The Prediction of Survival Outcome and Prognosis Factor in Association with Comorbidity Status in Patients with Colorectal Cancer: A Research-Based Study
Abstract
:1. Introduction
2. Methodology
3. Result
4. Discussion
5. Conclusions
6. Merits and Recommendations of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, J.; Zheng, R.; Zhang, S.; Zeng, H.; Wang, S.; Chen, R.; Sun, K.; Li, M.; Gu, J.; Zhuang, G. Colorectal Cancer Burden and Trends: Comparison between China and Major Burden Countries in the World. Chin. J. Cancer Res. 2021, 33, 1. [Google Scholar] [CrossRef] [PubMed]
- Santucci, C.; Boffetta, P.; Levi, F.; La Vecchia, C.; Negri, E.; Malvezzi, M. Colorectal Cancer Mortality in Young Adults Is Rising in The United States, Canada, United Kingdom, and Australia but not in Europe and Asia. Gastroenterology 2021, 160, 1860–1862.e2. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, T.; Ruszkowska, M.; Danielewicz, A.; Niedźwiedzka, E.; Arłukowicz, T.; Przybyłowicz, K.E. A Review of Colorectal Cancer in Terms of Epidemiology, Risk Factors, Development, Symptoms and Diagnosis. Cancers 2021, 13, 2025. [Google Scholar] [CrossRef]
- Todua, F.; Gagua, R.; Maglakelidze, M.; Maglakelidze, D. Cancer Incidence and Mortality-Major Patterns in Globocan 2012, Worldwide and Georgia. Bull. Georg. Natl. Acad. Sci. 2015, 9, 168–173. [Google Scholar]
- Afolabi, H.A.; Bin Zakariya, Z.; Shokri, A.B.A.; Hasim, M.N.B.M.; Vinayak, R.; Afolabi-Owolabi, O.T.; Elesho, R.F. The Rela-tionship between Obesity and other Medical Comorbidities. Obes. Med. 2020, 17, 100164. [Google Scholar] [CrossRef]
- Korn, E.L.; Freidlin, B.; Abrams, J.S. Overall Survival as the Outcome for Randomized Clinical Trials with Effective Subsequent Therapies. J. Clin. Oncol. 2011, 29, 2439. [Google Scholar] [CrossRef]
- Huang, C.; Zhao, J.; Zhu, Z. Prognostic Nomogram of Prognosis-Related Genes and Clinicopathological Characteristics to Predict the 5-Year Survival Rate of Colon Cancer Patients. Front. Surg. 2021, 8, 187. [Google Scholar] [CrossRef]
- Van Den Berg, I.; Coebergh Van Den Braak, R.R.; Van Vugt, J.L.; Ijzermans, J.N.; Buettner, S. Actual Survival After Resection of Primary Colorectal Cancer: Results from A Prospective Multicenter Study. World J. Surg. Oncol. 2021, 19, 96. [Google Scholar] [CrossRef]
- Wong, S.-W.; Ling, D.-Y.; Yeow, R.-Q.; Chong, R.-W.; Aziz MR, A.; Aziz, N.A.; Poh, K.-S.; Roslani, A.C. Clinicopathological Patterns and Survival Outcomes of Colorectal Cancer among Young Adults in Malaysia: An Institutional Cohort Study. Singap. Med. J 2021, 1, 18. [Google Scholar] [CrossRef]
- Artiles-Armas, M.; Roque-Castellano, C.; Fariña-Castro, R.; Conde-Martel, A.; Acosta-Mérida, M.A.; Marchena-Gómez, J. Impact of Frailty on 5-Year Survival in Patients Older than 70 Years Undergoing Colorectal Surgery for Cancer. World J. Surg. Oncol. 2021, 19, 106. [Google Scholar] [CrossRef]
- Zygulska, A.L.; Pierzchalski, P. Novel Diagnostic Biomarkers in Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 852. [Google Scholar] [CrossRef]
- Firth, M.; Blackmore, T.; Chepulis, L.; Keenan, R.; Stokes, T.; Elwood, M.; Weller, D.; Emery, J.; Lawrenson, R. Why does New Zealand Have Such Poor Outcomes from Colorectal Cancer?: The Importance of the Pre-Diagnostic Period. J. Prim. Health Care 2021, 13, 15–26. [Google Scholar] [CrossRef]
- Bach, S.; Paulis, I.; Sluiter, N.; Tibbesma, M.; Martin, I.; Van De Wiel, M.; Tuynman, J.; Bahce, I.; Kazemier, G.; Steenbergen, R. Detection of Colorectal Cancer in Urine Using DNA Methylation Analysis. Sci. Rep. 2021, 11, 2363. [Google Scholar] [CrossRef] [PubMed]
- De Groot, V.; Beckerman, H.; Lankhorst, G.J.; Bouter, L.M. How to Measure Comorbidity: A Critical Review of Available Methods. J. Clin. Epidemiol. 2003, 56, 221–229. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, M.; Ales, K.L.; MacKenzie, C.R. A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Baldwin, L.-M.; Kalbunde, C.N.; Green, P.; Barlow, W.; Wright, G. In search of the perfect comorbidity measure for use with administrative claims data: Does it exist? Medical Care 2006, 44, 745–753. [Google Scholar] [CrossRef]
- Hall, W.H.; Ramachandran, R.; Narayan, S.; Jani, A.B.; Vijayakumar, S. An Electronic Application for Rapidly Calculating Charlson Comorbidity Score. BMC Cancer 2004, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Extermann, M. Measuring Comorbidity in Older Cancer Patients. Eur. J. Cancer 2000, 36, 453–471. [Google Scholar] [CrossRef]
- Araghi, M.; Soerjomataram, I.; Jenkins, M.; Brierley, J.; Morris, E.; Bray, F.; Arnold, M. Global Trends in Colorectal Cancer Mortality: Projections to The Year 2035. Int. J. Cancer 2019, 144, 2992–3000. [Google Scholar] [CrossRef]
- Bass, G.; Fleming, C.; Conneely, J.; Martin, Z.; Mealy, K. Emergency First Presentation of Colorectal Cancer Predicts Signif-icantly Poorer Outcomes: A Review of 356 Consecutive Irish Patients. Dis. Colon Rectum 2009, 52, 678–684. [Google Scholar] [CrossRef]
- Phatak, U.R.; Kao, L.S.; Millas, S.G.; Wiatrek, R.L.; Ko, T.C.; Wray, C.J. Interaction Between Age and Race Alters Predicted Survival in Colorectal Cancer. Ann. Surg. Oncol. 2013, 20, 3363–3369. [Google Scholar] [CrossRef] [PubMed]
- Demir, H.; Caglayan, D.; Kaman, O.; Inanc, M.; Urvay, S.; Beypinar, I.; Demirci, A.; Davarci, S.; Araz, M.; Baykara, M. Evalu-ating The Effect of Tumor Size and Sidedness on Prognosis in Stage 2 Colon Cancer: A Retrospective Population Study. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 1328–1340. [Google Scholar]
- Barnous, R.; Somi, M.H.; Sanaat, Z.; Jabbaripoor, P.; Dolatkhah, N.; Dolatkhah, R. Epidemiological Aspects of Colorectal Cancer in East Azerbaijan, Northwest Iran: Five Year Survival Analysis. Univ. Med. 2021, 40, 190–199. [Google Scholar] [CrossRef]
- Burgers, K.; Moore, C.; Bednash, L. Care of the Colorectal Cancer Survivor. Am. Fam. Physician 2018, 97, 331–336. [Google Scholar] [PubMed]
- Lal, A.; Mccaffrey, N.; Gold, L.; Roder, D.; Buckley, E. Variations in Utilisation of Colorectal Cancer Services in South Aus-tralia indicated by MBS/PBS Benefits: A Benefit Incidence Analysis. Aust. New Zealand J. Public Health 2022, 46, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Motoyama, S.; Wada, Y.; Wakita, A.; Kawakita, Y.; Nagaki, Y.; Terata, K.; Imai, K.; Anbai, A.; Hashimoto, M. Neo-adjuvant chemoradiotherapy followed by esophagectomy with three-field lymph node dissection for thoracic esophageal squamous cell carcinoma patients with clinical stage III and with supraclavicular lymph node metastasis. Cancers 2021, 13, 983. [Google Scholar] [CrossRef] [PubMed]
- Tomita, N.; Ishida, H.; Tanakaya, K.; Yamaguchi, T.; Kumamoto, K.; Tanaka, T.; Hinoi, T.; Miyakura, Y.; Hasegawa, H.; Ta-kayama, T. Japanese Society for Cancer of The Colon and Rectum (JSCCR) Guidelines 2020 for the Clinical Practice of He-reditary Colorectal Cancer. Int. J. Clin. Oncol. 2021, 26, 1353–1419. [Google Scholar] [CrossRef]
- Hong, S.; Lee, Y.Y.; Lee, J.; Kim, Y.; Choi, K.S.; Jun, J.K.; Suh, M. Trends in Cancer Screening Rates Among Korean Men and Women: Results of the Korean National Cancer Screening Survey, 2004–2018. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2021, 53, 330. [Google Scholar] [CrossRef]
- Aliakbari, F.; Ghanbari, M.A.; Khayamzadeh, M.; Hajian, M.R.; Allameh, F.; Ahadi, M.; Sadeghzadeh, Z.; Akbari, M.E.; Soli-mani, M.; Nematollah, S. Five-Year Survival Rate of Prostate Cancer in Iran: Results of the National Cancer-Registry System during 2010–2015. Men’s Health J. 2020, 4, e4. [Google Scholar]
- Moghimi-Dehkordi, B.; Safaee, A. An Overview of Colorectal Cancer Survival Rates and Prognosis in Asia. World J. Gastro-Intest. Oncol. 2012, 4, 71. [Google Scholar] [CrossRef]
- Recio-Boiles, A.; Cagir, B. Colon Cancer; Statpearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Stintzing, S.; Tejpar, S.; Gibbs, P.; Thiebach, L.; Lenz, H.-J. Understanding the Role of Primary Tumour Localisation in Colo-rectal Cancer Treatment and Outcomes. Eur. J. Cancer 2017, 84, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Mohd, Y.; Balasubramanian, B.; Meyyazhagan, A.; Kuchi Bhotla, H.; Shanmugam, S.K.; Ramesh Kumar, M.K.; Pappusamy, M.; Alagamuthu, K.K.; Keshavarao, S.; Arumugam, V.A. Extricating the Association between the Prognostic Factors of Colorectal Cancer. J. Gastrointest. Cancer 2021, 52, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Michalopoulou, E.; Matthes, K.L.; Karavasiloglou, N.; Wanner, M.; Limam, M.; Korol, D.; Held, L.; Rohrmann, S. Impact of Comorbidities at Diagnosis on the 10-Year Colorectal Cancer Net Survival: A Population-Based Study. Cancer Epidemiol. 2021, 73, 101962. [Google Scholar] [CrossRef] [PubMed]
- Lakemeyer, L.; Sander, S.; Wittau, M.; Henne-Bruns, D.; Kornmann, M.; Lemke, J. Diagnostic and Prognostic Value of CEA and Ca19-9 in Colorectal Cancer. Diseases 2021, 9, 21. [Google Scholar] [CrossRef]
- Badic, B.; Oguer, M.; Cariou, M.; Kermarrec, T.; Bouzeloc, S.; Nousbaum, J.-B.; Robaszkiewicz, M.; Queneherve, L. Prognostic Factors for Stage III Colon Cancer in Patients 80 Years of Age and Older. Int. J. Colorectal Dis. 2021, 36, 811–819. [Google Scholar] [CrossRef]
- Li, Q.; Wang, G.; Luo, J.; Li, B.; Chen, W. Clinicopathological Factors Associated with Synchronous Distant Metastasis and Prognosis of Stage T1 Colorectal Cancer Patients. Sci. Rep. 2021, 11, 8722. [Google Scholar] [CrossRef]
- Ishizuka, D.; Shirai, Y.; Sakai, Y.; Hatakeyama, K. Colorectal Carcinoma Liver Metastases: Clinical Significance of Preopera-tive Measurement of Serum Carcinoembryonic Antigen and Carbohydrate Antigen 19-9 Levels. Int. J. Colorectal Dis. 2001, 16, 32–37. [Google Scholar] [CrossRef]
- Arru, M.; Aldrighetti, L.; Castoldi, R.; Di Palo, S.; Orsenigo, E.; Stella, M.; Pulitanò, C.; Gavazzi, F.; Ferla, G.; Di Carlo, V. Analysis of Prognostic Factors Influencing Long-Term Survival after Hepatic Resection for Metastatic Colorectal Cancer. World J. Surg. 2008, 32, 93–103. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, G.; Zheng, K.; Lou, Z.; Gao, X.H.; Meng, R.G.; Furnée, E.J.; Zhang, W. Prognostic Factors in Patients with Complete Response of The Tumour (Ypt0) after Neoadjuvant Chemoradiotherapy and Radical Resection of Rectal Cancer. ANZ J. Surg. 2021, 91, E190–E195. [Google Scholar] [CrossRef]
- Wiratkapun, S.; Kraemer, M.; Seow-Choen, F.; Ho, Y.-H.; Eu, K. High Preoperative Serum Carcinoembryonic Antigen Pre-dicts Metastatic Recurrence in Potentially Curative Colonic Cancer: Results of A Five-Year Study. Dis. Colon Rectum 2001, 44, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Konda, M.; Verma, R. Cancer Screening and Prevention: Sex and Gender Evidence in Lung, Breast, and Colorectal Cancer. In How Sex and Gender Impact Clinical Practice; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Lin, C.Y.; Lin, C.L.; Huang, W.T.; Peng, C.J.; Su, S.B.; Guo, H.R. Effect of Diabetes Mellitus Comorbidity on Outcomes in Stages II and III Colorectal Cancer. Asia-Pac. J. Clin. Oncol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Wrigley, H.; Roderick, P.; George, S.; Smith, J.; Mullee, M.; Goddard, J. Inequalities in Survival From Colorectal Cancer: A Comparison of the Impact of Deprivation, Treatment, and Host Factors on Observed and Cause Specific Survival. J. Epidemi-ol. Community Health 2003, 57, 301–309. [Google Scholar] [CrossRef]
- Luke, C.; Koczwara, B.; Moore, J.; Olver, I.; Penniment, M.; Pittman, K.; Price, T.; Rieger, N.; Roediger, B.; Wattchow, D. Treatment and Survival from Colorectal Cancer: The Experience of Patients at South Australian Teaching Hospitals between 1980 and 2002. Clin. Oncol. 2005, 17, 372–381. [Google Scholar] [CrossRef]
- Syriopoulou, E.; Morris, E.; Finan, P.J.; Lambert, P.C.; Rutherford, M.J. Understanding the Impact of Socioeconomic Differ-ences in Colorectal Cancer Survival: Potential Gain in Life-Years. Br. J. Cancer 2019, 120, 1052–1058. [Google Scholar] [CrossRef] [PubMed]
- Demir, H.; Beypinar, I.; Urvay, S.; Davarci, S.; Baykara, M. Prognostic Role of Pre-Operative Serum Ferritin Level in Stage 2 Colon Cancer. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 6473–6479. [Google Scholar] [PubMed]
- Zhao, S.; Chen, X.; Wen, D.; Zhang, C.; Wang, X. Oncologic Nomogram for Stage I Rectal Cancer To Assist Patient Selection for Adjuvant (Chemo) Radiotherapy Following Local Excision. Front. Oncol. 2021, 11, 532. [Google Scholar] [CrossRef]
- Ma, B.; Li, Y.; Meng, Q. The Predictive and Prognostic Value of Sex in Localized Colorectal Cancer: A SEER-based Analysis. Transl. Cancer Res. 2021, 10, 2108–2119. [Google Scholar] [CrossRef]
- Mehrkhani, F.; Nasiri, S.; Donboli, K.; Meysamie, A.; Hedayat, A. Prognostic Factors in Survival of Colorectal Cancer Pa-tients after Surgery. Colorectal Dis. 2009, 11, 157–161. [Google Scholar] [CrossRef]
- Miao, Y.; Xu, Z.; Feng, W.; Zheng, M.; Xu, Z.; Gao, H.; Li, W.; Zhang, Y.; Zong, Y.; Lu, A. Platelet Infiltration Predicts Surviv-al in Postsurgical Colorectal Cancer Patients. Int. J. Cancer 2022, 150, 509–520. [Google Scholar] [CrossRef]
- Macedo, F.; Sequeira, H.; Ladeira, K.; Bonito, N.; Viana, C.; Martins, S. Metastatic lymph node ratio as a better prognostic tool than the TNM system in colorectal cancer. Future Oncol. 2021, 17, 1519–1532. [Google Scholar] [CrossRef]
- Emrich, L.J.; Priore, R.L.; Murphy, G.P.; Brady, M.F. Prognostic Factors in Patients with Advanced Stage Prostate Cancer. Cancer Res. 1985, 45, 5173–5179. [Google Scholar] [PubMed]
- Basdanis, G.; Papadopoulos, V.; Michalopoulos, A.; Fahantidis, E.; Apostolidis, S.; Berovalis, P.; Zatagias, A.; Karamanlis, E. Colorectal Cancer in Patients over 70 Years of Age: Determinants of Outcome. Tech. Coloproctology 2004, 8, S112–S115. [Google Scholar] [CrossRef]
- Park, Y.J.; Park, K.J.; Park, J.-G.; Lee, K.U.; Choe, K.J.; Kim, J.-P. Prognostic Factors in 2230 Korean Colorectal Cancer Pa-tients: Analysis of Consecutively Operated Cases. World J. Surg. 1999, 23, 721–726. [Google Scholar] [CrossRef]
- Nitsche, U.; Maak, M.; Schuster, T.; Künzli, B.; Langer, R.; Slotta-Huspenina, J.; Janssen, K.-P.; Friess, H.; Rosenberg, R. Pre-diction of Prognosis Is not Improved by the Seventh and Latest Edition of the TNM Classification for Colorectal Cancer in A Single-Center Collective. Ann. Surg. 2011, 254, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, H.-M.; Lee, S.Y.; Kim, C.H.; Kim, H.R. Prognostic Significance of Enlarged Paraaortic Lymph Nodes Detected During Left-Sided Colorectal Cancer Surgery: A Single-Center Retrospective Cohort Study. World J. Surg. Oncol. 2021, 19, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Al-Mhanna, S.B.; Ghazali, W.S.W.; Mohamed, M.; Rabaan, A.A.; Santali, E.Y.; Alestad, J.H.; Santali, E.Y.; Arshad, S.; Ahmed, N.; Afolabi, H.A. Effectiveness of Physical Activity on Immunity Markers and Quality of life in Cancer Patient: A Systematic Review. PeerJ 2022, 10, e13664. [Google Scholar] [CrossRef] [PubMed]
- White, A.; Ironmonger, L.; Steele, R.J.; Ormiston-Smith, N.; Crawford, C.; Seims, A. A Review of Sex-Related Differences in Colorectal Cancer Incidence, Screening Uptake, Routes to Diagnosis, Cancer Stage and Survival in the UK. BMC Cancer 2018, 18, 906. [Google Scholar] [CrossRef]
- Cook, M.B.; Dawsey, S.M.; Freedman, N.D.; Inskip, P.D.; Wichner, S.M.; Quraishi, S.M.; Devesa, S.S.; McGlynn, K.A. Sex Dis-parities in Cancer Incidence by Period and Age. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1174–1182. [Google Scholar] [CrossRef]
- Murphy, G.; Devesa, S.S.; Cross, A.J.; Inskip, P.D.; Mcglynn, K.A.; Cook, M.B. Sex Disparities in Colorectal Cancer Incidence by Anatomic Subsite, Race and Age. Int. J. Cancer 2011, 128, 1668–1675. [Google Scholar] [CrossRef]
- Bates, B.; Cox, L.; Nicholson, S.; Page, P.; Prentice, A.; Steer, T.; Swan, G. National Diet and Nutrition Survey: Results from Years 5 and 6 (Combined) of the Rolling Programme (2012/2013–2013/2014); Public Health England: London, UK, 2016. [Google Scholar]
- Al-Mhanna, S.B.; Zakara, Z.; Afolabi, H.A.; Toyin, A.-O.O.; Elesho, R.F. A Mini Review on Covid-19 Infection and Severe Outcome on Cancer Patient. Indian J. Forensic Med. Toxicol. 2021, 15, 2447–2454. [Google Scholar]
- Cancer Research UK (CRUK); National Cancer Intelligence Network (NCIN). Excess Cancer Burden in Men; Cancer Research UK; NCIN; Leeds Met University; Men’s Health Forum: London, UK, 2013. [Google Scholar]
- Rattanasompattikul, M.; Feroze, U.; Molnar, M.Z.; Dukkipati, R.; Kovesdy, C.P.; Nissenson, A.R.; Norris, K.C.; Kopple, J.D.; Kalantar-Zadeh, K. Charlson Comorbidity Score Is A Strong Predictor of Mortality in Hemodialysis Patients. Int. Urol. Nephrol. 2012, 44, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Reid, B.C.; Alberg, A.J.; Klassen, A.C.; Samet, J.M.; Rozier, R.G.; Garcia, I.; Winn, D.M. Comorbidity and Survival of Elderly Head and Neck Carcinoma Patients. Cancer 2001, 92, 2109–2116. [Google Scholar] [CrossRef]
- Magliano, M. Obesity and Arthritis. Menopause Int. 2008, 14, 149–154. [Google Scholar] [CrossRef] [PubMed]
| Comorbid/Diseases | Assigned Weight Index | Comorbid/Diseases | Assigned Weight Index |
|---|---|---|---|
| 1. Congestive heart failure | 1 | 10. Hemiplegia/ Paraplegia | 1 |
| 2. Peripheral vascular disease | 1 | 11. Dementia | 1 |
| 3. Chronic pulmonary obstructive disease | 1 | 12. Moderate or severe renal disease | 2 |
| 4. Myocardial infarction | 1 | 13. Diabetes with end organ damage | 2 |
| 5. Rheumatologic disease | 1 | 14. Moderate–severe liver disease | 2 |
| 6. Cerebrovascular disease | 1 | 15. Any tumour, including leukaemia and lymphoma | 2 |
| 7. Peptic ulcer disease | 1 | 16. Metastatic solid tumour | 6 |
| 8. Mild liver disease | 1 | 17. AIDS | 6 |
| 9. Diabetes (uncomplicated) | 1 |
| Charlson Comorbidity Score-Category Range (CCSCR) | Charlson Comorbidity Score (CCS) |
|---|---|
| None | 0 |
| Mild | 1 or 2 |
| Moderate | 3 or 4 |
| Severe | ≥5 |
| Male | Female | Male | Female | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics | Total a | N (%) | N (%) | p-Value | Characteristics | Total | N (%) | N (%) | p-Value |
| Overall | N =114 | 74 (64.9) | 40 (35.1) | Overall | N = 114 | 74 (64.9) | 40 (35.1) | ||
| Age group 16–49 50–69.9 70–100 | 26 (22.8) 52 (45.6) 36 (31.6) | 18 (69.2) 36 (69.2) 20 (55.6) | 8 (30.8) 16 (30.8) 16 (44.4) | 0.364 b | Metastases Yes No | 58 (50.9) 56 (49.1) | 46 (79.3) 28 (50.0) | 12 (20.7) 28 (50.0) | 0.001 *b |
| Ethnicity Malay Chinese Indian | 102 (89.5) 6 (5.3) 6 (5.3) | 66 (64.7) 4 (66.7) 4 (66.7) | 36 (35.3) 2 (33.3) 2 (33.3) | 0.991 b | Metastasis site Liver Lungs Others None | 36 (31.6) 14 (12.3) 8 (7.0) 56 (49.1) | 28 (77.8) 12 (85.7) 6 (75.0) 28 (50.0) | 8 (22.2) 2 (14.3) 2 (25.0) 28 (50.0) | 0.011 *b |
| Occupation Working Retiree | 46 (40.4) 68 (59.6) | 34 (73.9) 40 (58.8) | 12 (26.1) 28 (41.2) | 0.098 b | Per rectal bleeding Yes No | 50 (43.9) 64 (56.1) | 34 (68.0) 40 (62.5) | 16 (32.0) 24 (37.5) | 0.541 b |
| Habitual Smoking Ex-smoker Non-smoker | 46 (40.4) 16 (14.0) 52 (45.6) | 44 (95.7) 10 (62.5) 20 (38.5) | 2 (4.3) 6 (37.5) 32 (61.5) | 0.001 *b | CEA level ≤ 5 > 5 | 42 (36.8) 72 (63.2) | 20 (47.6) 52 (72.2) | 22 (52.4) 20 (27.8) | 0.032 *b |
| Tumour site Left Right Rectum | 54 (47.4) 22 (19.3) 38 (33.3) | 20 (37.0) 16 (72.7) 38 (100) | 34 (63.0) 6 (27.3) 0 (0) | 0.001 *b | Treatment modality Surgery only Surgery + Chemo/Radio Surgery + Chemo + Radio Chemo or Radio | 32 (28.1) 62 (54.4) 14 (12.3) 6 (5.3) | 20 (62.5) 40 (64.5) 12 (85.7) 2 (33.3) | 12 (37.5) 22 (35.5) 2 (14.3) 4 (66.7) | 0.046*b |
| Duke staging Duke A Duke B Duke C Duke D | 6 (5.3) 22 (19.3) 76 (66.7) 10 (8.8) | 4 (66.7) 16 (72.7) 46 (60.5) 8 (80.00 | 2 (33.3) 6 (27.3) 30 (39.5) 2 (20.0) | 0.524 b | Comorbidity HTN DM Others None | 24 (21.1) 12 (10.5) 48 (42.1) 30 (26.3) | 14 (58.3) 10 (83.3) 26 (54.2) 24 (80.0) | 10 (41.7) 2 (16.7) 22 (45.8) 6 (20.0) | 0.053 *b |
| TNM stage Stage 1 Stage 2 Stage 3 Stage 4 | 2 (1.8) 22 (19.3) 78 (68.4) 12 (10.5) | 2 (100.0) 12 (54.5) 50 (64.1) 10 (83.3) | 0 (0) 10 (45.5) 28 (35.9) 2 (16.7) | 0.269 b | Comorbidity Number Single ≥2 None | 40 (35.1) 44 (38.6) 30 (26.3) | 28 (70.0) 22 (50.0) 24 (80.0) | 12 (30.0) 22 (50.0) 6 (20.0) | 0.021 *b |
| Tumour grading Well Moderately Poorly | 28 (24.6) 74 (64.9) 12 (10.5) | 22 (78.6) 42 (56.8) 10 (83.3) | 6 (21.4) 32 (43.2) 2 (16.7) | 0.044 *b | Family history Malignancy Other malignancy Adenomatous polyposis None | 28 (24.6) 20 (17.5) 4 (3.5) 62 (54.4) | 18 (64.3) 12 (60.0) 4 (100) 40 (64.5) | 10 (35.7) 8 (40.0) 0 (0) 22 (35.5) | 0.497 b |
| Survival status Dead Alive | 20 (17.5) 94 (82.5) | 16 (80.0) 56 (59.6) | 4 (20.0) 38 (40.4) | 0.172 b | Clinical features Abdominal Pain/cramp Abdominal Bloating/ distention Weight loss Loss of appetite/ anorexia Anaemia/Pale Tenesmus/constipation/ diarrhoea Bleeding per rectum Nausea/Vomiting/Fatigue Shortness of breath | 34 (9.4) 30 (8.3) 52 (14.4) 56 (8.3) 4 (1.1) 64 (17.8) 48 (13.3) 60 (16.7) 12 (3.3) | 20 (58.8) 16 (53.3) 15 (28.8) 22 (39.3) 1 (25) 40 (62.5) 36 (75) 22 (36.7) 5 (41.7) | 14 (41.2) 14 (46.7) 37 (71.2) 24 (42.9) 3 (75) 24 (37.5) 22 (25) 38 (63.3) 7 (58.3) | 0.043 *b |
| Tumour histology Adenocarcinoma Mucinous adenocarcinoma Signet-cell adenocarcinoma | 98 (86.0) 12 (10.5) 4 (3.5) | 58 (59.2) 12 (100) 4 (100) | 40 (40.8) 0 (0) 0 (0) | 0.007 *b | |||||
| Lymphovascular Invasion Present Absent | 70 (61.4) 44 (38.6) | 44 (62.9) 30 (68.2) | 26 (37.1) 14 (31.8) | 0.562 b |
| Variables | Survival Rate (%) | 95% Confidence Interval (CI) | p-Value | |
|---|---|---|---|---|
| Lower Limit | Upper Limit | |||
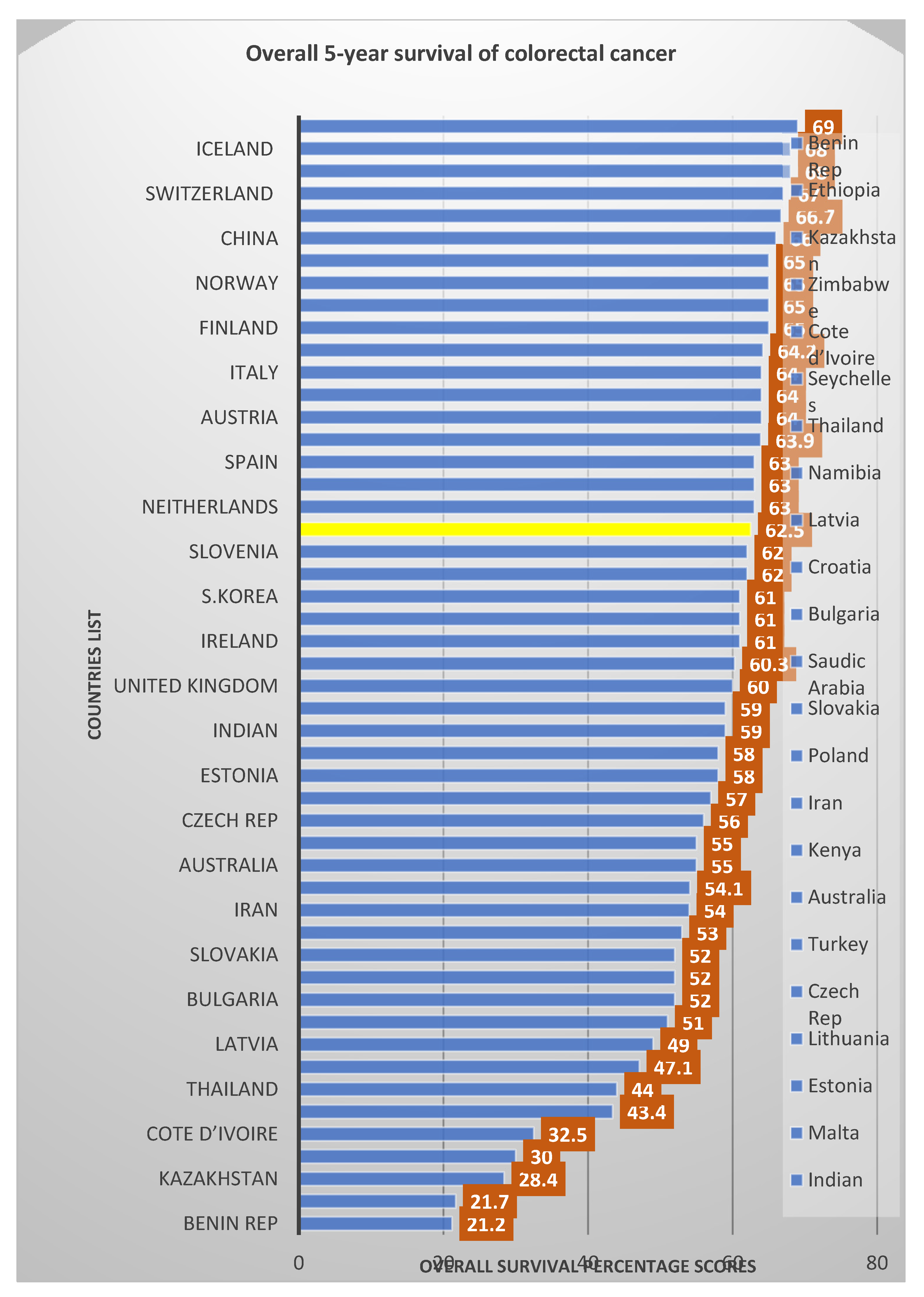
| Overall (62.5%) | ||||
| Gender Male Female | 62.1 67.0 | 48.992 59.519 | 58.129 68.581 | 0.026 * |
| Age group 50–69.9 70–100 | 57.6 54.1 | 51.942 47.541 | 63.224 60.590 | 0.366 |
| Metastases status Present Absent | 32.1 64.5 | 22.963 50.312 | 54.628 81.143 | 0.042 * |
| Per rectal bleeding No Yes | 52.7 42.8 | 28.9 29.5 | 73.631 59.391 | 0.041 * |
| Duke staging Duke B Duke C | 62.0 59.1 | 46.742 44.572 | 72.258 63.560 | 0.914 |
| Tumour site Left Right Rectum | 67.0 64.0 50.0 | 54.950 52.253 45.696 | 65.563 67.211 55.721 | - 0.754 0.058 |
| Factors | CHR (95% CI) | Wald | p-Value * | Factors | CHR (95% CI) | Wald | p-Value | AHR (95% CI) | p-Value *† |
|---|---|---|---|---|---|---|---|---|---|
| Age group 16–49 50–69.9 70–100 | 1 0.66 (0.25, 1.74) 0.28 (0.06, 1.25) | 2.78 0.69 | 0.095 0.405 | Gender Female Male | 1 4.68 (2.06, 12.81) | 4.20 | 0.020 | 1 2.62 (1.56, 9.81) | 0.040 *† |
| Ethnicity Indian Malay Chinese | 1 0.28 (0.03, 2.25) 0.00 (-) | 1.44 0.00 | 0.231 0.981 | Metastases No Yes | 1 4.60 (2.18, 10.94) | 5.10 | 0.024 | 1 3.76 (1.89, 7.32) | 0.010 *† |
| Occupation Retiree Working | 1 0.52 (0.20, 1.36) | 1.79 | 0.181 | Lymphovascular Invasion Absent Present | 1 3.09 (1.44, 8.75) | 0.04 | 0.043 | 1 2.94 (1.99, 5.92) | 0.021 *† |
| Habitual Non-smoker Smoking Ex-smoker | 1 1.90 (0.53, 4.30) 2.13 (0.68, 6.73) | 0.58 1.67 | 0.048 0.096 | CEA level ≤ 5 > 5 | 1 3.25 (1.99, 7.20) | 0.22 | 0.002 | 1 2.43 (1.49, 5.80) | 0.001 *† |
| Tumour grading Well Moderately Poorly | 1 1.04 (0.29, 3.70) 1.90 (0.23, 6.35) | 0.04 0.01 | 0.835 0.047 | Tumour site Rectum Left Right | 1 0.31 (0.10, 0.98) 0.34 (0.09, 1.24) | 3.99 2.69 | 0.046 0.101 | - | - |
| Duke staging Duke A Duke B Duke C Duke D | 1 1.44 (0.26, 8.01) 0.84 (0.19, 3.80) 1.78 (0.25, 12.86) | 0.32 0.17 0.05 | 0.680 0.818 0.570 | Metastasis site None Others Lungs Liver | 1 3.04 (1.93, 9.90) 4.94 (1.98, 20.36) 5.42 (1.03, 33.99) | 3.39 4.88 5..98 | 0.010 0.020 0.046 | 1 3.11 (1.46, 7.51) 4.42 (1.20, 12.36) 5.04 (1.71, 19.05) | 0.031 *† 0.020 *† 0.039 *† |
| TNM stage Stage 1 Stage 2 Stage 3 Stage 4 | 1 0.99 (0.18, 5.54) 1.05 (0.24, 4.70) 1.06 (0.09, 11.94) | 0.00 0.01 0.00 | 0.999 0.949 0.966 | Family history None Malignancy Other malignancy Adenomatous polyposis | 1 1.21 (0.48, 3.09) 0.00 (-) 3.04 (0.64, 14.40) | 0.16 0.00 1.97 | 0.686 0.979 0.161 | - | - |
| Comorbidity Type None HTN DM Others | 1 1.97 (0.55, 7.02) 0.00 (-) 1.02 (0.31, 3.35) | 1.10 0.00 0.00 | 0.295 0.988 0.981 | Treatment modality Chemo or Radio Surgery only Surgery + Chemo/Radio Surgery + Chemo + Radio | 1 0.53 (0.06, 4.66) 0.50 (0.06, 4.17) 1.67 (0.19, 14.60) | 0.33 0.41 0.21 | 0.568 0.520 0.645 | - | - |
| Comorbidity N None Single ≥2 | 1 1.17 (0.33, 4.18) 1.23 (0.38, 3.98) | 0.809 0.035 | Tumour Histopathology Unidentified Adenocarcinoma Mucinous adenocarcinoma Signet adenocarcinoma | 1 0.17 (1.95, 8.18) 0.23 (2.38, 10.98) 0.93 (2.38, 7.98) | 0.83 0.72 0.41 | 0.12 0.411 0.321 | - | - | |
| Per rectal bleeding No Yes | 1 1.06 (0.42, 2.68) | 0.02 | 0.901 |
| Variables | Male n (%) | Female n (%) | ᵡ (df) | p-Value |
|---|---|---|---|---|
| Charlson Comorbidity Index Score CCIS | 8.6 (7) | 0.373 | ||
| 0 | 9 (14.9) | 13 (24.5) | ||
| 1 | 6 (9.8) | 9 (17.0) | ||
| 2 | 13 (21.3) | 8 (15.1) | ||
| 3 | 9 (14.8) | 4 (7.5) | ||
| 4 | 10 (16.4) | 11 (20.8) | ||
| 5 | 9 (14.8) | 5 (9.4) | ||
| 6 | 5 (8.2) | 3 (5.7) |
| Variables | Male n (%) | Female n (%) | ᵡ (df) | p-Value |
|---|---|---|---|---|
| Charlson Comorbidity Score-Category Range (CCSCR) | 3.6 (3) | 0.434 | ||
| None (CCS: 0) | 9 (14.8) | 13 (24.5) | ||
| Mild (CCS: 1–2) | 19 (31.1) | 17 (32.1) | ||
| Moderate (3–4) | 19 (31.1) | 15 (28.3) | ||
| Severe (CCS:>5) | 14 (23.0) | 8 (15.1) |
| Charlson Comorbidity Score-Category Range (CCSCR) | Mean (SD) | F (df) | p-Value |
|---|---|---|---|
| None | 42.08 (13.48) | 0.92 (4.83) | 0.489 |
| Mild | 41.19 (7.29) | ||
| Moderate | 41.50 (6.57) | ||
| Severe | 44.84 (5.76) |
| Charlson Comorbidity Score-Category Range (CCSCR) | Mean (SD) | F (df) | p-Value |
|---|---|---|---|
| None | 33.34 (9.48) | 0.74 (3.63) | 0.050 |
| Mild | 35.76 (6.19) | ||
| Moderate | 38.37 (5.46) | ||
| Severe | 40.52 (4.90) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afolabi, H.; Salleh, S.M.; Zakaria, Z.; Ch’ng, E.S.; Mohd Nafi, S.N.; Abdul Aziz, A.A.B.; Al-Mhanna, S.B.; Wada, Y.; Abdulrahman, A.S. The Prediction of Survival Outcome and Prognosis Factor in Association with Comorbidity Status in Patients with Colorectal Cancer: A Research-Based Study. Healthcare 2022, 10, 1693. https://doi.org/10.3390/healthcare10091693
Afolabi H, Salleh SM, Zakaria Z, Ch’ng ES, Mohd Nafi SN, Abdul Aziz AAB, Al-Mhanna SB, Wada Y, Abdulrahman AS. The Prediction of Survival Outcome and Prognosis Factor in Association with Comorbidity Status in Patients with Colorectal Cancer: A Research-Based Study. Healthcare. 2022; 10(9):1693. https://doi.org/10.3390/healthcare10091693
Chicago/Turabian StyleAfolabi, Hafeez, Salzihan Md Salleh, Zaidi Zakaria, Ewe Seng Ch’ng, Siti Norasikin Mohd Nafi, Ahmad Aizat Bin Abdul Aziz, Sameer Badri Al-Mhanna, Yusuf Wada, and Abdulwali Sabo Abdulrahman. 2022. "The Prediction of Survival Outcome and Prognosis Factor in Association with Comorbidity Status in Patients with Colorectal Cancer: A Research-Based Study" Healthcare 10, no. 9: 1693. https://doi.org/10.3390/healthcare10091693






