Conduction Velocity of Spinal Reflex in Patients with Acute Lateral Ankle Sprain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Conduction Velocity of Spinal Reflex
2.3. Statistical Analysis
3. Results
3.1. Participant Characteristics
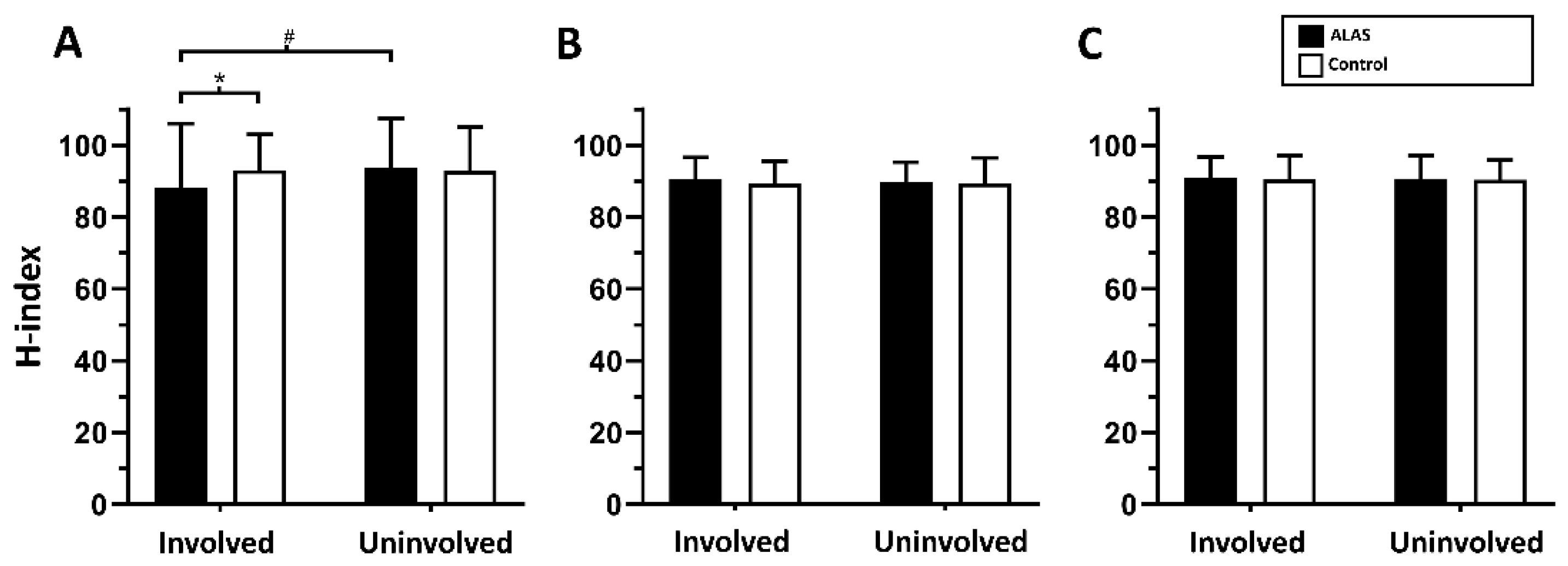
3.2. Conduction Velocity of Spinal Reflex Response Following ALAS
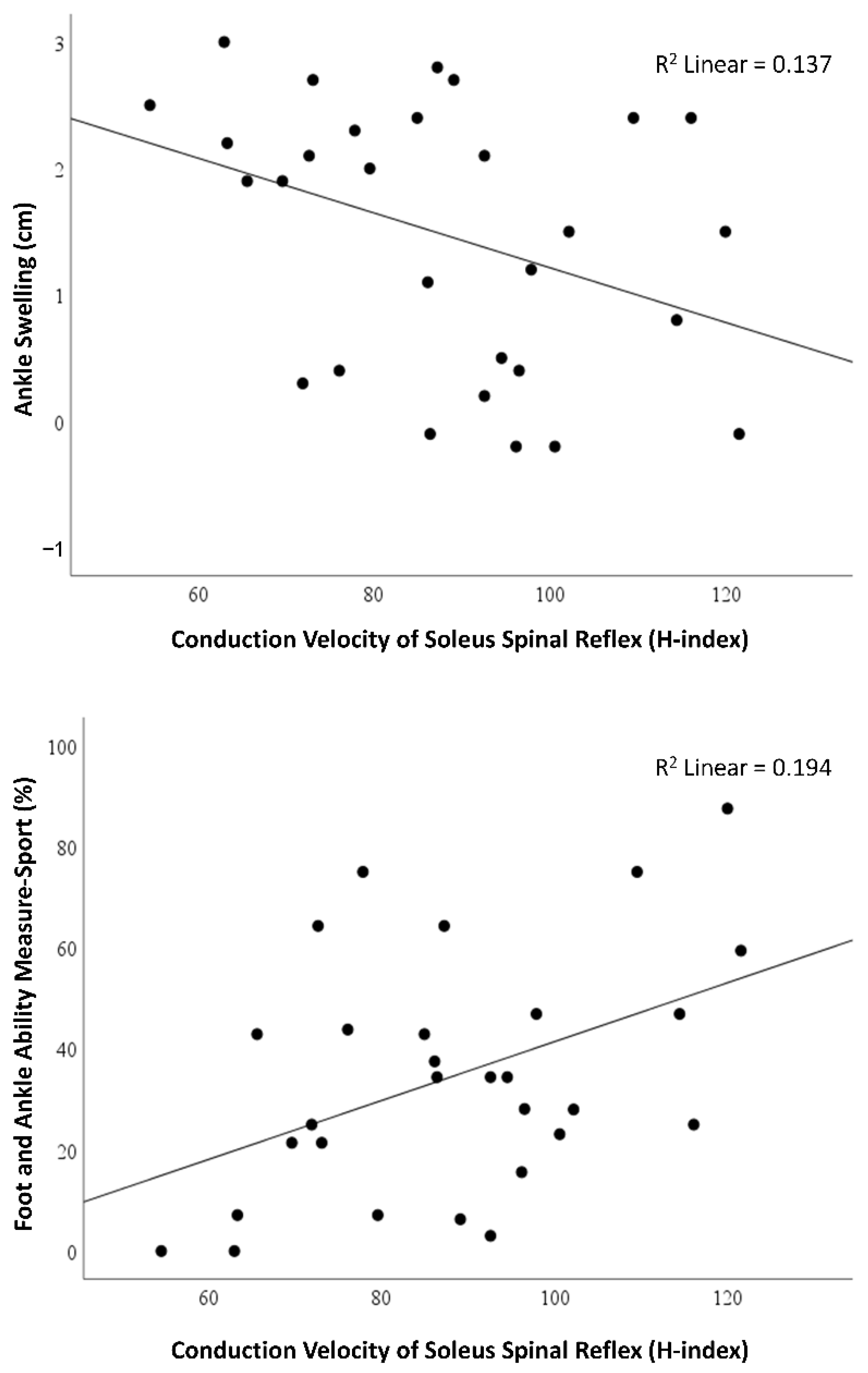
3.3. Relationships of Soleus Conduction Velocity of Spinal Reflex with Acute Symptoms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Herzog, M.M.; Kerr, Z.Y.; Marshall, S.W.; Wikstrom, E.A. Epidemiology of Ankle Sprains and Chronic Ankle Instability. J. Athl. Train. 2019, 54, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Holland, B.; Needle, A.R.; Battista, R.A.; West, S.T.; Christiana, R.W. Physical activity levels among rural adolescents with a history of ankle sprain and chronic ankle instability. PLoS ONE 2019, 14, e0216243. [Google Scholar] [CrossRef] [PubMed]
- Houston, M.N.; Hoch, J.M.; Hoch, M.C. Patient-Reported Outcome Measures in Individuals with Chronic Ankle Instability: A Systematic Review. J. Athl. Train. 2015, 50, 1019–1033. [Google Scholar] [CrossRef] [PubMed]
- Hubbard-Turner, T.; Turner, M.J. Physical Activity Levels in College Students with Chronic Ankle Instability. J. Athl. Train. 2015, 50, 742–747. [Google Scholar] [CrossRef]
- Wikstrom, E.; Song, K.; Tennant, J.; Dederer, K.; Paranjape, C.; Pietrosimone, B. T1ρ MRI of the talar articular cartilage is increased in those with chronic ankle instability. Osteoarthr. Cartil. 2019, 27, 646–649. [Google Scholar] [CrossRef]
- Needle, A.R.; Lepley, A.S.; Grooms, D. Central Nervous System Adaptation After Ligamentous Injury: A Summary of Theories, Evidence, and Clinical Interpretation. Sports Med. 2016, 47, 1271–1288. [Google Scholar] [CrossRef]
- Kim, K.-M.; Kim, J.-S.; Cruz-Díaz, D.; Ryu, S.; Kang, M.; Taube, W. Changes in Spinal and Corticospinal Excitability in Patients with Chronic Ankle Instability: A Systematic Review with Meta-Analysis. J. Clin. Med. 2019, 8, 1037. [Google Scholar] [CrossRef]
- Knikou, M. The H-reflex as a probe: Pathways and pitfalls. J. Neurosci. Methods 2008, 171, 1–12. [Google Scholar] [CrossRef]
- Hall, R.C.; Nyland, J.; Nitz, A.J.; Pinerola, J.; Johnson, D.L. Relationship Between Ankle Invertor H-Reflexes and Acute Swelling Induced by Inversion Ankle Sprain. J. Orthop. Sports Phys. Ther. 1999, 29, 339–344. [Google Scholar] [CrossRef]
- Klykken, L.W.; Pietrosimone, B.G.; Kim, K.-M.; Ingersoll, C.D.; Hertel, J. Motor-Neuron Pool Excitability of the Lower Leg Muscles After Acute Lateral Ankle Sprain. J. Athl. Train. 2011, 46, 263–269. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-S.; Kim, K.-M.; Chang, E.; Jung, H.C.; Lee, J.-M.; Needle, A.R. Spinal Reflex Excitability of Lower Leg Muscles Following Acute Lateral Ankle Sprain: Bilateral Inhibition of Soleus Spinal Reflex Excitability. Healthcare 2022, 10, 1171. [Google Scholar] [CrossRef] [PubMed]
- Pietrosimone, B.; Lepley, A.S.; Kuenze, C.; Harkey, M.S.; Hart, J.M.; Blackburn, J.T.; Norte, G. Arthrogenic Muscle Inhibition Following Anterior Cruciate Ligament Injury. J. Sport Rehabil. 2022, 1, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Guiheneuc, P.; Bathien, N. Two patterns of results in polyneuropathies investigated with the H reflex: Correlation between proximal and distal conduction velocities. J. Neurol. Sci. 1976, 30, 83–94. [Google Scholar] [CrossRef]
- Scaglioni, G.; Ferri, A.; Minetti, A.E.; Martin, A.; Van Hoecke, J.; Capodaglio, P.; Sartorio, A.; Narici, M.V. Plantar flexor activation capacity and H reflex in older adults: Adaptations to strength training. J. Appl. Physiol. 2002, 92, 2292–2302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Manor, B.; Li, L. H-Index Is Important for Postural Control for People with Impaired Foot Sole Sensation. PLoS ONE 2015, 10, e0121847. [Google Scholar] [CrossRef]
- Hoch, M.C.; McKeon, P. Peroneal Reaction Time after Ankle Sprain: A systematic review and meta-analysis. Med. Sci. Sports Exerc. 2014, 46, 546–556. [Google Scholar] [CrossRef]
- Tatro-Adams, D.; McGann, S.F.; Carbone, W. Reliability of the Figure-of-Eight Method of Ankle Measurement. J. Orthop. Sports Phys. Ther. 1995, 22, 161–163. [Google Scholar] [CrossRef]
- Delgado, D.A.; Lambert, B.S.; Boutris, N.; McCulloch, P.C.; Robbins, A.B.; Moreno, M.R.; Harris, J.D. Validation of Digital Visual Analog Scale Pain Scoring with a Traditional Paper-based Visual Analog Scale in Adults. JAAOS Glob. Res. Rev. 2018, 2, e088. [Google Scholar] [CrossRef]
- Martin, R.L.; Irrgang, J.J.; Burdett, R.G.; Conti, S.F.; Van Swearingen, J.M. Evidence of Validity for the Foot and Ankle Ability Measure (FAAM). Foot Ankle Int. 2005, 26, 968–983. [Google Scholar] [CrossRef]
- Hertel, J.; Braham, R.A.; Hale, S.A.; Olmsted-Kramer, L.C. Simplifying the Star Excursion Balance Test: Analyses of Subjects With and Without Chronic Ankle Instability. J. Orthop. Sports Phys. Ther. 2006, 36, 131–137. [Google Scholar] [CrossRef] [Green Version]
- Zehr, E.P. Considerations for use of the Hoffmann reflex in exercise studies. Eur. J. Appl. Physiol. 2002, 86, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Association: Hillsdale, MI, USA, 1988. [Google Scholar]
- Troni, W. Analysis of conduction velocity in the H pathway: Part 1. Methodology and results in normal subjects. J. Neurol. Sci. 1981, 51, 223–233. [Google Scholar] [CrossRef]
- Kleinrensink, G.J.; Stoeckart, R.; Meulstee, J.; Kaulesar Sukul, D.M.; Vleeming, A.; Snijders, C.J.; van Noort, A. Lowered motor conduction velocity of the peroneal nerve after inversion trauma. Medicine and science in sports and exercise. Med. Sci. Sports Exerc. 1994, 26, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Nitz, A.J.; Dobner, J.J.; Kersey, D. Nerve injury and Grades II and III ankle sprains. Am. J. Sports Med. 1985, 13, 177–182. [Google Scholar] [CrossRef]
- Kaplan, P.E. Sensory and motor residual latency measurements in helathy patients and patients with neuropathy-part 1. J. Neurol. Neurosurg. Psychiatry 1976, 39, 338–340. [Google Scholar] [CrossRef]
- Colak, T.; Bamaç, B.; Alemdar, M.; Selekler, H.M.; Ozbek, A.; Colak, S.; Dinçer, O. Nerve conduction studies of the axillary, musculocutaneous and radial nerves in elite ice hockey players. J. Sports Med. Phys. Fit. 2009, 49, 224–231. [Google Scholar]
- Hortobagyi, T.; Tracy, J.; Hamilton, G.; Lambert, J. Fatigue Effects on Muscle Excitability. Endoscopy 1996, 17, 409–414. [Google Scholar] [CrossRef]
- Palmieri, R.M.; Ingersoll, C.D.; Hoffman, M.A.; Cordova, M.L.; Porter, D.A.; Edwards, J.E.; Babington, J.P.; Krause, B.A.; Stone, M.B. Arthrogenic muscle response to a simulated ankle joint effusion. Br. J. Sports Med. 2004, 38, 26–30. [Google Scholar] [CrossRef]
- Deandrade, J.R.; Grant, C.; Dixon, A.S.J. Joint Distension and Reflex Muscle Inhibition in the Knee. J. Bone Jt. Surg. 1965, 47, 313–322. [Google Scholar] [CrossRef]
- Spencer, J.D.; Hayes, K.C.; Alexander, I.J. Knee joint effusion and quadriceps reflex inhibition in man. Arch. Phys. Med. Rehabil. 1984, 65, 171–177. [Google Scholar]
- Rice, D.A.; McNair, P.J. Quadriceps Arthrogenic Muscle Inhibition: Neural Mechanisms and Treatment Perspectives. Semin. Arthritis Rheum. 2010, 40, 250–266. [Google Scholar] [CrossRef]
- Burke, D. Clinical uses of H reflexes of upper and lower limb muscles. Clin. Neurophysiol. Pr. 2016, 1, 9–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wikstrom, E.A.; Naik, S.; Lodha, N.; Cauraugh, J.H. Bilateral balance impairments after lateral ankle trauma: A systematic review and meta-analysis. Gait Posture 2010, 31, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Evans, T.; Hertel, J.; Sebastianelli, W. Bilateral Deficits in Postural Control following Lateral Ankle Sprain. Foot Ankle Int. 2004, 25, 833–839. [Google Scholar] [CrossRef]
- Chantanachai, T.; Sturnieks, D.L.; Lord, S.R.; Payne, N.; Webster, L.; Taylor, M.E. Risk factors for falls in older people with cognitive impairment living in the community: Systematic review and meta-analysis. Ageing Res. Rev. 2021, 71, 101452. [Google Scholar] [CrossRef] [PubMed]
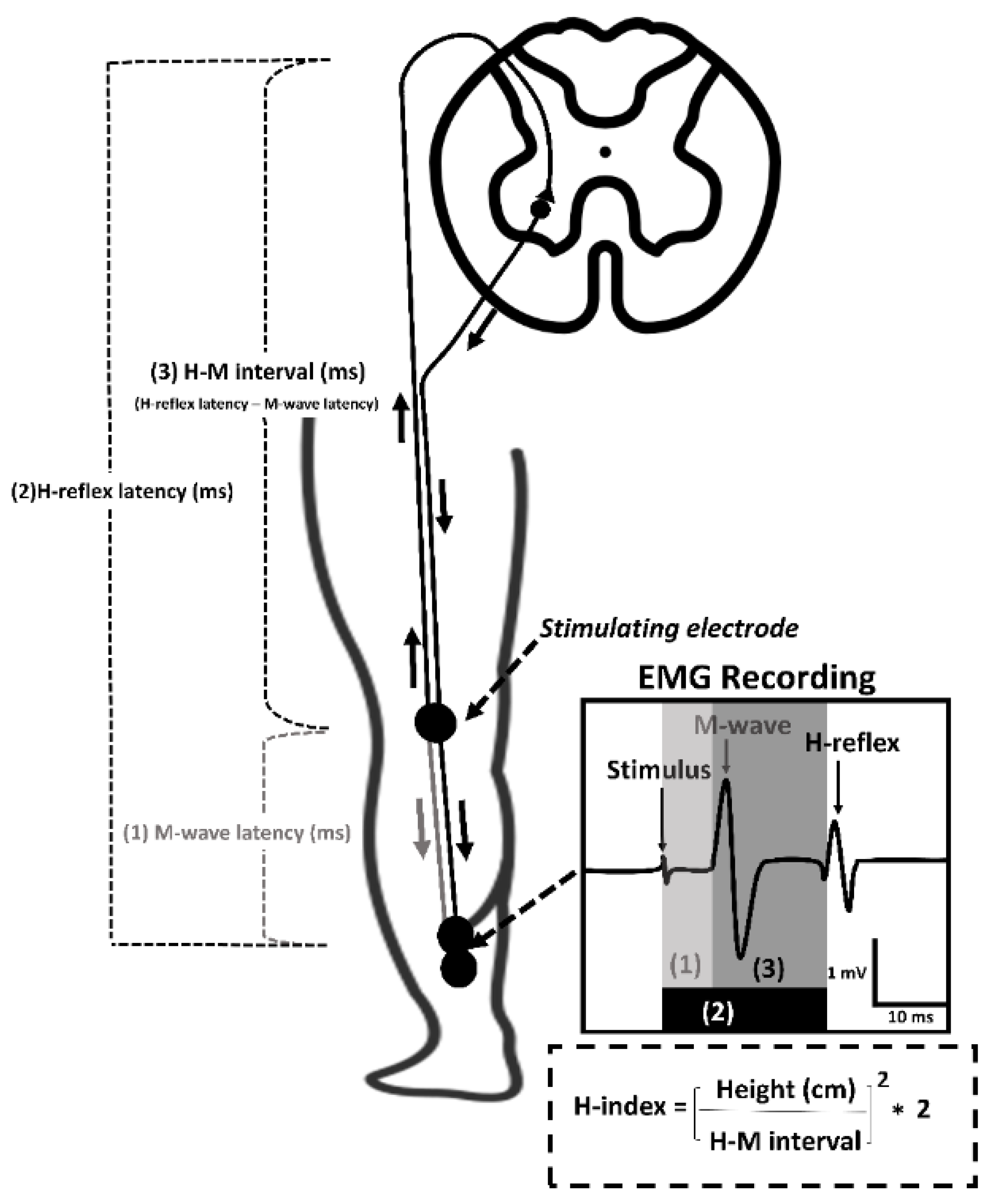
| Muscle | Latency (ms) | ALAS | Control | ||
|---|---|---|---|---|---|
| Involved | Uninvolved | Involved | Uninvolved | ||
| Soleus | H-reflex latency | 36.5 ± 3.9 *† | 35.4 ± 3.3 | 35.3 ± 3.6 | 35.3 ± 3.6 |
| M-wave latency | 9.7 ± 1.1 | 9.6 ± 1.1 | 9.7 ± 1.6 | 9.7 ± 1.7 | |
| Fibularis longus | H-reflex latency | 31.7 ± 2.4 | 32.0 ± 2.4 | 31.5 ± 2.0 | 31.6 ± 1.9 |
| M-wave latency | 5.7 ± 0.9 | 5.7 ± 0.9 | 5.6 ± 0.7 | 5.7 ± 0.7 | |
| Tibialis anterior | H-reflex latency | 32.9 ± 2.0 | 33.0 ± 1.9 | 33.0 ± 2.6 | 33.0 ± 2.6 |
| M-wave latency | 6.8 ± 0.8 | 6.8 ± 0.9 | 7.0 ± 1.3 | 7.0 ± 1.3 | |
| Soleus H-Index a | ||
|---|---|---|
| r (95% Confidence Interval) | p | |
| Ankle swelling (cm) | −0.371 * (−0.649 to −0.005) | 0.048 |
| VAS score for pain (cm) | 0.148 (−0.231 to 0.488) | 0.443 |
| FAAM-ADL (%) | 0.167 (−0.213 to 0.503) | 0.387 |
| FAAM-Sport (%) | 0.440 * (0.088 to 0.695) | 0.017 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-S.; Kim, K.-M.; Chang, E.; Jung, H.C.; Lee, J.-M.; Needle, A.R. Conduction Velocity of Spinal Reflex in Patients with Acute Lateral Ankle Sprain. Healthcare 2022, 10, 1794. https://doi.org/10.3390/healthcare10091794
Kim J-S, Kim K-M, Chang E, Jung HC, Lee J-M, Needle AR. Conduction Velocity of Spinal Reflex in Patients with Acute Lateral Ankle Sprain. Healthcare. 2022; 10(9):1794. https://doi.org/10.3390/healthcare10091794
Chicago/Turabian StyleKim, Joo-Sung, Kyung-Min Kim, Eunwook Chang, Hyun Chul Jung, Jung-Min Lee, and Alan R. Needle. 2022. "Conduction Velocity of Spinal Reflex in Patients with Acute Lateral Ankle Sprain" Healthcare 10, no. 9: 1794. https://doi.org/10.3390/healthcare10091794
APA StyleKim, J.-S., Kim, K.-M., Chang, E., Jung, H. C., Lee, J.-M., & Needle, A. R. (2022). Conduction Velocity of Spinal Reflex in Patients with Acute Lateral Ankle Sprain. Healthcare, 10(9), 1794. https://doi.org/10.3390/healthcare10091794








