Digital Care Programs for Chronic Hip Pain: A Prospective Longitudinal Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
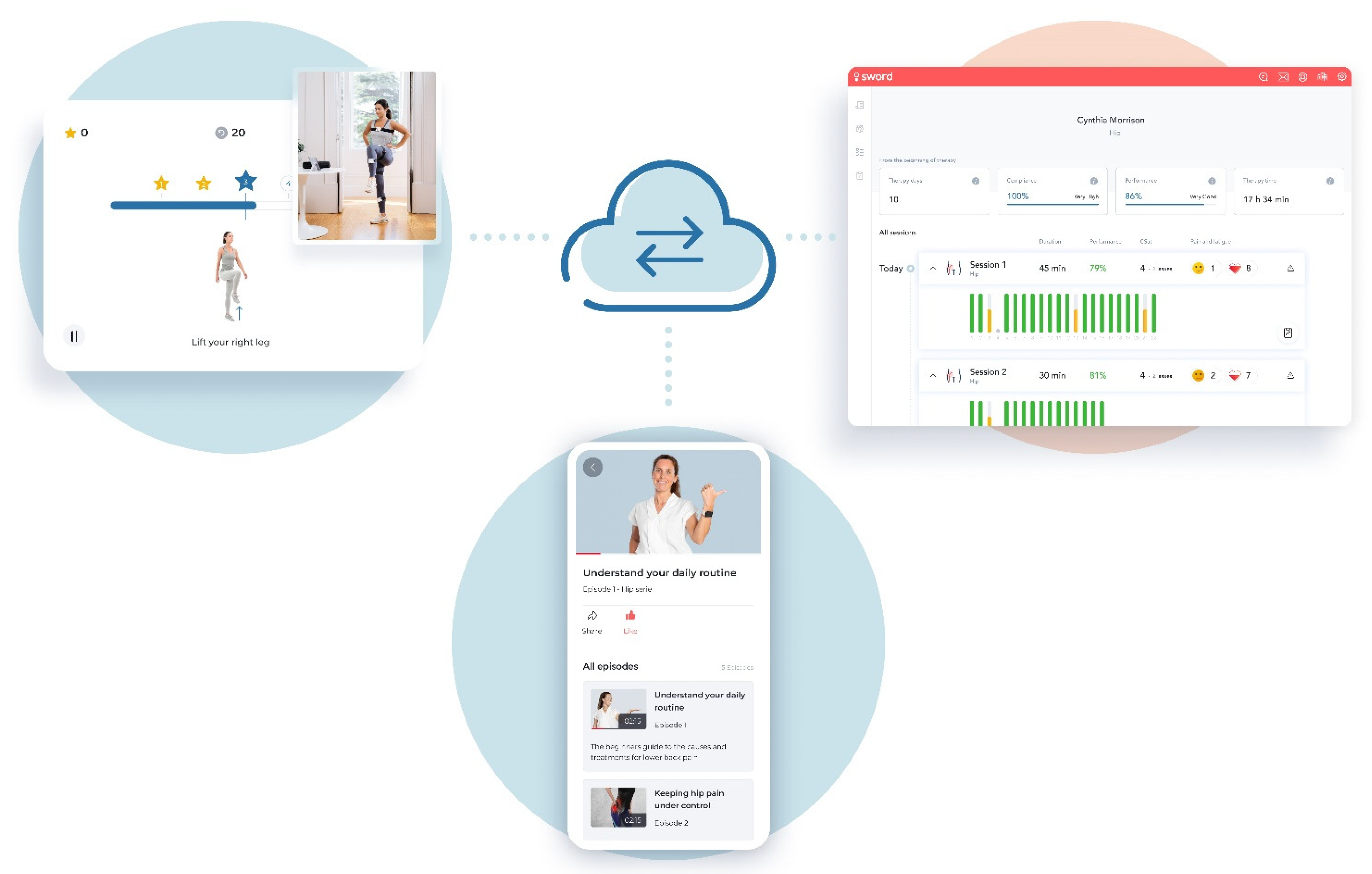
2.3. Intervention
2.4. Outcomes Measures
- Pain intensity assessed by the 11-point Numerical Pain Rating Score (NPRS) through the question “Please rate your average pain over the last 7 days: 0 (no pain at all) to 10 (worst pain imaginable)” [44].
- Self-reported surgery intent assessed by the question “How likely are you to seek surgery to address your condition in the next 12 months?” (range 0 (not likely)–100 (extremely likely)).
- Generalized Anxiety Disorder 7-item scale (GAD-7) (scores 0–21) was applied to assess anxiety severity in clinical practice and research, and was chosen due to strong validity and reliability: Cronbach’s alpha = 0.92 (excellent), ICC = 0.83 (excellent) [45,46]. Patient Health 9-item questionnaire (PHQ-9) (scores 0–27) was chosen for its strong scale validity (area under the curve in diagnosing major depression = 0.95), and reliability (Cronbach’s alpha = 0.89 (excellent)) as a brief measure of depression severity. Both scales evaluated symptomatology in the past two weeks. A cut-off threshold of ≥5 indicates at least mild anxiety/depression, respectively [45,47].
- Work productivity assessed by Work Productivity and Activity Impairment questionnaire for general health (WPAI), comprising the sub-scores: WPAI overall (combines presenteeism and absenteeism), WPAI work (presenteeism), WPAI time (absenteeism) in employed participants, and WPAI activities (non-work related activities impairment) in the entire cohort (range 0–100%, higher scores depicting greater impairment) [48].
- Patient engagement assessed by completion of the program (completion rate), cumulative time dedicated to exercise, number of completed exercise sessions, and number of sessions per week.
- Satisfaction by the question: “On a scale from 0 to 10, how likely is it that you would recommend this intervention to a friend or neighbor?”.
2.5. Safety and Adverse Events
2.6. Data Availability
2.7. Statistical Analyses
3. Results
3.1. Participants
3.2. Clinical Outcomes
3.2.1. Primary Outcome
HOOS
3.2.2. Secondary Outcomes
Pain
Surgery Intent
Mental Health
Work productivity
4. Discussion
4.1. Main Findings
4.2. Comparison with the Literature
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dawson, J. Epidemiology of hip and knee pain and its impact on overall health status in older adults. Rheumatology 2004, 43, 497–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- United States Bone and Joint Initiative. The Burden of Musculoskeletal Diseases in the United States (BMUS), 3rd ed.; United States Bone and Joint Initiative: Lombard, IL, USA, 2015. [Google Scholar]
- Plinsinga, M.L.; Coombes, B.K.; Mellor, R.; Nicolson, P.; Grimaldi, A.; Hodges, P.; Bennell, K.; Vicenzino, B. Psychological factors not strength deficits are associated with severity of gluteal tendinopathy: A cross-sectional study. Eur. J. Pain 2018, 22, 1124–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gudmundsson, P.; Nakonezny, P.A.; Lin, J.; Owhonda, R.; Richard, H.; Wells, J. Functional improvement in hip pathology is related to improvement in anxiety, depression, and pain catastrophizing: An intricate link between physical and mental well-being. BMC Musculoskelet. Disord. 2021, 22, 133. [Google Scholar] [CrossRef]
- Hampton, S.N.; Nakonezny, P.A.; Richard, H.M.; Wells, J.E. Pain catastrophizing, anxiety, and depression in hip pathology. Bone Jt. J. 2019, 101-b, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Heerey, J.J.; Kemp, J.L.; Mosler, A.B.; Jones, D.M.; Pizzari, T.; Souza, R.B.; Crossley, K.M. What is the prevalence of imaging-defined intra-articular hip pathologies in people with and without pain? A systematic review and meta-analysis. Br. J. Sports Med. 2018, 52, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Ratcliff, T.L.; Chhabra, A.; Okpara, S.O.; Lawson, P.; Kayfan, S.; Xi, Y.; Mulligan, E.P.; Wells, J.E. Correlation of the Imaging Features of Femoroacetabular Impingement Syndrome With Clinical Findings and Patient Functional Scores. Orthopedics 2021, 44, e577–e582. [Google Scholar] [CrossRef] [PubMed]
- Kemp, J.L.; Risberg, M.A.; Mosler, A.; Harris-Hayes, M.; Serner, A.; Moksnes, H.; Bloom, N.; Crossley, K.M.; Gojanovic, B.; Hunt, M.A.; et al. Physiotherapist-led treatment for young to middle-aged active adults with hip-related pain: Consensus recommendations from the International Hip-related Pain Research Network, Zurich 2018. Br. J. Sports Med. 2020, 54, 504–511. [Google Scholar] [CrossRef]
- The Royal Australian College of General Practitioners. Guideline for the Management of Knee and Hip Osteoarthritis, 2nd ed.; RACGP: East Melbourne, VIC, Australia, 2018. [Google Scholar]
- Hochberg, M.C.; Altman, R.D.; April, K.T.; Benkhalti, M.; Guyatt, G.; McGowan, J.; Towheed, T.; Welch, V.; Wells, G.; Tugwell, P. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2012, 64, 465–474. [Google Scholar] [CrossRef] [Green Version]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef] [Green Version]
- Geenen, R.; Overman, C.L.; Christensen, R.; Åsenlöf, P.; Capela, S.; Huisinga, K.L.; Husebø, M.E.P.; Köke, A.J.A.; Paskins, Z.; Pitsillidou, I.A.; et al. EULAR recommendations for the health professional’s approach to pain management in inflammatory arthritis and osteoarthritis. Ann. Rheum. Dis. 2018, 77, 797–807. [Google Scholar] [CrossRef] [Green Version]
- Maradit Kremers, H.; Larson, D.R.; Crowson, C.S.; Kremers, W.K.; Washington, R.E.; Steiner, C.A.; Jiranek, W.A.; Berry, D.J. Prevalence of Total Hip and Knee Replacement in the United States. J. Bone Jt. Surg. Am. Vol. 2015, 97, 1386–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steiner, C.; Andrews, R.; Barrett, M.; Weiss, A. HCUP Projections: Mobility/Orthopedic Procedures 2003 to 2012; US Agency for Healthcare Research and Quality: Rockville, MD, USA, 2012.
- Beswick, A.D.; Wylde, V.; Gooberman-Hill, R.; Blom, A.; Dieppe, P. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open 2012, 2, e000435. [Google Scholar] [CrossRef] [PubMed]
- Griffin, D.R.; Dickenson, E.J.; Wall, P.D.H.; Achana, F.; Donovan, J.L.; Griffin, J.; Hobson, R.; Hutchinson, C.E.; Jepson, M.; Parsons, N.R.; et al. Hip arthroscopy versus best conservative care for the treatment of femoroacetabular impingement syndrome (UK FASHIoN): A multicentre randomised controlled trial. Lancet 2018, 391, 2225–2235. [Google Scholar] [CrossRef]
- Mansell, N.S.; Rhon, D.I.; Meyer, J.; Slevin, J.M.; Marchant, B.G. Arthroscopic Surgery or Physical Therapy for Patients With Femoroacetabular Impingement Syndrome: A Randomized Controlled Trial with 2-Year Follow-up. Am. J. Sports Med. 2018, 46, 1306–1314. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.J.R.; Ayyar Gupta, V.; Fernquest, S.; Rombach, I.; Dutton, S.J.; Mansour, R.; Wood, S.; Khanduja, V.; Pollard, T.C.B.; McCaskie, A.W.; et al. Arthroscopic hip surgery compared with physiotherapy and activity modification for the treatment of symptomatic femoroacetabular impingement: Multicentre randomised controlled trial. BMJ 2019, 364, l185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurley, M.; Dickson, K.; Hallett, R.; Grant, R.; Hauari, H.; Walsh, N.; Stansfield, C.; Oliver, S. Exercise interventions and patient beliefs for people with hip, knee or hip and knee osteoarthritis: A mixed methods review. Cochrane Database Syst. Rev. 2018, 4, Cd010842. [Google Scholar] [CrossRef] [PubMed]
- Fransen, M.; McConnell, S.; Hernandez-Molina, G.; Reichenbach, S. Exercise for osteoarthritis of the hip. Cochrane Database Syst. Rev. 2014, 4, CD007912. [Google Scholar] [CrossRef] [Green Version]
- Jönsson, T.; Eek, F.; Dell’Isola, A.; Dahlberg, L.E.; Ekvall Hansson, E. The Better Management of Patients with Osteoarthritis Program: Outcomes after evidence-based education and exercise delivered nationwide in Sweden. PLoS ONE 2019, 14, e0222657. [Google Scholar] [CrossRef] [Green Version]
- Nicholas, M.K.; Asghari, A.; Corbett, M.; Smeets, R.J.; Wood, B.M.; Overton, S.; Perry, C.; Tonkin, L.E.; Beeston, L. Is adherence to pain self-management strategies associated with improved pain, depression and disability in those with disabling chronic pain? Eur. J. Pain 2012, 16, 93–104. [Google Scholar] [CrossRef]
- Pisters, M.F.; Veenhof, C.; Schellevis, F.G.; Twisk, J.W.; Dekker, J.; De Bakker, D.H. Exercise adherence improving long-term patient outcome in patients with osteoarthritis of the hip and/or knee. Arthritis Care Res. 2010, 62, 1087–1094. [Google Scholar] [CrossRef] [Green Version]
- Jack, K.; McLean, S.M.; Moffett, J.K.; Gardiner, E. Barriers to treatment adherence in physiotherapy outpatient clinics: A systematic review. Man. Ther. 2010, 15, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Jordan, J.L.; Holden, M.A.; Mason, E.E.; Foster, N.E. Interventions to improve adherence to exercise for chronic musculoskeletal pain in adults. Cochrane Database Syst. Rev. 2010, 2010, Cd005956. [Google Scholar] [CrossRef] [PubMed]
- Nero, H.; Dahlberg, J.; Dahlberg, L.E. A 6-Week Web-Based Osteoarthritis Treatment Program: Observational Quasi-Experimental Study. J. Med. Internet Res. 2017, 19, e422. [Google Scholar] [CrossRef]
- Wang, X.; Hunter, D.J.; Vesentini, G.; Pozzobon, D.; Ferreira, M.L. Technology-assisted rehabilitation following total knee or hip replacement for people with osteoarthritis: A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2019, 20, 506. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Lee, R.L.; Hunter, S.; Chan, S.W. The effectiveness of internet-based telerehabilitation among patients after total joint arthroplasty: A systematic review and meta-analysis of randomised controlled trials. J. Telemed. Telecare 2021. [Google Scholar] [CrossRef]
- Jiang, Y.; Ramachandran, H.J.; Teo, J.Y.C.; Leong, F.L.; Lim, S.T.; Nguyen, H.D.; Wang, W. Effectiveness of a nurse-led smartphone-based self-management programme for people with poorly controlled type 2 diabetes: A randomized controlled trial. J. Adv. Nurs. 2022, 78, 1154–1165. [Google Scholar] [CrossRef]
- Sadiq, S.; Ahmad, A.; Ahmed, A.; Khan, I.; Asim, H.M.; Aziz, A. Role of tele-rehabilitation in patients following total hip replacement: Systematic review of clinical trials. J. Pak. Med. Assoc. 2022, 72, 101–107. [Google Scholar] [CrossRef]
- Allen, K.D.; Yancy, W.S., Jr.; Bosworth, H.B.; Coffman, C.J.; Jeffreys, A.S.; Datta, S.K.; McDuffie, J.; Strauss, J.L.; Oddone, E.Z. A Combined Patient and Provider Intervention for Management of Osteoarthritis in Veterans: A Randomized Clinical Trial. Ann. Intern. Med. 2016, 164, 73–83. [Google Scholar] [CrossRef] [Green Version]
- Bossen, D.; Veenhof, C.; Van Beek, K.E.; Spreeuwenberg, P.M.; Dekker, J.; De Bakker, D.H. Effectiveness of a web-based physical activity intervention in patients with knee and/or hip osteoarthritis: Randomized controlled trial. J. Med. Internet Res. 2013, 15, e257. [Google Scholar] [CrossRef]
- Cronström, A.; Nero, H.; Dahlberg, L.E. Factors Associated With Patients’ Willingness to Consider Joint Surgery After Completion of a Digital Osteoarthritis Treatment Program: A Prospective Cohort Study. Arthritis Care Res. 2019, 71, 1194–1201. [Google Scholar] [CrossRef] [Green Version]
- Kloek, C.J.J.; Bossen, D.; Spreeuwenberg, P.M.; Dekker, J.; de Bakker, D.H.; Veenhof, C. Effectiveness of a Blended Physical Therapist Intervention in People With Hip Osteoarthritis, Knee Osteoarthritis, or Both: A Cluster-Randomized Controlled Trial. Phys. Ther. 2018, 98, 560–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelle, T.; Bevers, K.; van der Palen, J.; van den Hoogen, F.H.J.; van den Ende, C.H.M. Effect of the dr. Bart application on healthcare use and clinical outcomes in people with osteoarthritis of the knee and/or hip in the Netherlands; a randomized controlled trial. Osteoarthr. Cartil. 2020, 28, 418–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correia, F.D.; Nogueira, A.; Magalhaes, I.; Guimaraes, J.; Moreira, M.; Barradas, I.; Teixeira, L.; Tulha, J.; Seabra, R.; Lains, J.; et al. Home-based Rehabilitation With A Novel Digital Biofeedback System versus Conventional In-person Rehabilitation after Total Knee Replacement: A feasibility study. Sci. Rep. 2018, 8, 11299. [Google Scholar] [CrossRef] [Green Version]
- Correia, F.D.; Nogueira, A.; Magalhaes, I.; Guimaraes, J.; Moreira, M.; Barradas, I.; Molinos, M.; Teixeira, L.; Tulha, J.; Seabra, R.; et al. Medium-Term Outcomes of Digital Versus Conventional Home-Based Rehabilitation After Total Knee Arthroplasty: Prospective, Parallel-Group Feasibility Study. JMIR Rehabil. Assist. Technol. 2019, 6, e13111. [Google Scholar] [CrossRef]
- Correia, F.D.; Molinos, M.; Luís, S.; Carvalho, D.; Carvalho, C.; Costa, P.; Seabra, R.; Francisco, G.; Bento, V.; Lains, J. Digitally Assisted Versus Conventional Home-Based Rehabilitation After Arthroscopic Rotator Cuff Repair: A Randomized Controlled Trial. Am. J. Phys. Med. Rehabil. 2022, 101, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Janela, D.; Molinos, M.; Lains, J.; Francisco, G.E.; Bento, V.; Dias Correia, F. Telerehabilitation of acute musculoskeletal multi-disorders: Prospective, single-arm, interventional study. BMC Musculoskelet. Disord. 2022, 23, 29. [Google Scholar] [CrossRef]
- Janela, D.; Costa, F.; Molinos, M.; Moulder, R.G.; Lains, J.; Francisco, G.E.; Bento, V.; Cohen, S.P.; Correia, F.D. Asynchronous and Tailored Digital Rehabilitation of Chronic Shoulder Pain: A Prospective Longitudinal Cohort Study. J. Pain. Res. 2022, 15, 53–66. [Google Scholar] [CrossRef]
- Correia, F.D.; Molinos, M.; Neves, C.; Janela, D.; Carvalho, D.; Luis, S.; Francisco, G.E.; Lains, J.; Bento, V. Digital Rehabilitation for Acute Ankle Sprains: Prospective Longitudinal Cohort Study. JMIR Rehabil. Assist. Technol. 2021, 8, e31247. [Google Scholar] [CrossRef]
- Dias Correia, F.; Nogueira, A.; Magalhaes, I.; Guimaraes, J.; Moreira, M.; Barradas, I.; Molinos, M.; Teixeira, L.; Pires, J.; Seabra, R.; et al. Digital Versus Conventional Rehabilitation After Total Hip Arthroplasty: A Single-Center, Parallel-Group Pilot Study. JMIR Rehabil. Assist. Technol. 2019, 6, e14523. [Google Scholar] [CrossRef] [Green Version]
- Klässbo, M.; Larsson, E.; Mannevik, E. Hip disability and osteoarthritis outcome score. An extension of the Western Ontario and McMaster Universities Osteoarthritis Index. Scand. J. Rheumatol. 2003, 32, 46–51. [Google Scholar] [CrossRef]
- Williamson, A.; Hoggart, B. Pain: A review of three commonly used pain rating scales. J. Clin. Nurs. 2005, 14, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.; Löwe, B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch. Intern. Med. 2006, 166, 1092–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bijker, L.; Sleijser-Koehorst, M.L.S.; Coppieters, M.W.; Cuijpers, P.; Scholten-Peeters, G.G.M. Preferred Self-Administered Questionnaires to Assess Depression, Anxiety and Somatization in People With Musculoskeletal Pain—A Modified Delphi Study. J. Pain. 2020, 21, 409–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.; Ospina, M.; Dennett, L.; Waye, A.; Jacobs, P. A systematic review of the measurement properties of self report instruments that assess presenteeism. Am. J. Manag. Care 2015, 21, e171–e185. [Google Scholar]
- McNeish, D.; Matta, T. Differentiating between mixed-effects and latent-curve approaches to growth modeling. Behav. Res. Methods 2018, 50, 1398–1414. [Google Scholar] [CrossRef]
- Pfaffel, A.; Schober, B.; Spiel, C. A Comparison of Three Approaches to Correct for Direct and Indirect Range Restrictions: A Simulation Study. Pract. Assess. Res. Eval. 2016, 21, 6. [Google Scholar]
- Xiao, J.; Bulut, O. Evaluating the Performances of Missing Data Handling Methods in Ability Estimation From Sparse Data. Educ. Psychol. Meas. 2020, 80, 932–954. [Google Scholar] [CrossRef]
- Dahlberg, L.E.; Dell’Isola, A.; Lohmander, L.S.; Nero, H. Improving osteoarthritis care by digital means—Effects of a digital self-management program after 24- or 48-weeks of treatment. PLoS ONE 2020, 15, e0229783. [Google Scholar] [CrossRef] [Green Version]
- Dworkin, R.H.; Turk, D.C.; Wyrwich, K.W.; Beaton, D.; Cleeland, C.S.; Farrar, J.T.; Haythornthwaite, J.A.; Jensen, M.P.; Kerns, R.D.; Ader, D.N.; et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J. Pain. 2008, 9, 105–121. [Google Scholar] [CrossRef]
- Niemeijer, A.; Lund, H.; Stafne, S.N.; Ipsen, T.; Goldschmidt, C.L.; Jørgensen, C.T.; Juhl, C.B. Adverse events of exercise therapy in randomised controlled trials: A systematic review and meta-analysis. Br. J. Sports Med. 2020, 54, 1073–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villadsen, A.; Overgaard, S.; Holsgaard-Larsen, A.; Christensen, R.; Roos, E.M. Immediate efficacy of neuromuscular exercise in patients with severe osteoarthritis of the hip or knee: A secondary analysis from a randomized controlled trial. J. Rheumatol. 2014, 41, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Teirlinck, C.H.; Luijsterburg, P.A.; Dekker, J.; Bohnen, A.M.; Verhaar, J.A.; Koopmanschap, M.A.; van Es, P.P.; Koes, B.W.; Bierma-Zeinstra, S.M. Effectiveness of exercise therapy added to general practitioner care in patients with hip osteoarthritis: A pragmatic randomized controlled trial. Osteoarthr. Cartil. 2016, 24, 82–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skou, S.T.; Roos, E.M. Good Life with osteoArthritis in Denmark (GLA:D™): Evidence-based education and supervised neuromuscular exercise delivered by certified physiotherapists nationwide. BMC Musculoskelet. Disord. 2017, 18, 72. [Google Scholar] [CrossRef] [Green Version]
- Bennell, K.L.; Nelligan, R.K.; Rini, C.; Keefe, F.J.; Kasza, J.; French, S.; Forbes, A.; Dobson, F.; Abbott, J.H.; Dalwood, A.; et al. Effects of internet-based pain coping skills training before home exercise for individuals with hip osteoarthritis (HOPE trial): A randomised controlled trial. Pain 2018, 159, 1833–1842. [Google Scholar] [CrossRef]
- Cranen, K.; Drossaert, C.H.; Brinkman, E.S.; Braakman-Jansen, A.L.; Ijzerman, M.J.; Vollenbroek-Hutten, M.M. An exploration of chronic pain patients’ perceptions of home telerehabilitation services. Health Expect. 2012, 15, 339–350. [Google Scholar] [CrossRef] [Green Version]
- Dahlberg, L.E.; Grahn, D.; Dahlberg, J.E.; Thorstensson, C.A. A Web-Based Platform for Patients with Osteoarthritis of the Hip and Knee: A Pilot Study. JMIR Res. Protoc. 2016, 5, e115. [Google Scholar] [CrossRef] [Green Version]
- Cheng, A.L.; Schwabe, M.; Doering, M.M.; Colditz, G.A.; Prather, H. The Effect of Psychological Impairment on Outcomes in Patients with Prearthritic Hip Disorders: A Systematic Review and Meta-analysis. Am. J. Sports Med. 2019, 48, 2563–2571. [Google Scholar] [CrossRef]
- Bartley, E.J.; Palit, S.; Staud, R. Predictors of Osteoarthritis Pain: The Importance of Resilience. Curr. Rheumatol. Rep. 2017, 19, 57. [Google Scholar] [CrossRef]
- Dell’Isola, A.; Jönsson, T.; Ranstam, J.; Dahlberg, L.E.; Ekvall Hansson, E. Education, Home Exercise, and Supervised Exercise for People With Hip and Knee Osteoarthritis As Part of a Nationwide Implementation Program: Data From the Better Management of Patients with Osteoarthritis Registry. Arthritis Care Res. 2020, 72, 201–207. [Google Scholar] [CrossRef]
- Pekkala, J.; Rahkonen, O.; Pietiläinen, O.; Lahelma, E.; Blomgren, J. Sickness absence due to different musculoskeletal diagnoses by occupational class: A register-based study among 1.2 million Finnish employees. Occup. Environ. Med. 2018, 75, 296. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, A.; Higgins, N.M.; Rothwell, C.; Ashton, J.; Breen, R.; Corcoran, O.; FitzGerald, O.; Gallagher, P.; Desmond, D. Work Outcomes in Patients Who Stay at Work Despite Musculoskeletal Pain. J. Occup. Rehabil. 2018, 28, 559–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mota, R.E.M.; Tarricone, R.; Ciani, O.; Bridges, J.F.P.; Drummond, M. Determinants of demand for total hip and knee arthroplasty: A systematic literature review. BMC Health Serv. Res. 2012, 12, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snijders, G.F.; den Broeder, A.A.; van Riel, P.L.; Straten, V.H.; de Man, F.H.; van den Hoogen, F.H.; van den Ende, C.H. Evidence-based tailored conservative treatment of knee and hip osteoarthritis: Between knowing and doing. Scand. J. Rheumatol. 2011, 40, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Svege, I.; Nordsletten, L.; Fernandes, L.; Risberg, M.A. Exercise therapy may postpone total hip replacement surgery in patients with hip osteoarthritis: A long-term follow-up of a randomised trial. Ann. Rheum. Dis. 2015, 74, 164–169. [Google Scholar] [CrossRef] [Green Version]
- Pisters, M.F.; Veenhof, C.; Schellevis, F.G.; De Bakker, D.H.; Dekker, J. Long-term effectiveness of exercise therapy in patients with osteoarthritis of the hip or knee: A randomized controlled trial comparing two different physical therapy interventions. Osteoarthr. Cartil. 2010, 18, 1019–1026. [Google Scholar] [CrossRef] [Green Version]
- Cronström, A.; Dahlberg, L.E.; Nero, H.; Hammarlund, C.S. “I was considering surgery because I believed that was how it was treated”: A qualitative study on willingness for joint surgery after completion of a digital management program for osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1026–1032. [Google Scholar] [CrossRef] [Green Version]
- Rolfson, O.; Wissig, S.; van Maasakkers, L.; Stowell, C.; Ackerman, I.; Ayers, D.; Barber, T.; Benzakour, T.; Bozic, K.; Budhiparama, N.; et al. Defining an International Standard Set of Outcome Measures for Patients With Hip or Knee Osteoarthritis: Consensus of the International Consortium for Health Outcomes Measurement Hip and Knee Osteoarthritis Working Group. Arthritis Care Res. 2016, 68, 1631–1639. [Google Scholar] [CrossRef]
- Iacobucci, D. Structural equations modeling: Fit Indices, sample size, and advanced topics. J. Consum. Psychol. 2010, 20, 90–98. [Google Scholar] [CrossRef]
- Brown, T.A. Confirmatory Factor Analysis for Applied Research; The Guilford Press: New York, NY, USA, 2006; pp. 81–88. [Google Scholar]


| Characteristic | Entire Cohort (N = 534) |
|---|---|
| Age (years), mean (SD) | 50.2 (11.3) |
| Age categories (years), N (%): | |
| <25 | 3 (0.6) |
| 25–40 | 122 (22.8) |
| 40–60 | 292 (54.7) |
| >60 | 117 (21.9) |
| Sex, N (%) | |
| Female | 363 (68.0) |
| Male | 171 (32.0) |
| BMI, mean (SD) a | 29.1 (6.4) |
| BMI categories, N (%) a: | |
| Underweight (<18.5) | 2 (0.4) |
| Normal (18.5–25) | 147 (27.7) |
| Overweight (25–30) | 188 (35.4) |
| Obese (30–40) | 159 (29.9) |
| Morbidly obese (>40) | 35 (6.6) |
| Laterality | |
| Left | 150 (28.1) |
| Right | 185 (34.6) |
| Both | 199 (37.3) |
| Employment status, N (%): | |
| Employed (part-time or full-time) | 480 (89.9) |
| Unemployed (not working or retired) | 54 (10.1) |
| Hip pain-related condition, N (%): | |
| Hip Osteoarthritis | 106 (19.9%) |
| Others b | 428 (80.1%) |
| Psychopathology comorbidity | |
| GAD-7 ≥ 5 | 135 (25.3%) |
| GAD-7 ≥ 10 | 46 (8.6%) |
| PHQ-9 ≥ 5 | 102 (19.1%) |
| PHQ-9 ≥ 10 | 34 (6.4%) |
| Outcome, Mean (95%CI) | n | Baseline | End-of-Program | Mean Change | % Change |
|---|---|---|---|---|---|
| HOOS-Pain | 515 | 65.59 (64.33; 66.84) | 78.91 (77.17; 80.65) | 13.32 (11.67; 14.97) | 20.3% |
| HOOS-Function | 251 | 75.08 (73.17; 77.00) | 86.09 (83.89; 88.30) | 11.01 (8.61; 13.41) | 14.7% |
| HOOS-Qol | 515 | 52.44 (50.86; 54.02) | 66.52 (64.22; 68.81) | 14.08 (12.03; 16.12) | 26.8% |
| HOOS-Sport | 251 | 65.37 (62.84; 67.90) | 78.92 (76.20; 81.63) | 13.55 (10.76; 16.33) | 20.7% |
| HOOS-Symptoms | 251 | 68.18 (66.25; 70.12) | 78.62 (76.38; 80.85) | 10.43 (8.20; 12.67) | 15.3% |
| Pain Level | 534 | 4.82 (4.65; 4.98) | 2.60 (2.33; 2.87) | 2.22 (1.93; 2.50) | 46.0% |
| Surgery Intent > 0 | 201 | 23.67 (20.10; 27.23) | 7.07 (3.38; 10.77) | 16.59 (12.92; 20.27) | 70.1% |
| Surgery Intent | 534 | 8.84 (7.14; 10.54) | 3.16 (1.67; 4.65) | 5.68 (4.01; 7.34) | 64.3% |
| GAD-7 ≥ 5 | 135 | 9.19 (8.43; 9.94) | 4.22 (3.07; 5.36) | 4.97 (3.79; 6.15) | 54.1% |
| GAD-7 | 534 | 3.05 (2.68; 3.42) | 1.92 (1.51; 2.33) | 1.13 (0.68; 1.57) | 36.9% |
| PHQ-9 ≥ 5 | 102 | 9.86 (8.85; 10.87) | 4.48 (3.27; 5.46) | 5.38 (4.23; 6.54) | 54.6% |
| PHQ-9 | 534 | 2.66 (2.29; 3.03) | 1.55 (1.22; 1.87) | 1.16 (0.75; 1.49) | 41.9% |
| WPAI Overall > 0 | 224 | 30.18 (27.37; 33.00) | 11.94 (8.63; 15.24) | 18.25 (14.92; 21.58) | 60.5% |
| WPAI Overall | 430 | 15.82 (13.80; 17.84) | 9.05 (6.35; 11.75) | 6.77 (3.89; 9.66) | 42.8% |
| WPAI Work > 0 | 218 | 29.43 (26.72; 32.14) | 11.97 (8.72; 15.22) | 17.46 (14.18; 20.74) | 59.3% |
| WPAI Work | 430 | 14.91 (13.00; 16.81) | 9.05 (6.90; 11.47) | 5.86 (2.93; 8.80) | 39.3% |
| WPAI Activity > 0 | 390 | 35.70 (33.41; 37.98) | 17.14 (13.75; 20.52) | 18.56 (15.11; 22.01) | 52.0% |
| WPAI Activity | 534 | 26.07 (23.94; 28.20) | 14.68 (11.88; 17.47) | 11.39 (8.40; 14.38) | 43.7% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janela, D.; Costa, F.; Areias, A.C.; Molinos, M.; Moulder, R.G.; Lains, J.; Bento, V.; Scheer, J.K.; Yanamadala, V.; Cohen, S.P.; et al. Digital Care Programs for Chronic Hip Pain: A Prospective Longitudinal Cohort Study. Healthcare 2022, 10, 1595. https://doi.org/10.3390/healthcare10081595
Janela D, Costa F, Areias AC, Molinos M, Moulder RG, Lains J, Bento V, Scheer JK, Yanamadala V, Cohen SP, et al. Digital Care Programs for Chronic Hip Pain: A Prospective Longitudinal Cohort Study. Healthcare. 2022; 10(8):1595. https://doi.org/10.3390/healthcare10081595
Chicago/Turabian StyleJanela, Dora, Fabíola Costa, Anabela C. Areias, Maria Molinos, Robert G. Moulder, Jorge Lains, Virgílio Bento, Justin K. Scheer, Vijay Yanamadala, Steven P. Cohen, and et al. 2022. "Digital Care Programs for Chronic Hip Pain: A Prospective Longitudinal Cohort Study" Healthcare 10, no. 8: 1595. https://doi.org/10.3390/healthcare10081595






