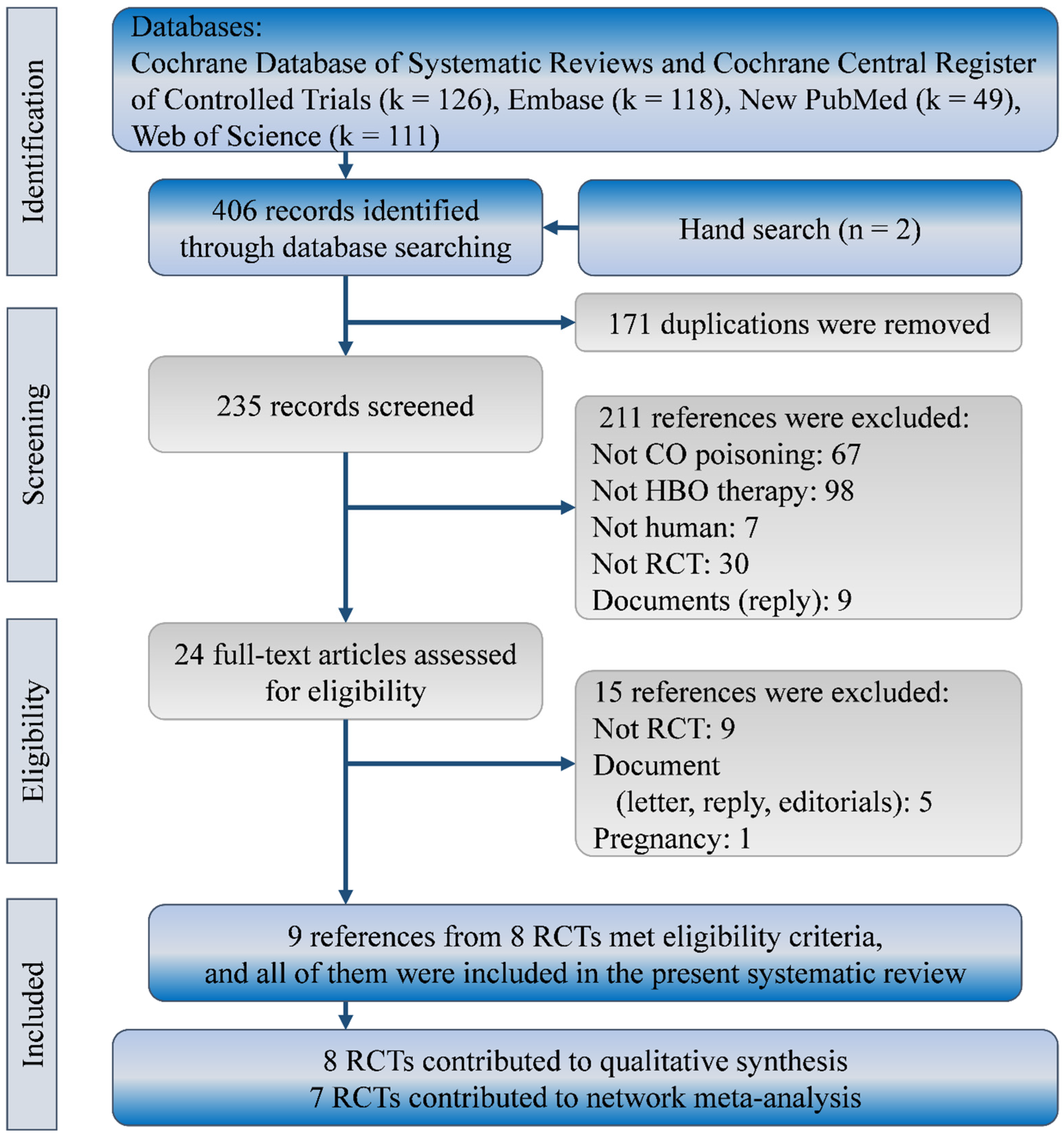
3. Results
3.1. Characteristics and Quality of Included Studies
3.2. Mortality
3.3. Headache Recovery and General Fatigue
3.4. Memory and Concentration Impairments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Satetment
Conflicts of Interest
Abbreviations
| CI | confidence intervals |
| CO | carbon monoxide |
| HBO | hyperbaric oxygen |
| NBO | normobaric oxygen |
| RCT | randomized clinical trial |
| RR | risk ratio |
| SUCRA | surface under the cumulative ranking |
References
- Centers for Disease Control and Prevention. Carbon Monoxide (CO) Poisoning Prevention. 2020. Available online: https://www.cdc.gov/nceh/features/copoisoning/index.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Ffeatures%2Fcopoisoning%2Findex.html (accessed on 4 December 2021).
- Thom, S.R.; Taber, R.L.; Mendiguren, I.I.; Clark, J.M.; Hardy, K.R.; Fisher, A.B. Delayed neuropsychologic sequelae after carbon monoxide poisoning: Prevention by treatment with hyperbaric oxygen. Ann. Emerg. Med. 1995, 25, 474–480. [Google Scholar] [CrossRef]
- Kreshak, A.A.; Lawrence, S.M.; Ontiveros, S.T.; Castellano, T.; VanHoesen, K.B. Perinatal Carbon Monoxide Poisoning: Treatment of a 2-Hour-Old Neonate with Hyperbaric Oxygen. AJP Rep. 2022, 12, e113–e116. [Google Scholar] [CrossRef] [PubMed]
- Arslan, A. Hyperbaric oxygen therapy in carbon monoxide poisoning in pregnancy: Maternal and fetal outcome. Am. J. Emerg. Med. 2021, 43, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Bağci, Z.; Arslan, A.; Neşelioğlu, S. Pediatric Carbon Monoxide Poisoning: Effects of Hyperbaric Oxygen Therapy on Thiol/Disulfide Balance. Pediatr. Emerg. Care 2022, 38, 104–107. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Clinical Guidance for Carbon Monoxide (CO) Poisoning. 2020. Available online: https://www.cdc.gov/disasters/co_guidance.html (accessed on 25 June 2022).
- Hampson, N.B.; Piantadosi, C.A.; Thom, S.R.; Weaver, L.K. Practice recommendations in the diagnosis, management, and prevention of carbon monoxide poisoning. Am. J. Respir. Crit. Care Med. 2012, 186, 1095–1101. [Google Scholar] [CrossRef] [Green Version]
- Drinhaus, H.; Nüsgen, S.; Hinkelbein, J. Guidelines desirable for treatment of carbon monoxide poisoning. Anaesthesist 2016, 65, 301–302. [Google Scholar] [CrossRef]
- Jüttner, B.; Busch, H.J.; Callies, A.; Dormann, H.; Janisch, T.; Kaiser, G.; Körner-Göbel, H.; Kluba, K.; Kluge, S.; Leidel, B.A.; et al. S2k guideline diagnosis and treatment of carbon monoxide poisoning. Ger. Med. Sci. 2021, 19, Doc13. [Google Scholar] [CrossRef]
- Buboltz, J.B.; Robins, M. Hyperbaric Treatment of Carbon Monoxide Toxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Scheinkestel, C.D.; Bailey, M.; Myles, P.S.; Jones, K.; Cooper, D.J.; Millar, I.L.; Tuxen, D.V. Hyperbaric or normobaric oxygen for acute carbon monoxide poisoning: A randomised controlled clinical trial. Med. J. Aust. 1999, 170, 203–210. [Google Scholar] [CrossRef]
- Annane, D.; Chadda, K.; Gajdos, P.; Jars-Guincestre, M.C.; Chevret, S.; Raphael, J.C. Hyperbaric oxygen therapy for acute domestic carbon monoxide poisoning: Two randomized controlled trials. Intensive Care Med. 2011, 37, 486–492. [Google Scholar] [CrossRef]
- Annane, D.; Chevret, S.; Jars-Guincestre, C.; Chillet, P.; Elkharrat, D.; Gajdos, P.; Raphael, C. Prognostic factors in unintentional mild carbon monoxide poisoning. Intensive Care Med. 2001, 27, 1776–1781. [Google Scholar] [CrossRef]
- Hampson, N.B.; Dunford, R.G.; Ross, D.E.; Wreford-Brown, C.E. A prospective, randomized clinical trial comparing two hyperbaric treatment protocols for carbon monoxide poisoning. Undersea Hyperb. Med. 2006, 33, 27–32. [Google Scholar]
- Weaver, L.K.; Hopkins, R.O.; Chan, K.J.; Churchill, S.; Gregory Elliott, C.; Clemmer, T.P.; Orme, J.F., Jr.; Thomas, F.O.; Morris, A.H. Hyperbaric oxygen for acute carbon monoxide poisoning. N. Engl. J. Med. 2002, 347, 1057–1067. [Google Scholar] [CrossRef]
- Wang, W.; Cheng, J.; Zhang, J.; Wang, K. Effect of Hyperbaric Oxygen on Neurologic Sequelae and All-Cause Mortality in Patients with Carbon Monoxide Poisoning: A Meta-Analysis of Randomized Controlled Trials. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 7684–7693. [Google Scholar] [CrossRef]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Shim, S.R.; Kim, S.J.; Lee, J.; Rücker, G. Network meta-analysis: Application and practice using R software. Epidemiol. Health 2019, 41, e2019013. [Google Scholar] [CrossRef] [Green Version]
- Sadeghirad, B.; Brignardello-Petersen, R.; Johnston, B.; Guyatt, G.; Beyene, J. Comparing Bayesian and Frequentist Approaches for network meta-analysis: An empirical study. Cochrane Database Syst. Rev. 2017, 9 (Suppl. S1), 18515. [Google Scholar] [CrossRef]
- Harrer, M.; Cuijpers, P.; Furukawa, T.; Ebert, D. Doing meta-analysis in R: A hands-on guide. In Network Meta-Analysis in R; 2019; Available online: https://zenodo.org/record/2551803#.Ys_LJ7q-vIV (accessed on 4 December 2021).
- Salanti, G.; Schmid, C.H. Research Synthesis Methods special issue on network meta-analysis: Introduction from the editors. Res. Synth. Methods 2012, 3, 69–70. [Google Scholar] [CrossRef]
- Gordon, G.; Drummond, R.; Maureen, O.; Deborah, J. Users’ Guides to the Medical Literature: Essentials of Evidence-Based Clinical Practice; McGraw-Hill Education: New York, NY, USA, 2014. [Google Scholar]
- Rouse, B.; Chaimani, A.; Li, T. Network meta-analysis: An introduction for clinicians. Intern. Emerg. Med. 2017, 12, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Birmingham, C.M.; Hoffman, R.S. Hyperbaric oxygen therapy for acute domestic carbon monoxide poisoning: Two randomized controlled trials. Intensive Care Med. 2011, 37, 1219. [Google Scholar] [CrossRef] [Green Version]
- Ducasse, J.L.; Celsis, P.; Marc-Vergnes, J.P. Non-comatose patients with acute carbon monoxide poisoning: Hyperbaric or normobaric oxygenation? Undersea Hyperb. Med. 1995, 22, 9–15. [Google Scholar]
- Raphael, J.C.; Elkharrat, D.; Jars-Guincestre, M.C.; Chastang, C.; Chasles, V.; Vercken, J.B.; Gajdos, P. Trial of normobaric and hyperbaric oxygen for acute carbon monoxide intoxication. Lancet 1989, 2, 414–419. [Google Scholar] [CrossRef]
- Huang, C.C.; Ho, C.H.; Chen, Y.C.; Lin, H.J.; Hsu, C.C.; Wang, J.J.; Su, S.B.; Guo, H.R. Hyperbaric Oxygen Therapy Is Associated with Lower Short- and Long-Term Mortality in Patients with Carbon Monoxide Poisoning. Chest 2017, 152, 943–953. [Google Scholar] [CrossRef]
- Fujita, M.; Todani, M.; Kaneda, K.; Suzuki, S.; Wakai, S.; Kikuta, S.; Sasaki, S.; Hattori, N.; Yagishita, K.; Kuwata, K.; et al. Use of hyperbaric oxygen therapy for preventing delayed neurological sequelae in patients with carbon monoxide poisoning: A multicenter, prospective, observational study in Japan. PLoS ONE 2021, 16, e0253602. [Google Scholar] [CrossRef]
- Han, S.; Nah, S.; Choi, S.; Kim, G.W.; Lee, Y.H. Optimal sessions of hyperbaric oxygen therapy in patients with carbon monoxide poisoning: A prospective observational study. Am. J. Emerg. Med. 2021, 44, 132–136. [Google Scholar] [CrossRef]
- Weaver, L.K. Clinical practice. Carbon monoxide poisoning. N. Engl. J. Med. 2009, 360, 1217–1225. [Google Scholar] [CrossRef] [Green Version]
- Fineschi, V.; Agricola, E.; Baroldi, G.; Bruni, G.; Cerretani, D.; Mondillo, S.; Parolini, M.; Turillazzi, E. Myocardial findings in fatal carbon monoxide poisoning: A human and experimental morphometric study. Int. J. Leg. Med. 2000, 113, 276–282. [Google Scholar] [CrossRef]
- Kavakli, H.S.; Erel, O.; Delice, O.; Gormez, G.; Isikoglu, S.; Tanriverdi, F. Oxidative stress increases in carbon monoxide poisoning patients. Hum. Exp. Toxicol. 2011, 30, 160–164. [Google Scholar] [CrossRef]
- Cha, Y.S.; Chang, J.S.; Kim, H.; Park, K.S. Application of Mitochondrial and Oxidative Stress Biomarkers in the Evaluation of Neurocognitive Prognosis Following Acute Carbon Monoxide Poisoning. Metabolites 2022, 12, 201. [Google Scholar] [CrossRef]
- Teksam, O.; Sabuncuoğlu, S.; Girgin, G.; Özgüneş, H. Evaluation of oxidative stress and antioxidant parameters in children with carbon monoxide poisoning. Hum. Exp. Toxicol. 2019, 38, 1235–1243. [Google Scholar] [CrossRef]
- Kim, S.J.; Thom, S.R.; Kim, H.; Hwang, S.O.; Lee, Y.; Park, E.J.; Lee, S.J.; Cha, Y.S. Effects of Adjunctive Therapeutic Hypothermia Combined with Hyperbaric Oxygen Therapy in Acute Severe Carbon Monoxide Poisoning. Crit. Care Med. 2020, 48, e706–e714. [Google Scholar] [CrossRef]
- Lee, Y.; Cha, Y.S.; Kim, S.H.; Kim, H. Effect of Hyperbaric Oxygen Therapy Initiation Time in Acute Carbon Monoxide Poisoning. Crit. Care Med. 2021, 49, e910–e919. [Google Scholar] [CrossRef] [PubMed]
- Hampson, N.B.; Hampson, L.A. Characteristics of headache associated with acute carbon monoxide poisoning. Headache 2002, 42, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, Y.H.; Lv, H.Y.; Chen, L.T. Effect of hyperbaric oxygen on lipid peroxidation and visual development in neonatal rats with hypoxia-ischemia brain damage. Biomed. Rep. 2016, 5, 136–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atalay, H.; Koseoglu, M.; Avci, A.; Erbay, H.; Canbolat, O. Does hyperbaric oxygen therapy reduce lipid peroxidation in experimentally carbon monoxide (CO) poisoned rat brain? Eur. J. Anaesthesiol. 2001, 18, 48. [Google Scholar] [CrossRef]
- Ciarlone, G.E.; Hinojo, C.M.; Stavitzski, N.M.; Dean, J.B. CNS function and dysfunction during exposure to hyperbaric oxygen in operational and clinical settings. Redox Biol. 2019, 27, 101159. [Google Scholar] [CrossRef]
- Bitterman, N. CNS oxygen toxicity. Undersea Hyperb. Med. 2004, 31, 63–72. [Google Scholar]
- Stone, J.; Gurunathan, U.; Glass, K.; Munn, Z.; Tugwell, P.; Doi, S.A.R. Stratification by quality induced selection bias in a meta-analysis of clinical trials. J. Clin. Epidemiol. 2019, 107, 51–59. [Google Scholar] [CrossRef] [Green Version]


| Inclusion | Sex | CO Exposure | CO | Coma | ||||
|---|---|---|---|---|---|---|---|---|
| Author | Location | Years | Group | (M/F) | Age | Time | Level | (n/N) |
| Annane | France | NR | NBO | ALL: | ALL: | ALL: | ALL: | ALL: |
| 2001 | (4 years) | 1-HBO | 149/158 | 49.7 | 5.9 h | 22% | Unclear | |
| Annane | France | 1989 to | NBO | 39/47 | 34 | 4 h | 22% | 3/86 |
| 2011 | 2000 | 1-HBO | 80/114 | 35.1 | 4.4 h | 22% | 93/194 | |
| 2-HBO | 44/61 | 37 | 3 h | 26% | 104/105 | |||
| Ducasse | France | NR | NBO | 8/5 | 31.6 | <12 h | 24% | 9/13 |
| 1995 | 1-HBO | 7/6 | 28.3 | <12 h | 23% | 8/13 | ||
| Hampson | USA | 1995 to | 1-HBO | 10/8 | 47.2 | 2 h | 22% | Unclear |
| 2006 | 2002 | 2-HBO | 4/8 | 43.1 | 2 h | 24% | ||
| Raphael | France | NR | NBO | 91/79 | 35.6 | 6.2 h | 22% | 0/170 |
| 1989 | 1-HBO | 143/175 | 36.4 | 7.1 h | 23% | 43/318 | ||
| 2-HBO | 56/85 | 37 | 5.3 h | 25% | 39/141 | |||
| Scheinkestel | Australia | 1993 to | NBO | 67/20 | 34.8 | 2.5 h | 22% | 49/87 |
| 1999 | 1995 | 1-HBO | 89/15 | 37.8 | 2.6 h | 21% | 53/104 | |
| Thom | USA | 1989 to | NBO | 18/14 | 39 | NR | 20% | Unclear |
| 1995 | 1993 | 1-HBO | 16/17 | 35 | 25% | |||
| Weaver | USA | 1992 to | NBO | 54/22 | 36 | NR | 25% | 38/76 |
| 2002 | 1999 | 3-HBO | 54/22 | 35 | 25% | 37/76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, Y.-W.; Chung, P.-Y.; Hou, S.-K.; Chang, M.-L.; Kang, Y.-N. Should We Use Hyperbaric Oxygen for Carbon Monoxide Poisoning Management? A Network Meta-Analysis of Randomized Controlled Trials. Healthcare 2022, 10, 1311. https://doi.org/10.3390/healthcare10071311
Ho Y-W, Chung P-Y, Hou S-K, Chang M-L, Kang Y-N. Should We Use Hyperbaric Oxygen for Carbon Monoxide Poisoning Management? A Network Meta-Analysis of Randomized Controlled Trials. Healthcare. 2022; 10(7):1311. https://doi.org/10.3390/healthcare10071311
Chicago/Turabian StyleHo, Yu-Wan, Ping-Yen Chung, Sen-Kuang Hou, Ming-Long Chang, and Yi-No Kang. 2022. "Should We Use Hyperbaric Oxygen for Carbon Monoxide Poisoning Management? A Network Meta-Analysis of Randomized Controlled Trials" Healthcare 10, no. 7: 1311. https://doi.org/10.3390/healthcare10071311






