The Impact of Postoperative Complications on Survival after Simultaneous Resection of Colorectal Cancer and Liver Metastases
Abstract
:1. Introduction
2. Materials and Methods
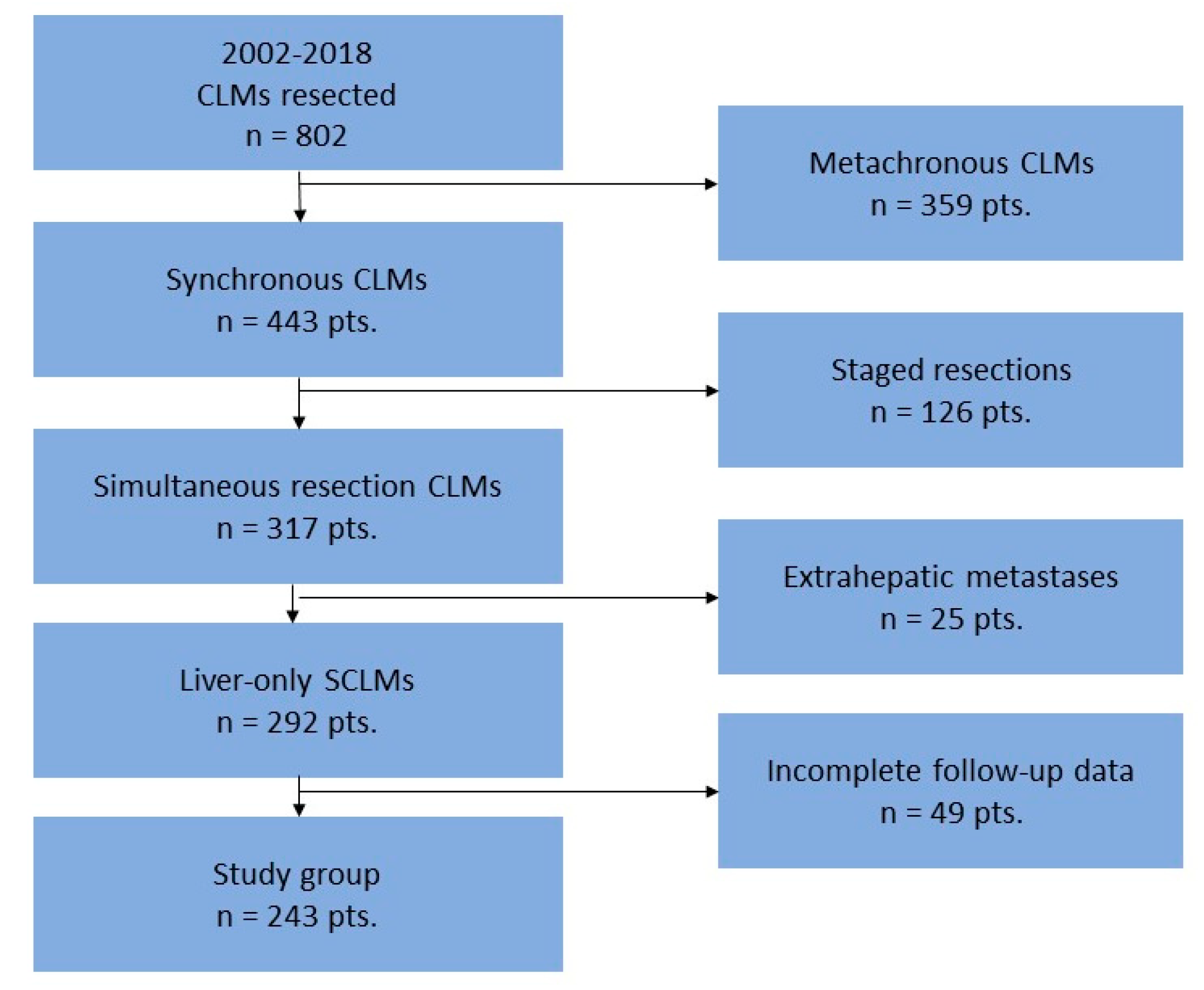
2.1. Study Design and Population
2.2. Treatment Allocation
2.3. Data
2.4. Postoperative Complications
2.5. Outcome Measurements
2.6. Statistical Analysis
3. Results
3.1. Study Cohort
3.2. Description of the Surgery
3.3. Short-Term Outcomes
3.4. Long-Term Outcomes
3.5. Univariate Analysis
3.6. Multivariate Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Alexandrescu, S.T.; Anastase, D.T.; Grigorie, R.T.; Zlate, C.A.; Andrei, S.; Costea, R.; Gramaticu, I.M.; Croitoru, A.E.; Popescu, I. Influence of the Primary Tumor Location on the Pattern of Synchronous Metastatic Spread in Patients with Stage IV Colorectal Carcinoma, according to the 8 th Edition of the AJCC Staging System. J. Gastrointest. Liver Dis. 2020, 29, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Raghav, P.K.; Kumar, R.; Kumar, V.; Raghava, G.P.S. Docking-based approach for identification of mutations that disrupt binding between Bcl-2 and Bax proteins: Inducing apoptosis in cancer cells. Mol. Genet. Genom. Med. 2019, 7, e910. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Mao, M.; Xu, G.; Lin, F.; Sun, P.; Baklaushev, V.P.; Chekhonin, V.P.; Peltzer, K.; Zhang, J.; Zhang, C. The incidence, associated factors, and predictive nomogram for early death in stage IV colorectal cancer. Int. J. Color. Dis. 2019, 34, 1189–1201. [Google Scholar] [CrossRef]
- Nordlinger, B.; Guiguet, M.; Vaillant, J.C.; Balladur, P.; Boudjema, K.; Bachellier, P.; Jaeck, D. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Française de Chirurgie. Cancer 1996, 77, 1254–1262. [Google Scholar] [CrossRef]
- Fong, Y.; Fortner, J.; Sun, R.L.; Brennan, M.F.; Blumgart, L.H. Clinical Score for Predicting Recurrence after Hepatic Resection for Metastatic Colorectal Cancer: Analysis of 1001 consecutive cases. Ann. Surg. 1999, 230, 309–318, discussion 318–321. [Google Scholar] [CrossRef]
- Iwatsuki, S.; Dvorchik, I.; Madariaga, J.R.; Marsh, J.W.; Dodson, F.; Bonham, A.C.; Geller, D.A.; Gayowski, T.J.; Fung, J.J.; Starzl, T.E. Hepatic resection for metastatic colorectal adenocarcinoma: A proposal of a prognostic scoring system. J. Am. Coll. Surg. 1999, 189, 291–299. [Google Scholar] [CrossRef]
- Weber, J.C.; Bachellier, P.; Oussoultzoglou, E.; Jaeck, D. Simultaneous resection of colorectal primary tumour and synchronous liver metastases. Br. J. Surg. 2003, 90, 956–962. [Google Scholar] [CrossRef]
- Martin, R.C., 2nd; Augenstein, V.; Reuter, N.P.; Scoggins, C.R.; McMasters, K.M. Simultaneous Versus Staged Resection for Synchronous Colorectal Cancer Liver Metastases. J. Am. Coll. Surg. 2009, 208, 842–850, discussion 50–52. [Google Scholar] [CrossRef]
- Alexandrescu, S.; Diaconescu, A.; Zarnescu, N.O.; Ionel, Z.; Zlate, C.; Hrehoret, D.; Braşoveanu, V.; Grigorie, R.; Botea, F.; Tomescu, D.; et al. Prognostic Factors for Survival after Resection of Liver Metastases from Colorectal Cancer: A Single Institution Analysis of 655 Cases. Surg. Gastroenterol. Oncol. 2017, 22, 291–302. [Google Scholar] [CrossRef]
- Boudjema, K.; Locher, C.; Sabbagh, C.; Ortega-Deballon, P.; Heyd, B.; Bachellier, P.; Métairie, S.; Paye, F.; Bourlier, P.; Adam, R.; et al. Simultaneous Versus Delayed Resection for Initially Resectable Synchronous Colorectal Cancer Liver Metastases: A Prospective, Open-label, Randomized, Controlled Trial. Ann. Surg. 2021, 273, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Mentha, G.; Majno, P.E.; Andres, A.; Rubbia-Brandt, L.; Morel, P.; Roth, A.D. Neoadjuvant chemotherapy and resection of advanced synchronous liver metastases before treatment of the colorectal primary. Br. J. Surg. 2006, 93, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Serrano, P.E.; Parpia, S.; Karanicolas, P.; Gallinger, S.; Wei, A.C.; Simunovic, M.; Bhandari, M.; Levine, M. Simultaneous resection for synchronous colorectal cancer liver metastases: A feasibility clinical trial. J. Surg. Oncol. 2022, 125, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Deng, Y.; Chen, J.; Zhao, J.; Bi, X.; Zhou, J.; Li, Z.; Huang, Z.; Zhang, Y.; Chen, X.; et al. Impact of Postoperative Infectious Complications on Long-Term Outcomes for Patients Undergoing Simultaneous Resection for Colorectal Cancer Liver Metastases: A Propensity Score Matching Analysis. Front. Oncol. 2021, 11, 793653. [Google Scholar] [CrossRef]
- Kleive, D.; Aas, E.; Angelsen, J.-H.; Bringeland, E.A.; Nesbakken, A.; Nymo, L.S.; Schultz, J.K.; Søreide, K.; Yaqub, S. Simultaneous Resection of Primary Colorectal Cancer and Synchronous Liver Metastases: Contemporary Practice, Evidence and Knowledge Gaps. Oncol. Ther. 2021, 9, 111–120. [Google Scholar] [CrossRef]
- Griffiths, C.; Bogach, J.; Simunovic, M.; Parpia, S.; Ruo, L.; Hallet, J.; Serrano, P.E. Simultaneous resection of colorectal cancer with synchronous liver metastases; a practice survey. HPB 2020, 22, 728–734. [Google Scholar] [CrossRef]
- Sasaki, K.; Morioka, D.; Conci, S.; Margonis, G.A.; Sawada, Y.; Ruzzenente, A.; Kumamoto, T.; Iacono, C.; Andreatos, N.; Guglielmi, A.; et al. The Tumor Burden Score: A New “Metro-ticket” Prognostic Tool for Colorectal Liver Metastases Based on Tumor Size and Number of Tumors. Ann. Surg. 2018, 267, 132–141. [Google Scholar] [CrossRef]
- Clavien, P.A.; Strasberg, S.M. Severity Grading of Surgical Complications. Ann. Surg. 2009, 250, 197–198. [Google Scholar] [CrossRef]
- Kim, H.-Y. Statistical notes for clinical researchers: Chi-squared test and Fisher’s exact test. Restor. Dent. Endod. 2017, 42, 152–155. [Google Scholar] [CrossRef]
- Ignatavicius, P.; Oberkofler, C.E.; Chapman, W.C.; DeMatteo, R.P.; Clary, B.M.; D’Angelica, M.I.; Tanabe, K.K.; Hong, J.C.; Aloia, T.A.; Pawlik, T.M.; et al. Choices of Therapeutic Strategies for Colorectal Liver Metastases Among Expert Liver Surgeons: A Throw of the Dice? Ann. Surg. 2020, 272, 715–722. [Google Scholar] [CrossRef]
- Tsilimigras, D.I.; Sahara, K.; Hyer, J.M.; Diaz, A.; Moris, D.; Bagante, F.; Guglielmi, A.; Ruzzenente, A.; Alexandrescu, S.; Poultsides, G.; et al. Trends and outcomes of simultaneous versus staged resection of synchronous colorectal cancer and colorectal liver metastases. Surgery 2021, 170, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Snyder, R.A.; Hao, S.; Irish, W.; Zervos, E.E.; Tuttle-Newhall, J.E.; Parikh, A.A. Thirty-Day Morbidity after Simultaneous Resection of Colorectal Cancer and Colorectal Liver Metastasis: American College of Surgeons NSQIP Analysis. J. Am. Coll. Surg. 2020, 230, 617–627e9. [Google Scholar] [CrossRef] [PubMed]
- Driedger, M.R.; Yamashita, T.S.; Starlinger, P.; Mathis, K.L.; Smoot, R.L.; Cleary, S.P.; Nagorney, D.M. Synchronous resection of colorectal cancer primary and liver metastases: An outcomes analysis. HPB 2021, 23, 1277–1284. [Google Scholar] [CrossRef]
- Dorcaratto, D.; Mazzinari, G.; Fernández-Moreno, M.-C.; Muñoz, E.; Garcés-Albir, M.; Ortega, J.; Sabater, L. Impact of Postoperative Complications on Survival and Recurrence After Resection of Colorectal Liver Metastases: Systematic Review and Meta-analysis. Ann. Surg. 2019, 270, 1018–1027. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Moreno, M.C.; Dorcaratto, D.; Garcés-Albir, M.M.; Muñoz, E.; Arvizu, R.; Ortega, J.; Sabater, L. Impact of type and severity of postoperative complications on long-term outcomes after colorectal liver metastases resection. J. Surg. Oncol. 2020, 122, 212–225. [Google Scholar] [CrossRef]
- Memeo, R.; de Blasi, V.; Adam, R.; Goéré, D.; Laurent, A.; De’Angelis, N.; Piardi, T.; Lermite, E.; Herrero, A. Postoperative Infectious Complications Impact Long-Term Survival in Patients Who Underwent Hepatectomies for Colorectal Liver Metastases: A Propensity Score Matching Analysis. J. Gastrointest. Surg. 2018, 22, 2045–2054. [Google Scholar] [CrossRef] [PubMed]
- Roxburgh, C.S.D.; Richards, C.H.; Moug, S.J.; Foulis, A.K.; McMillan, D.C.; Horgan, P.G. Determinants of short- and long-term outcome in patients undergoing simultaneous resection of colorectal cancer and synchronous colorectal liver metastases. Int. J. Color. Dis. 2012, 27, 363–369. [Google Scholar] [CrossRef]
- Nitsche, U.; Weber, C.; Kaufmann, B.; von Figura, G.; Assfalg, V.; Miller, G.; Friess, H.; Hüser, N.; Hartmann, D. Simultaneous Versus Staged Resection of Colorectal Cancer Liver Metastasis: A Retrospective Single-Center Study. J. Surg. Res. 2020, 255, 346–354. [Google Scholar] [CrossRef]
- Lodewick, T.M.; de Jong, M.C.; Van Dam, R.M.; Bemelmans, M.H.A.; Neumann, U.P.; Damink, S.W.M.O.; DeJong, C.H.C. Effects of Postoperative Morbidity on Long-Term Outcome Following Surgery for Colorectal Liver Metastases. World J. Surg. 2015, 39, 478–486. [Google Scholar] [CrossRef]
- Pang, T.C.; Spiro, C.; Ramacciotti, T.; Choi, J.; Drummond, M.; Sweeney, E.; Samra, J.S.; Hugh, T.J. Complications following liver resection for colorectal metastases do not impact on longterm outcome. HPB 2015, 17, 185–193. [Google Scholar] [CrossRef]
- Yamashita, S.; Sheth, R.A.; Niekamp, A.S.; Aloia, T.A.; Chun, Y.S.; Lee, J.E.; Vauthey, J.-N.; Conrad, C. Comprehensive Complication Index Predicts Cancer-specific Survival After Resection of Colorectal Metastases Independent of RAS Mutational Status. Ann. Surg. 2017, 266, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Dupré, A.; Malik, H.Z.; Jones, R.P.; Diaz-Nieto, R.; Fenwick, S.W.; Poston, G.J. Influence of the primary tumour location in patients undergoing surgery for colorectal liver metastases. Eur. J. Surg. Oncol. 2018, 44, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, A.; Kaido, T.; Hamaguchi, Y.; Okumura, S.; Shirai, H.; Kamo, N.; Yagi, S.; Taura, K.; Okajima, H.; Uemoto, S. Impact of Visceral Adiposity as Well as Sarcopenic Factors on Outcomes in Patients Undergoing Liver Resection for Colorectal Liver Metastases. World J. Surg. 2018, 42, 1180–1191. [Google Scholar] [CrossRef]
- Shubert, C.R.; Habermann, E.B.; Bergquist, J.R.; Thiels, C.A.; Thomsen, K.M.; Kremers, W.K.; Kendrick, M.L.; Cima, R.R.; Nagorney, D.M. A NSQIP Review of Major Morbidity and Mortality of Synchronous Liver Resection for Colorectal Metastasis Stratified by Extent of Liver Resection and Type of Colorectal Resection. J. Gastrointest. Surg. 2015, 19, 1982–1994. [Google Scholar] [CrossRef] [PubMed]
- Capussotti, L.; Ferrero, A.; Viganò, L.; Ribero, D.; Tesoriere, R.L.; Polastri, R. Major Liver Resections Synchronous with Colorectal Surgery. Ann. Surg. Oncol. 2007, 14, 195–201. [Google Scholar] [CrossRef]
- Reddy, S.K.; Pawlik, T.M.; Zorzi, D.; Gleisner, A.L.; Ribero, D.; Assumpcao, L.; Barbas, A.S.; Abdalla, E.K.; Choti, M.A.; Vauthey, J.-N.; et al. Simultaneous Resections of Colorectal Cancer and Synchronous Liver Metastases: A Multi-institutional Analysis. Ann. Surg. Oncol. 2007, 14, 3481–3491. [Google Scholar] [CrossRef]
- Torzilli, G.; McCormack, L.; Pawlik, T. Parenchyma-sparing liver resections. Int. J. Surg. 2020, 82, 192–197. [Google Scholar] [CrossRef]
- Torzilli, G.; Procopio, F.; Viganò, L.; Costa, G.; Fontana, A.P.; Cimino, M.; Donadon, M.; Del Fabbro, D. The Liver Tunnel: Intention-to-treat Validation of a New Type of Hepatectomy. Ann. Surg. 2019, 269, 331–336. [Google Scholar] [CrossRef]
- Petrelli, F.; Zaniboni, A.; Ghidini, A.; Ghidini, M.; Turati, L.; Pizzo, C.; Ratti, M.; Libertini, M.; Tomasello, G. Timing of Adjuvant Chemotherapy and Survival in Colorectal, Gastric, and Pancreatic Cancer. A Systematic Review and Meta-Analysis. Cancers 2019, 11, 550. [Google Scholar] [CrossRef]
- Ito, H.; Are, C.; Gonen, M.; D’Angelica, M.; DeMatteo, R.P.; Kemeny, N.E.; Fong, Y.; Blumgart, L.H.; Jarnagin, W.R. Effect of Postoperative Morbidity on Long-term Survival After Hepatic Resection for Metastatic Colorectal Cancer. Ann. Surg. 2008, 247, 994–1002. [Google Scholar] [CrossRef]
- Minagawa, M.; Yamamoto, J.; Miwa, S.; Sakamoto, Y.; Kokudo, N.; Kosuge, T.; Miyagawa, S.-I.; Makuuchi, M. Selection Criteria for Simultaneous Resection in Patients with Synchronous Liver Metastasis. Arch. Surg. 2006, 141, 1006–1012, discussion 13. [Google Scholar] [CrossRef] [PubMed]
- Allard, M.A.; Adam, R.; Giuliante, F.; Lapointe, R.; Hubert, C.; Ijzermans, J.N.M.; Mirza, D.; Elias, D.; Laurent, C.; Gruenberger, T.; et al. Long-term outcomes of patients with 10 or more colorectal liver metastases. Br. J. Cancer 2017, 117, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-F.; Mao, R.; Chen, X.; Zhao, J.-J.; Bi, X.-Y.; Li, Z.-Y.; Zhou, J.-G.; Zhao, H.; Huang, Z.; Sun, Y.-K.; et al. Prognostic Analysis of 102 Patients with Synchronous Colorectal Cancer and Liver Metastases Treated with Simultaneous Resection. Chin. Med. J. 2017, 130, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Brouquet, A.; Mortenson, M.M.; Vauthey, J.-N.; Rodriguez-Bigas, M.A.; Overman, M.J.; Chang, G.J.; Kopetz, S.; Garrett, C.; Curley, S.A.; Abdalla, E.K. Surgical Strategies for Synchronous Colorectal Liver Metastases in 156 Consecutive Patients: Classic, Combined or Reverse Strategy? J. Am. Coll. Surg. 2010, 210, 934–941. [Google Scholar] [CrossRef] [PubMed]


| Procedures with Anastomosis (n = 184) | Number (%) |
| Left colectomy | 67 (27.6%) |
| Right colectomy | 41 (16.9%) |
| Low anterior resection with colorectostomy and diverting ileostomy | 31 (12.8%) |
| Dixon operation | 20 (8.2%) |
| Subtotal colectomy | 19 (7.8%) |
| Transverse colectomy | 5 (2.1%) |
| Total colectomy | 1 (0.4%) |
| Procedures without anastomosis (n = 59) | Number (%) |
| Colorectal resection with colostomy (Hartmann operation) | 31 (12.8%) |
| Abdominoperineal resection | 26 (10.7%) |
| Total pelvic exenteration | 1 (0.4%) |
| Proctocolectomy | 1 (0.4%) |
| Postoperative Complications | Mild Complications (Grade I, II) Nr. of Complications (%) | Severe Complications (Grade III, IV, V) Nr. of Complications (%) | Decease (Grade V) n (%) |
|---|---|---|---|
| Surgical complications (n = 108) | |||
| Liver cut-surface collection | 16 (23.53%) | 24 (44.44%) | 1 (8.33%) |
| Biliary fistula | 14 (20.59%) | 4 (7.41%) | 0 |
| Anastomotic fistula | 13 (19.12%) | 16 (29.63%) | 3 (25%) |
| Wound complications | 11 (16.18%) | 3 (5.55%) | 0 |
| Pelvic abscess | 5 (7.35%) | 0 | 0 |
| Intraabdominal abscess | 0 | 1 (1.85%) | 0 |
| Partial bowel necrosis | 0 | 1 (1.85%) | 0 |
| Medical complications (n = 25) | |||
| Pulmonary complications | 4 (5.88%) | 8 (14.81%) | 4 (33.33%) |
| Renal complications | 3 (4.41%) | 1 (1.85%) | 1 (8.33%) |
| Digestive complications | 2 (2.94%) | 2 (3.70%) | 1 (8.33%) |
| Cardiac complications | 0 | 2 (3.70%) | 1 (8.33%) |
| Clostridium difficile | 2 (2.94%) | 1 (1.85%) | 1 (8.33%) |
| Comprehensive complication index [median (IQR)] | 20.90 (8.70–20.90) | 37.25 (26.20–60.57) | 100 |
| Total number of patients | 68 * | 54 ** | 12 *** |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | p Value | HR | 95% CI | p Value |
| Age | ||||||
| ≤65 y-o | 1 | - | - | |||
| >65 y-o | 0.932 | 0.673–1.289 | 0.668 | |||
| Gender | ||||||
| Male | 1 | - | - | |||
| Female | 0.853 | 0.619–1.175 | 0.330 | |||
| Primary tumor location | ||||||
| Colon | 1 | - | - | |||
| Rectum | 0.885 | 0.637–1.229 | 0.466 | |||
| T stage | ||||||
| T1–T3 | 1 | - | - | |||
| T4 | 1.045 | 0.592–1.844 | 0.878 | |||
| N stage | ||||||
| N0 or N1 | 1 | - | - | 1 | - | - |
| N2 | 1.494 | 1.082–2.064 | 0.015 | 1.466 | 1.061–2.026 | 0.020 |
| Distributions of SCLMs | ||||||
| Unilobar | 1 | - | - | 1 | - | - |
| Bilobar | 1.726 | 1.238–2.405 | 0.001 | 1.237 | 0.803–1.905 | 0.334 |
| Number of SCLMs | ||||||
| 1–3 lesions | 1 | - | - | 1 | - | - |
| ≥4 lesions | 2.274 | 1.513–3.419 | 0.001 | 2.177 | 1.438–3.294 | <0.001 |
| Size of SCLMs | ||||||
| <3 cm | 1 | - | - | |||
| ≥3 cm | 1.243 | 0.911–1.696 | 0.171 | |||
| TBS score | ||||||
| ≤3.2 | 1 | - | - | 1 | - | - |
| >3.2 | 1.411 | 1.032–1.929 | 0.031 | 1.142 | 0.795–1.642 | 0.472 |
| Type of hepatectomy | ||||||
| Anatomic | 1 | - | - | |||
| Non-anatomic | 0.905 | 0.594–1.378 | 0.640 | |||
| Extension of hepatectomy | ||||||
| Minor | 1 | - | - | 1 | - | - |
| Major | 1.518 | 0.990–2.329 | 0.056 | 0.975 | 0.582–1.635 | 0.924 |
| All postoperative complications | ||||||
| No | 1 | - | - | |||
| Yes | 1.199 | 0.878–1.637 | 0.254 | |||
| Clavien–Dindo severe complications | ||||||
| No | 1 | - | - | |||
| Yes | 0.976 | 0.645–1.477 | 0.909 | |||
| Septic complications | ||||||
| No | 1 | - | - | |||
| Yes | 1.075 | 0.773–1.496 | 0.667 | |||
| Hepatic complications | ||||||
| No | 1 | - | - | |||
| Yes | 1.038 | 0.723–1.489 | 0.842 | |||
| Hepatic septic complications | ||||||
| No | 1 | - | - | |||
| Yes | 0.988 | 0.649–1.504 | 0.954 | |||
| Comprehensive complication index (CCI) | 1.007 | 0.997–1.017 | 0.186 | |||
| Neoadjuvant treatment | ||||||
| No | 1 | - | - | 1 | - | - |
| Yes | 1.562 | 0.994–2.456 | 0.053 | 1.488 | 0.945–2.345 | 0.086 |
| Adjuvant treatment | ||||||
| Yes | 1 | - | - | |||
| No | 0.504 | 0.187–1.361 | 0.176 | |||
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | p Value | HR | 95% CI | p Value |
| Age | ||||||
| ≤65 y-o | 1 | - | - | |||
| >65 y-o | 1.255 | 0.919–1.714 | 0.153 | |||
| Gender | ||||||
| Male | 1 | - | - | |||
| Female | 1.031 | 0.760–1.398 | 0.847 | |||
| Primary tumor location | ||||||
| Colon | 1 | - | - | |||
| Rectum | 1.242 | 0.912–1.692 | 0.168 | |||
| T stage | ||||||
| T1–T3 | 1 | - | - | |||
| T4 | 1.010 | 0.584–1.747 | 0.970 | |||
| N stage | ||||||
| N0 or N1 | 1 | - | - | 1 | - | - |
| N2 | 1.652 | 1.208–2.260 | 0.002 | 1.610 | 1.175–2.206 | 0.003 |
| Distributions of SCLMs | ||||||
| Unilobar | 1 | - | - | 1 | - | - |
| Bilobar | 1.391 | 0.995–1.943 | 0.054 | 1.127 | 0.744–1.708 | 0.573 |
| Number of CLMs | ||||||
| 1–3 lesions | 1 | - | - | 1 | - | - |
| ≥4 lesions | 1.573 | 1.023–2.418 | 0.039 | 1.490 | 0.960–2.314 | 0.076 |
| Size of CLMs | ||||||
| <3 cm | 1 | - | - | |||
| ≥3 cm | 1.047 | 0.774–1.416 | 0.767 | |||
| TBS score | ||||||
| ≤3.2 | 1 | - | ||||
| >3.2 | 1.209 | 0.894–1.636 | 0.219 | |||
| Type of hepatectomy | ||||||
| Anatomic | 1 | - | - | |||
| Non-anatomic | 0.960 | 0.628–1.469 | 0.851 | |||
| Extension of hepatectomy | ||||||
| Minor | 1 | - | - | |||
| Major | 1.080 | 0.676–1.725 | 0.747 | |||
| All postoperative complications | ||||||
| No | 1 | - | - | |||
| Yes | 1.113 | 0.822–1.506 | 0.488 | |||
| Clavien–Dindo severe complications | ||||||
| No | 1 | - | - | |||
| Yes | 1.073 | 0.723–1.592 | 0.727 | |||
| Septic complications | ||||||
| No | 1 | - | - | |||
| Yes | 1.149 | 0.837–1.577 | 0.391 | |||
| Hepatic complications | ||||||
| No | 1 | - | - | |||
| Yes | 1.099 | 0.768–1.573 | 0.604 | |||
| Hepatic septic complications | ||||||
| No | 1 | - | - | |||
| Yes | 1.084 | 0.726–1.617 | 0.694 | |||
| Comprehensive complication index (CCI) | 1.006 | 0.996–1.016 | 0.243 | |||
| Neoadjuvant treatment | ||||||
| Yes | 1 | - | - | 1 | - | - |
| No | 0.595 | 0.389–0.913 | 0.017 | 0.660 | 0.429–1.015 | 0.058 |
| Adjuvant treatment | ||||||
| Yes | 1 | - | - | |||
| No | 1.521 | 0.895–2.587 | 0.121 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexandrescu, S.T.; Zarnescu, N.O.; Diaconescu, A.S.; Tomescu, D.; Droc, G.; Hrehoret, D.; Brasoveanu, V.; Popescu, I. The Impact of Postoperative Complications on Survival after Simultaneous Resection of Colorectal Cancer and Liver Metastases. Healthcare 2022, 10, 1573. https://doi.org/10.3390/healthcare10081573
Alexandrescu ST, Zarnescu NO, Diaconescu AS, Tomescu D, Droc G, Hrehoret D, Brasoveanu V, Popescu I. The Impact of Postoperative Complications on Survival after Simultaneous Resection of Colorectal Cancer and Liver Metastases. Healthcare. 2022; 10(8):1573. https://doi.org/10.3390/healthcare10081573
Chicago/Turabian StyleAlexandrescu, Sorin Tiberiu, Narcis Octavian Zarnescu, Andrei Sebastian Diaconescu, Dana Tomescu, Gabriela Droc, Doina Hrehoret, Vladislav Brasoveanu, and Irinel Popescu. 2022. "The Impact of Postoperative Complications on Survival after Simultaneous Resection of Colorectal Cancer and Liver Metastases" Healthcare 10, no. 8: 1573. https://doi.org/10.3390/healthcare10081573
APA StyleAlexandrescu, S. T., Zarnescu, N. O., Diaconescu, A. S., Tomescu, D., Droc, G., Hrehoret, D., Brasoveanu, V., & Popescu, I. (2022). The Impact of Postoperative Complications on Survival after Simultaneous Resection of Colorectal Cancer and Liver Metastases. Healthcare, 10(8), 1573. https://doi.org/10.3390/healthcare10081573








