Development of a Digital Healthcare Management System for Lower-Extremity Amputees: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Digital Healthcare Management System for Lower-Limb Amputees
2.2. Participants
2.3. Intervention
2.4. Measurement Methods and Items
2.5. Clinical Data
2.5.1. Muscle Strength
2.5.2. Thigh Circumference Test
2.5.3. Lipid Profile
2.5.4. Glycated Hemoglobin Examination
2.5.5. Pulmonary Functional Test
2.6. Self-Reported Measures
2.6.1. Quality of Life
2.6.2. Amount of Physical Activity
2.6.3. Satisfaction Survey on the Digital Healthcare Management System
2.6.4. Adherence
2.6.5. Data and Statistical Analysis
3. Results
3.1. Clinical Data
3.2. Self-Reported Measures
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Escamilla-Nunez, R.; Michelini, A.; Andrysek, J. Biofeedback Systems for Gait Rehabilitation of Individuals with Lower-Limb Amputation: A Systematic Review. Sensors 2020, 20, 1628. [Google Scholar] [CrossRef] [Green Version]
- Rietman, J.S.; Postema, K.; Geertzen, J.H. Gait Analysis in Prosthetics: Opinions, Ideas and Conclusions. Prosthet. Orthot. Int. 2002, 26, 50–57. [Google Scholar] [CrossRef] [Green Version]
- Sansam, K.; O’Connor, R.J.; Neumann, V.; Bhakta, B. Clinicians’ Perspectives on Decision Making in Lower Limb Amputee Rehabilitation. J. Rehabil. Med. 2014, 46, 447–453. [Google Scholar] [CrossRef] [Green Version]
- Naschitz, J.E.; Lenger, R. Why Traumatic Leg Amputees Are at Increased Risk for Cardiovascular Diseases. QJM Int. J. Med. 2008, 101, 251–259. [Google Scholar] [CrossRef]
- Esquenazi, A.; DiGiacomo, R. Rehabilitation After Amputation. J. Am. Podiatr. Med. Assoc. 2001, 91, 13–22. [Google Scholar] [CrossRef]
- Dang, A.; Arora, D.; Rane, P. Role of digital therapeutics and the changing future of healthcare. J. Fam. Med. Prim. Care 2020, 9, 2207. [Google Scholar] [CrossRef]
- Patel, N.A.; Butte, A.J. Characteristics and challenges of the clinical pipeline of digital therapeutics. NPJ Digit. Med. 2020, 3, 159. [Google Scholar] [CrossRef]
- Abbadessa, G.; Brigo, F.; Clerico, M.; De Mercanti, S.; Trojsi, F.; Tedeschi, G.; Bonavita, S.; Lavorgna, L. Digital therapeutics in neurology. J. Neurol. 2022, 269, 1209–1224. [Google Scholar] [CrossRef]
- Carl, J.R.; Jones, D.; Lindhiem, O.; Doss, B.; Weingardt, K.; Timmons, A.; Comer, J.S. Regulating digital therapeutics for mental health: Opportunities, challenges, and the essential role of psychologists. Br. J. Clin. Psychol. 2022, 61, 130–135. [Google Scholar] [CrossRef]
- Ellis, T.D.; Earhart, G.M. Digital therapeutics in parkinson’s disease: Practical applications and future potential. J. Park. Dis. 2021, 11, S95–S101. [Google Scholar] [CrossRef]
- Parcher, B.; Coder, M. Decision makers need an approach to determine digital therapeutic product quality, access, and appropriate use. J. Manag. Care Spec. Pharm. 2021, 27, 536–538. [Google Scholar] [CrossRef]
- Wellbeloved-Stone, C.A.; Weppner, J.L.; Valdez, R.S. A systematic review of telerehabilitation and mHealth interventions for spinal cord injury. Curr. Phys. Med. Rehabil. Rep. 2016, 4, 295–311. [Google Scholar] [CrossRef]
- Tanaka, H.; Monahan, K.D.; Seals, D.R. Age-predicted maximal heart rate revisited. J. Am. Coll. Cardiol. 2001, 37, 153–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Smet, L.; Fabry, G. Grip Force Reduction in Patients with Tennis Elbow: Influence of Elbow Position. J. Hand Ther. 1997, 10, 229–231. [Google Scholar] [CrossRef] [PubMed]
- McRae, R. Pocketbook of Orthopaedics and Fractures; Elsevier Health Sciences: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Ko, S.-H. 2021 Clinical Practice Guidelines for Diabetes Mellitus in Korea. J. Korean Diabetes 2021, 22, 244–249. [Google Scholar] [CrossRef]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef] [PubMed]
- Janssen, M.F.; Pickard, A.S.; Golicki, D.; Gudex, C.; Niewada, M.; Scalone, L.; Swinburn, P.; Busschbach, J. Measurement Properties of the EQ-5D-5L Compared to the EQ-5D-3L Across Eight Patient Groups: A Multi-country Study. Qual. Life Res. 2013, 22, 1717–1727. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Kim, H.J.; Lee, S.-I.; Jo, M.-W. Comparing the psychometric properties of the eq-5d-3l and eq-5d-5l in cancer patients in Korea. Qual. Life Res. 2012, 21, 1065–1073. [Google Scholar] [CrossRef]
- Lee, P.H.; Macfarlane, D.J.; Lam, T.H.; Stewart, S.M. Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): A Systematic Review. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 115. [Google Scholar] [CrossRef] [Green Version]
- Chun, M.Y. Validity and reliability of Korean version of international physical activity questionnaire short form in the elderly. Korean J. Fam. Med. 2012, 33, 144. [Google Scholar] [CrossRef]
- Ito, T.; Sugiura, H.; Ito, Y.; Noritake, K.; Ochi, N. Relationship Between the Skeletal Muscle Mass Index and Physical Activity of Japanese Children: A Cross-Sectional, Observational Study. PLoS ONE 2021, 16, e0251025. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.J.; Piasecki, M.; Atherton, P.J. The Age-Related Loss of Skeletal Muscle Mass and Function: Measurement and Physiology of Muscle Fibre Atrophy and Muscle Fibre Loss in Humans. Ageing Res. Rev. 2018, 47, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Deeg, D.J.; Lips, P.; Harris, T.B.; Bouter, L.M. Skeletal Muscle Mass and Muscle Strength in Relation to Lower-Extremity Performance in Older Men and Women. J. Am. Geriatr. Soc. 2000, 48, 381–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, M.; Kim, H.; Oh, B. Comparison of hand–grip strength between normal korean adults and those with type 2 diabetes: 2014-2015 korea national health and nutrition examination survey. Korean J. Fam. Pract. 2018, 8, 654–661. [Google Scholar] [CrossRef]
- Schafer, Z.A.; Perry, J.L.; Vanicek, N. A Personalised Exercise Programme for Individuals with Lower Limb Amputation Reduces Falls and Improves Gait Biomechanics: A Block Randomised Controlled Trial. Gait Posture 2018, 63, 282–289. [Google Scholar] [CrossRef]
- Yi, D.; Yim, J. Remote Home-Based Exercise Program to Improve the Mental State, Balance, and Physical Function and Prevent Falls in Adults Aged 65 Years and Older During the COVID-19 Pandemic in Seoul, Korea. Med. Sci. Monit. 2021, 27, e935496. [Google Scholar] [CrossRef]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P.; American College of Sports Medicine. American College of Sports Medicine Position Stand. Quantity and Quality of Exercise for Developing and Maintaining Cardiorespiratory, Musculoskeletal, and Neuromotor Fitness in Apparently Healthy Adults: Guidance for Prescribing Exercise. Med. Sci. Sports Exer. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- Lavie, C.J.; Arena, R.; Swift, D.L.; Johannsen, N.M.; Sui, X.; Lee, D.C.; Earnest, C.P.; Church, T.S.; O’Keefe, J.H.; Milani, R.V.; et al. Exercise and the Cardiovascular System: Clinical Science and Cardiovascular Outcomes. Circ. Res. 2015, 117, 207–219. [Google Scholar] [CrossRef] [Green Version]
- Durstine, J.L.; Grandjean, P.W.; Davis, P.G.; Ferguson, M.A.; Alderson, N.L.; DuBose, K.D. Blood Lipid and Lipoprotein Adaptations to Exercise: A Quantitative Analysis. Sport. Med. 2001, 31, 1033–1062. [Google Scholar] [CrossRef]
- Schellenberg, E.S.; Dryden, D.M.; Vandermeer, B.; Ha, C.; Korownyk, C. Lifestyle Interventions for Patients with and At Risk for Type 2 Diabetes: A Systematic Review and Meta-analysis. Ann. Intern. Med. 2013, 159, 543–551. [Google Scholar] [CrossRef] [Green Version]
- Muscella, A.; Stefàno, E.; Marsigliante, S. The Effects of Exercise Training on Lipid Metabolism and Coronary Heart Disease. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H76–H88. [Google Scholar] [CrossRef] [PubMed]
- Bergman, B.C.; Butterfield, G.E.; Wolfel, E.E.; Casazza, G.A.; Lopaschuk, G.D.; Brooks, G.A. Evaluation of Exercise and Training on Muscle Lipid Metabolism. Am. J. Physiol. 1999, 276, E106–E117. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, J.F.; Klein, S. Lipid Metabolism During Endurance Exercise. Am. J. Clin. Nutr. 2000, 72, 558S–563S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LeMura, L.M.; von Duvillard, S.P.; Andreacci, J.; Klebez, J.M.; Chelland, S.A.; Russo, J. Lipid and Lipoprotein Profiles, Cardiovascular Fitness, Body Composition, and Diet During and After Resistance, Aerobic and Combination Training in Young Women. Eur. J. Appl. Physiol. 2000, 82, 451–458. [Google Scholar] [CrossRef]
- Sunami, Y.; Motoyama, M.; Kinoshita, F.; Mizooka, Y.; Sueta, K.; Matsunaga, A.; Sasaki, J.; Tanaka, H.; Shindo, M. Effects of Low-Intensity Aerobic Training on the High-Density Lipoprotein Cholesterol Concentration in Healthy Elderly Subjects. Metabolism 1999, 48, 984–988. [Google Scholar] [CrossRef]
- Kim, J.W.; Ko, Y.C.; Seo, T.B.; Kim, Y.P. Effect of Circuit Training on Body Composition, Physical Fitness, and Metabolic Syndrome Risk Factors in Obese Female College Students. J. Exer. Rehabil. 2018, 14, 460–465. [Google Scholar] [CrossRef]
- Connelly, J.; Kirk, A.; Masthoff, J.; MacRury, S. The Use of Technology to Promote Physical Activity in Type 2 Diabetes Management: A Systematic Review. Diabet. Med. 2013, 30, 1420–1432. [Google Scholar] [CrossRef]
- Qiu, S.; Cai, X.; Chen, X.; Yang, B.; Sun, Z. Step Counter Use in Type 2 Diabetes: A Meta-analysis of Randomized Controlled Trials. BMC Med. 2014, 12, 36. [Google Scholar] [CrossRef] [Green Version]
- Cotes, J.E.; Chinn, D.J.; Miller, M.R. Lung Function: Physiology, Measurement and Application in Medicine; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Harms, C.A. Does Gender Affect Pulmonary Function and Exercise Capacity? Respir. Physiol. Neurobiol. 2006, 151, 124–131. [Google Scholar] [CrossRef]
- Stanghelle, J.K.; Hjeltnes, N.; Bangstad, H.J.; Michalsen, H. Effect of Daily Short Bouts of Trampoline Exercise During 8 Weeks on the Pulmonary Function and the Maximal Oxygen Uptake of Children with Cystic Fibrosis. Int. J. Sport. Med. 1988, 9 (Suppl. S1), 32–36. [Google Scholar] [CrossRef]
- Jang, W.H.; Lee, S.B.; Kim, D.W.; Lee, Y.H.; Uhm, Y.J.; Yang, S.W.; Kim, J.H.; Kim, J.B. ICT-Based Health Care Services for Individuals with Spinal Cord Injuries: A Feasibility Study. Sensors 2020, 20, 2491. [Google Scholar] [CrossRef] [PubMed]
- Group, W. The World Health Organization Quality of Life Assessment (WHOQOL): Position Paper from the World Health Organization. Soc. Sci. Med. 1995, 41, 1403–1409. [Google Scholar] [CrossRef]
- Grzebień, A.; Chabowski, M.; Malinowski, M.; Uchmanowicz, I.; Milan, M.; Janczak, D. Analysis of Selected Factors Determining Quality of Life in Patients After Lower Limb Amputation-A Review Article. Pol. Przegl. Chir. 2017, 89, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Kim, A.R.; Yang, M.A.; Lim, S.J.; Park, J.H. Impact of the COVID-19 Pandemic on the Lifestyle, Mental Health, and Quality of Life of Adults in South Korea. PLoS ONE 2021, 16, e0247970. [Google Scholar] [CrossRef]
- DiMatteo, M.R. Variations in Patients’ Adherence to Medical Recommendations: A Quantitative Review of 50 Years of Research. Med. Care 2004, 42, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.R.; Williams, S.L.; Haskard, K.B.; DiMatteo, M.R. The challenge of patient adherence. Ther. Clin. Risk. Manag. 2005, 1, 189. [Google Scholar] [PubMed]

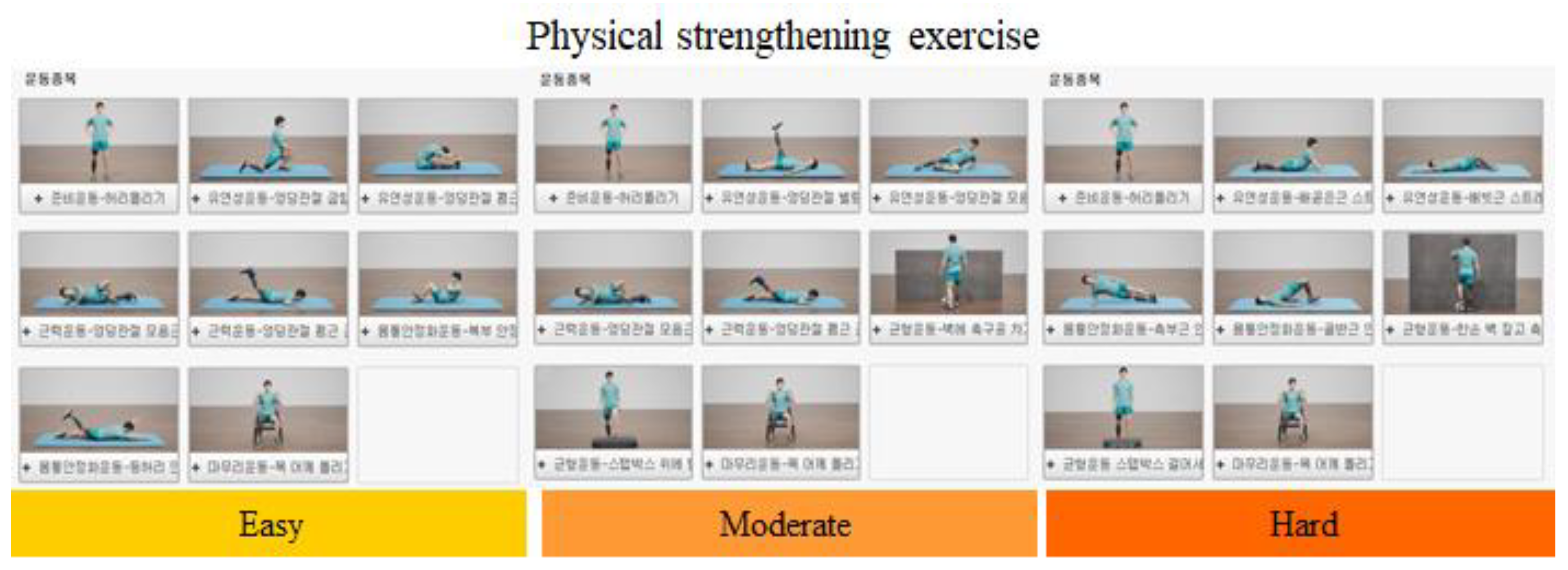
| No. | Type | Grade | Exercise Sequence |
|---|---|---|---|
| 1 | AK | Easy | 1. Twisting of waist, 2. Hip flexor stretching, 3. Hip extensor stretching, 4. Hip adductor strengthening exercise, 5. Hip abductor strengthening exercise, 6. Sit-up, 7. Superman back extension, 8. Twisting of neck and shoulders. |
| 2 | AK | Moderate | 1.Twisting of waist, 2. Hip abductor stretching, 3. Hip adductor stretching, 4. Hip extensor strengthening exercise, 5. Knee extensor strengthening exercise, 6. Kicking and rolling a ball, 7. Box step up and down, 8. Twisting of neck and shoulders. |
| 3 | AK | Hard | 1. Twisting of waist, 2. Rectus abdominis stretching, 3. Internal/external oblique muscle stretching, 4. Side plank, 5. Bridge exercise, 6. Balance training with a ball, 7. Box step up and down, 8. Twisting of neck and shoulders. |
| 4 | BK | Easy | 1. Twisting of waist, 2. Hip flexor stretching, 3. Hip extensor stretching, 4. Hip adductor strengthening exercise, 5. Hip extensor strengthening exercise, 6. Sit-up, 7. Superman back extension, 8. Twisting of neck and shoulders. |
| 5 | BK | Moderate | 1. Twisting of waist, 2. Hip abductor stretching, 3. Hip adductor stretching, 4. Hip extensor strengthening, 5. Knee extensor strengthening, 6. Kicking and rolling a ball, 7. Box step up and down, 8. Twisting of neck and shoulders. |
| 6 | BK | Hard | 1. Twisting of waist, 2. Knee flexor stretching, 3. Knee extensor stretching, 4. Side plank, 5. Bridge exercise, 6. Balance training with a ball, 7. Box jump up and down, 8. Twisting of neck and shoulders. |
| Baseline Variable | M ± SD or Frequency (%) |
|---|---|
| Age (years) | 46.1 ± 10.3 |
| Sex (female/male) | 0/14 |
| Weight (kg) | 73.4 ± 14.4 |
| Height (cm) | 170.1 ± 3.6 |
| BMI (kg/m2) | 29.1 ± 9.3 |
| Level of injury (%) | AK: 6 (42.9)/BK: 8 (57.1) |
| K-level (%) | Level 2: 10 (71.4)/Level 3: 4 (28.6) |
| Onset duration (days) | 676.5 ± 253.5 |
| Measures | Baseline | Post-Intervention | t-Value | p-Value |
|---|---|---|---|---|
| Muscle strength test | ||||
| Knee flexor (N) | 162.1 ± 60.4 | 189.9 ± 41.4 | 3.15 | 0.00 * |
| Knee extensor (N) | 266.4 ± 35.9 | 309.1 ± 27.8 | 5.20 | 0.00 * |
| Grip power (N) | 45.9 ± 87.1 | 47.6 ± 7.8 | 3.11 | 0.00 * |
| Thigh circumference (cm) | 45.9 ± 87.1 | 47.6 ± 7.8 | 3.11 | 0.00 * |
| Lipid test | ||||
| Total cholesterol (mg/dL) | 205.1 ± 24.9 | 202.0 ± 27.3 | −0.44 | 0.66 |
| HDL (mg/dL) | 48.9 ± 11.8 | 51.9 ± 15.2 | 1.71 | 0.11 |
| LDL (mg/dL) | 127.5 ± 19.3 | 122.2 ± 20.4 | −1.08 | 0.29 |
| TG (mg/dL) | 209.9 ± 125.6 | 186.1 ± 92.2 | −0.85 | 0.41 |
| HbA1C (%) | 5.8 ± 1.0 | 5.5 ± 0.6 | 2.32 | 0.03 * |
| PFT | ||||
| FVC (liter) | 4.2 ± 0.7 | 4.2 ± 0.9 | −0.08 | 0.93 |
| FEV1 (liter) | 3.4 ± 0.6 | 3.4 ± 0.6 | −0.95 | 0.35 |
| Assessment | Outcome Measure | Baseline | Post-12 Weeks | t-Value | p-Value |
|---|---|---|---|---|---|
| Self-reported | EQ-5D-3L (QoL) | 16.3 ± 3.2 | 16.9 ± 2.1 | 0.59 | 0.56 |
| IPAQ (Physical activity) | 4806 ± 3875.3 | 4855.1 ± 3849.9 | 0.11 | 0.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.H.; Kim, Y.R.; Han, M.H.; Lee, J.Y.; Kim, J.S.; Kang, Y.C.; Yoon, S.J.; Chang, Y.; Lee, G.; Cho, N.S. Development of a Digital Healthcare Management System for Lower-Extremity Amputees: A Pilot Study. Healthcare 2023, 11, 106. https://doi.org/10.3390/healthcare11010106
Kim JH, Kim YR, Han MH, Lee JY, Kim JS, Kang YC, Yoon SJ, Chang Y, Lee G, Cho NS. Development of a Digital Healthcare Management System for Lower-Extremity Amputees: A Pilot Study. Healthcare. 2023; 11(1):106. https://doi.org/10.3390/healthcare11010106
Chicago/Turabian StyleKim, Jin Hong, Yu Ri Kim, Mi Hyang Han, Ji Young Lee, Ji Sung Kim, Yong Cheol Kang, Seong Jun Yoon, Yunhee Chang, Gangpyo Lee, and Nam Soon Cho. 2023. "Development of a Digital Healthcare Management System for Lower-Extremity Amputees: A Pilot Study" Healthcare 11, no. 1: 106. https://doi.org/10.3390/healthcare11010106
APA StyleKim, J. H., Kim, Y. R., Han, M. H., Lee, J. Y., Kim, J. S., Kang, Y. C., Yoon, S. J., Chang, Y., Lee, G., & Cho, N. S. (2023). Development of a Digital Healthcare Management System for Lower-Extremity Amputees: A Pilot Study. Healthcare, 11(1), 106. https://doi.org/10.3390/healthcare11010106





