Sleep Should Be Focused on When Analyzing Physical Activity in Hospitalized Older Adults after Trunk and Lower Extremity Fractures—A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Data Collection
2.3. Physical Activity
2.4. Physical Function
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheung, C.L.; Ang, S.B.; Chadha, M.; Chow, E.S.; Chung, Y.S.; Hew, F.L.; Jaisamrarn, U.; Ng, H.; Takeuchi, Y.; Wu, C.H.; et al. An updated hip fracture projection in Asia: The Asian Federation of Osteoporosis Societies study. Osteoporos. Sarcopenia 2018, 4, 16–21. [Google Scholar] [CrossRef]
- Tsuda, T. Epidemiology of fragility fractures and fall prevention in the elderly: A systematic review of the literature. Curr. Orthop. Pract. 2017, 28, 580–585. [Google Scholar] [CrossRef]
- Covinsky, K.E.; Pierluissi, E.; Johnston, C.B. Hospitalization-associated disability: "She was probably able to ambulate, but I’m not sure. JAMA 2011, 306, 1782–1793. [Google Scholar] [CrossRef]
- Ekegren, C.L.; Climie, R.E.; Veitch, W.G.; Owen, N.; Dunstan, D.W.; Kimmel, L.A.; Gabbe, B.J. Sedentary behaviour and physical activity patterns in adults with traumatic limb fracture. AIMS Med. Sci. 2019, 6, 1–12. [Google Scholar] [CrossRef]
- Ekegren, C.L.; Climie, R.E.; Simpson, P.M.; Owen, N.; Dunstan, D.W.; Veitch, W.; Gabbe, B.J. Physical Activity and Sedentary Behavior 6 Months After Musculoskeletal Trauma: What Factors Predict Recovery? Phys Ther. 2020, 100, 332–345. [Google Scholar] [CrossRef]
- Abe, T.; Shibao, Y.; Takeuchi, Y.; Mataki, Y.; Amano, K.; Hioki, S.; Miura, K.; Noguchi, H.; Funayama, T.; Koda, M.; et al. Initial hospitalization with rigorous bed rest followed by bracing and rehabilitation as an option of conservative treatment for osteoporotic vertebral fractures in elderly patients: A pilot one arm safety and feasibility study. Arch. Osteoporos. 2018, 13, 134. [Google Scholar] [CrossRef] [Green Version]
- Ekegren, C.L.; Edwards, E.R.; Kimmel, L.; Gabbe, B.J. Do levels of sedentary behaviour and physical activity differ according to weight-bearing status after lower limb fracture? A prospective cohort study. Orthop. Trauma Rehabil. 2021, 28, 22104917211020436. [Google Scholar] [CrossRef]
- Wu, Y.; Smits, E.J.; Window, P.; Beningfield, A.; Johnston, V.; McRae, P. Mobility levels of acute medical patients: Is behavioural mapping comparable to accelerometry? Clin. Rehabil. 2021, 35, 595–605. [Google Scholar] [CrossRef]
- Tremblay, M.S.; Aubert, S.; Barnes, J.D.; Saunders, T.J.; Carson, V.; Latimer-Cheung, A.E.; Chastin, S.; Altenburg, T.M.; Chinapaw, M.; SBRN Terminology Consensus Project Participants. Sedentary Behavior Research Network (SBRN)—Terminology Consensus Project process and outcome. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodilsen, A.C.; Pedersen, M.M.; Petersen, J.; Beyer, N.; Andersen, O.; Smith, L.L.; Kehlet, H.; Bandholm, T. Acute hospitalization of the older patient: Changes in muscle strength and functional performance during hospitalization and 30 days after discharge. Am. J. Phys. Med. Rehabil. 2013, 92, 789–796. [Google Scholar] [CrossRef]
- Brown, C.J.; Redden, D.T.; Flood, K.L.; Allman, R.M. The underrecognized epidemic of low mobility during hospitalization of older adults. J. Am. Geriatr. Soc. 2009, 57, 1660–1665. [Google Scholar] [CrossRef] [PubMed]
- van Delft, L.; Bor, P.; Valkenet, K.; Slooter, A.; Veenhof, C. The Effectiveness of Hospital in Motion, a Multidimensional Implementation Project to Improve Patients’ Movement Behavior During Hospitalization. Phys. Ther. 2020, 100, 2090–2098. [Google Scholar] [CrossRef] [PubMed]
- Kaneya, S.; Hashidate, H. Single question about total lying time for assessing physical inactivity in community-dwelling older adults: A study of reliability and discriminant validity from sleeping time. J. Phys. Ther. Sci. 2020, 32, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Ikezoe, T.; Asakawa, Y.; Shima, H.; Kishibuchi, K.; Ichihashi, N. Daytime physical activity patterns and physical fitness in institutionalized elderly women: An exploratory study. Arch. Gerontol. Geriatr. 2013, 57, 221–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, M.J.; Blankenship, J.M.; Kline, P.W.; Melanson, E.L.; Christiansen, C.L. Patterns of Sitting, Standing, and Stepping After Lower Limb Amputation. Phys Ther. 2021, 101, pzaa212. [Google Scholar] [CrossRef]
- Diaz, K.M.; Howard, V.J.; Hutto, B.; Colabianchi, N.; Vena, J.E.; Safford, M.M.; Blair, S.N.; Hooker, S.P. Patterns of Sedentary Behavior and Mortality in U.S. Middle-Aged and Older Adults: A National Cohort Study. Ann. Intern. Med. 2017, 167, 465–475. [Google Scholar] [CrossRef]
- Wilson, J.J.; McMullan, I.; Blackburn, N.E.; Skjødt, M.; Caserotti, P.; Giné-Garriga, M.; Farche, A.; Klenk, J.; Dallmeier, D.; Deidda, M.; et al. Associations of sedentary behavior bouts with community-dwelling older adults’ physical function. Scand. J. Med. Sci. Sports. 2021, 31, 153–162. [Google Scholar] [CrossRef]
- Santos, D.A.; Silva, A.M.; Baptista, F.; Santos, R.; Vale, S.; Mota, J.; Sardinha, L.B. Sedentary behavior and physical activity are independently related to functional fitness in older adults. Exp. Gerontol. 2012, 47, 908–912. [Google Scholar] [CrossRef]
- Kato, S. Development of the revised version of Hasegawa’s Dementia Scale (HDS-R). Jpn. Geriatr. Psychiatr. Med. 1991, 2, 1339–1347. [Google Scholar]
- Trost, S.G.; McIver, K.L.; Pate, R.R. Conducting accelerometer-based activity assessments in field-based research. Med. Sci. Sports Exerc. 2005, 37, S531–S543. [Google Scholar] [CrossRef]
- Shimizu, N.; Hashidate, H.; Ota, T.; Yatsunami, M. Daytime physical activity at admission is associated with improvement of gait independence 1 month later in people with subacute stroke: A longitudinal study. Top. Stroke Rehabil. 2020, 27, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Ohkawara, K.; Oshima, Y.; Hikihara, Y.; Ishikawa-Takata, K.; Tabata, I.; Tanaka, S. Real-time estimation of daily physical activity intensity by a triaxial accelerometer and a gravity-removal classification algorithm. Br. J. Nutr. 2011, 105, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Nagayoshi, S.; Oshima, Y.; Ando, T.; Aoyama, T.; Nakae, S.; Usui, C.; Kumagai, S.; Tanaka, S. Validity of estimating physical activity intensity using a triaxial accelerometer in healthy adults and older adults. BMJ Open Sport Exerc. Med. 2019, 5, e000592. [Google Scholar] [CrossRef]
- Kocherginsky, M.; Huisingh-Scheetz, M.; Dale, W.; Lauderdale, D.S.; Waite, L. Measuring Physical Activity with Hip Accelerometry among U.S. Older Adults: How Many Days Are Enough? PLoS ONE 2017, 12, e0170082. [Google Scholar] [CrossRef] [Green Version]
- Gomersall, S.; Maher, C.; English, C.; Rowlands, A.; Olds, T. Time regained: When people stop a physical activity program, how does their time use change? A randomised controlled trial. PLoS ONE 2015, 10, e0126665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berg, K.; Wood-Dauphine, S.; Williams, J.I.; Gayton, D. Measuring balance in the elderly: Preliminary development of an instrument. Physiother. Can. 1989, 41, 304–311. [Google Scholar] [CrossRef]
- Berg, K.; Wood-Dauphine, S.; Williams, J.I. The Balance Scale: Reliability assessment with elderly residents and patients with an acute stroke. Scand. J. Rehabil. Med. 1995, 27, 27–36. [Google Scholar]
- Berg, K.; Wood-Dauphine, S.; Williams, J.I.; Maki, B. Measuring balance in the elderly: Validation of an instrument. Can. J. Public Health 1992, 83, S7–S11. [Google Scholar]
- Podsiadlo, D.; Richardson, S. The timed "Up & Go": A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef]
- Chan, Y.H. Biostatistics 104: Correlational analysis. Singap. Med. J. 2003, 44, 614–619. [Google Scholar]
- Baldwin, C.E.; Phillips, A.C.; Edney, S.M.; Lewis, L.K. Recommendations for older adults’ physical activity and sedentary behaviour during hospitalisation for an acute medical illness: An international Delphi study. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- Diaz, K.M.; Duran, A.T.; Colabianchi, N.; Judd, S.E.; Howard, V.J.; Hooker, S.P. Potential Effects on Mortality of Replacing Sedentary Time with Short Sedentary Bouts or Physical Activity: A National Cohort Study. Am. J. Epidemiol. 2019, 188, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Powell, C.; Browne, L.D.; Carson, B.P.; Dowd, K.P.; Perry, I.J.; Kearney, P.M.; Harrington, J.M.; Donnelly, A.E. Use of Compositional Data Analysis to Show Estimated Changes in Cardiometabolic Health by Reallocating Time to Light-Intensity Physical Activity in Older Adults. Sports Med. 2020, 50, 205–217. [Google Scholar] [CrossRef]
- Zusman, E.Z.; Dawes, M.; Fleig, L.; McAllister, M.M.; Cook, W.L.; Guy, P.; Brasher, P.; McKay, H.A.; Khan, K.M.; Ashe, M.C. Older Adults’ Sedentary Behavior and Physical Activity after Hip Fracture: Results from an Outpatient Rehabilitation Randomized Controlled Trial. J. Geriatr. Phys. Ther. 2019, 42, E32–E38. [Google Scholar] [CrossRef] [PubMed]
- Zusman, E.Z.; Dawes, M.G.; Edwards, N.; Ashe, M.C. A systematic review of evidence for older adults’ sedentary behavior and physical activity after hip fracture. Clin. Rehabil. 2018, 32, 679–691. [Google Scholar] [CrossRef] [PubMed]
| Variable | All Participants (n = 25) | |
|---|---|---|
| Basic information | ||
| Age, years | 79.4 ± 7.5 (80, 73–85) | |
| Sex, male/female | 4/21 | |
| Body mass index, kg/m2 | 21.7 ± 3.3 (21.3, 20.4–23.1) | |
| Type of fracture, pelvis fracture/vertebral compression fracture/hip fracture/distal femoral fracture/tibial plateau fracture | 3/14/7/1 | |
| Discharge parameters | ||
| Destination, home/facility | 25/0 | |
| Length of hospital stay, days | 52.8 ± 18.9 (49, 44–63) | |
| Functional Independence Measure | Motor score | 82.3 ± 7.6 (85, 77–89) |
| Cognitive score | 33.3 ± 2.5 (34, 32–35) | |
| Total score | 115.6 ± 9.3 (120, 109–124) | |
| Physical function at gait independence | ||
| Berg balance scale | 50 ± 5.2 (51, 46–55) | |
| Timed Up and Go test, s | 14.6 ± 6.8 (12.9, 9–17.5) | |
| Physical activity | ||
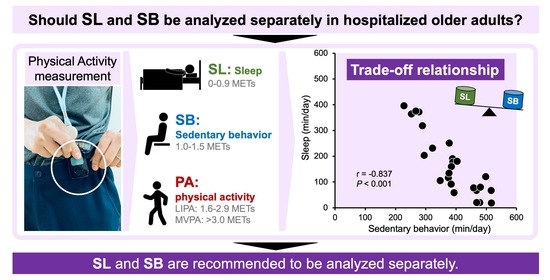
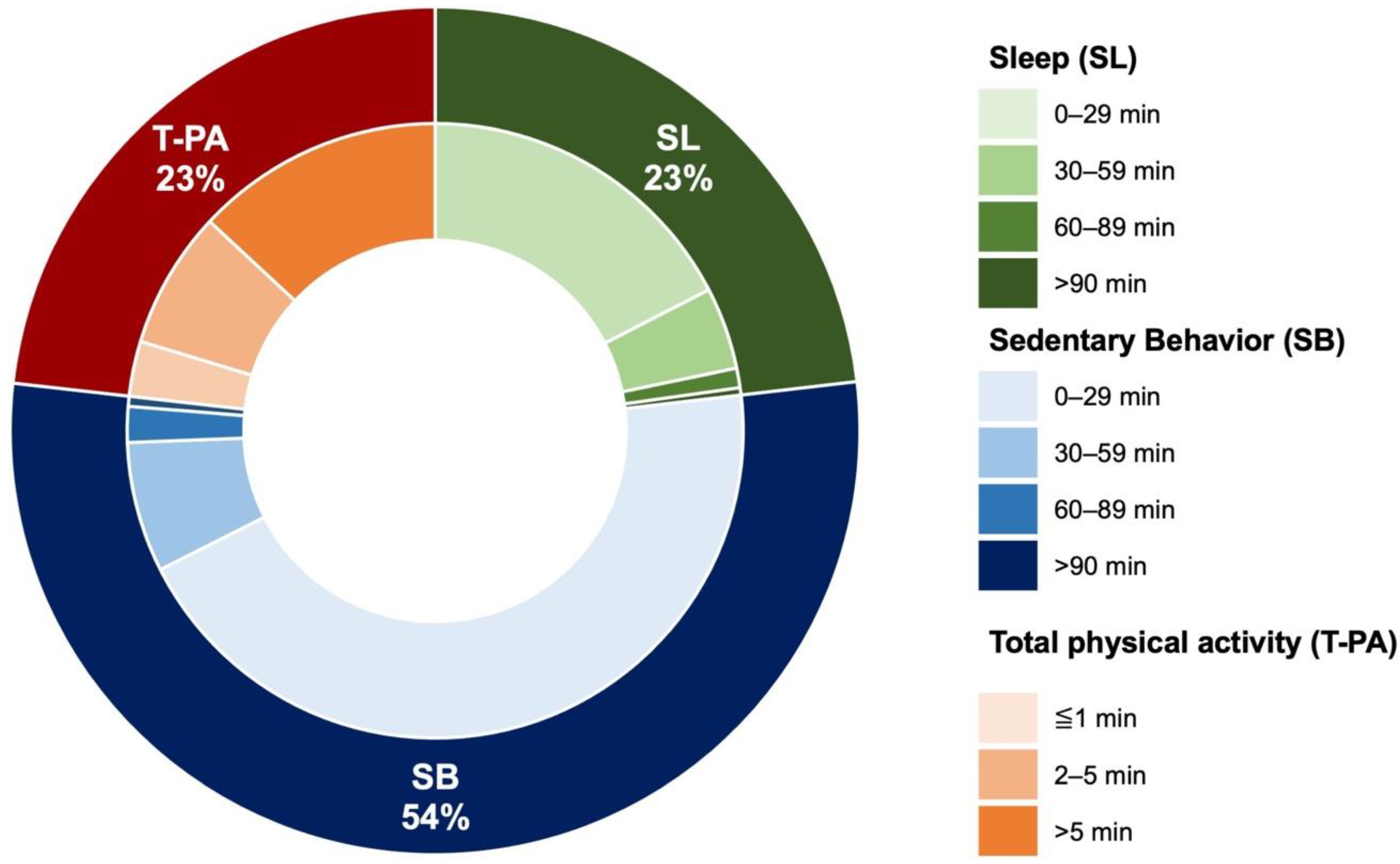
| SL (0–0.9 METs), min/day | 167.5 ± 119.2 (134, 72–241) | |
| SB (1–1.5 METs), min/day | 385.8 ± 85.5 (385, 309–468) | |
| LIPA (1.6–2.9 METs), min/day | 163.9 ± 56.7 (163, 113–216) | |
| MVPA (>3.0 METs), min/day | 3.8 ± 3 (3, 2–5) | |
| SL | SB | LIPA | MVPA | |||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| Age | −0.081 | 0.699 | 0.106 | 0.615 | −0.017 | 0.935 | 0.24 | 0.91 |
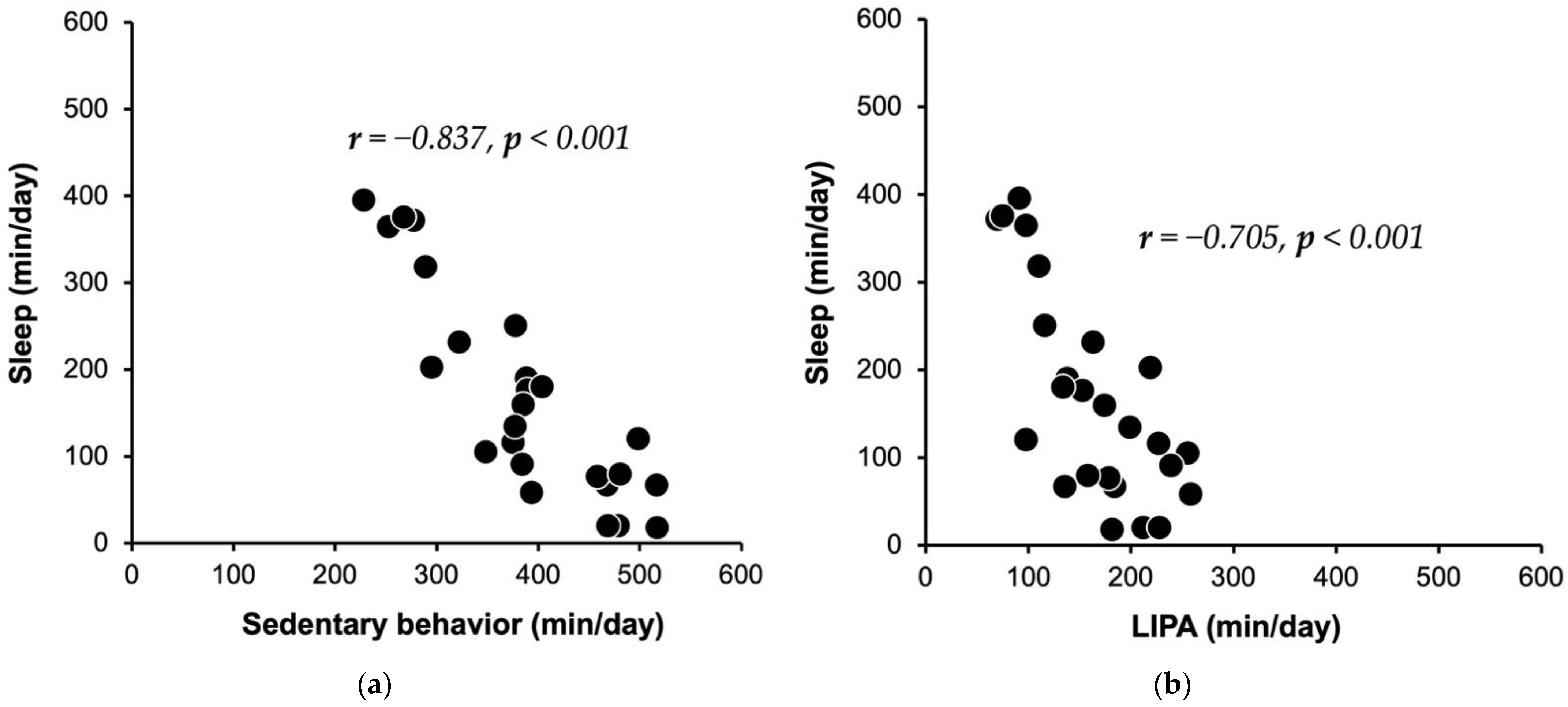
| SL | −0.837 | <0.001 | −0.705 | <0.001 | −0.183 | 0.381 | ||
| SB | 0.346 | 0.091 | −0.031 | 0.885 | ||||
| LIPA | 0.429 | 0.032 | ||||||
| MVPA | ||||||||
| Berg Balance Scale | Timed up and Go Test | |||
|---|---|---|---|---|
| r | p | r | p | |
| SL | 0.354 | 0.083 | −0.283 | 0.17 |
| SB | −0.432 | 0.031 | 0.364 | 0.074 |
| LIPA | −0.098 | 0.641 | 0.152 | 0.467 |
| MVPA | 0.036 | 0.864 | −0.109 | 0.605 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaizu, Y.; Kasuga, T.; Takahashi, Y.; Otani, T.; Miyata, K. Sleep Should Be Focused on When Analyzing Physical Activity in Hospitalized Older Adults after Trunk and Lower Extremity Fractures—A Pilot Study. Healthcare 2022, 10, 1429. https://doi.org/10.3390/healthcare10081429
Kaizu Y, Kasuga T, Takahashi Y, Otani T, Miyata K. Sleep Should Be Focused on When Analyzing Physical Activity in Hospitalized Older Adults after Trunk and Lower Extremity Fractures—A Pilot Study. Healthcare. 2022; 10(8):1429. https://doi.org/10.3390/healthcare10081429
Chicago/Turabian StyleKaizu, Yoichi, Takeaki Kasuga, Yu Takahashi, Tomohiro Otani, and Kazuhiro Miyata. 2022. "Sleep Should Be Focused on When Analyzing Physical Activity in Hospitalized Older Adults after Trunk and Lower Extremity Fractures—A Pilot Study" Healthcare 10, no. 8: 1429. https://doi.org/10.3390/healthcare10081429
APA StyleKaizu, Y., Kasuga, T., Takahashi, Y., Otani, T., & Miyata, K. (2022). Sleep Should Be Focused on When Analyzing Physical Activity in Hospitalized Older Adults after Trunk and Lower Extremity Fractures—A Pilot Study. Healthcare, 10(8), 1429. https://doi.org/10.3390/healthcare10081429