3D Printing in Otolaryngology Surgery: Descriptive Review of Literature to Define the State of the Art
Abstract
:1. Introduction
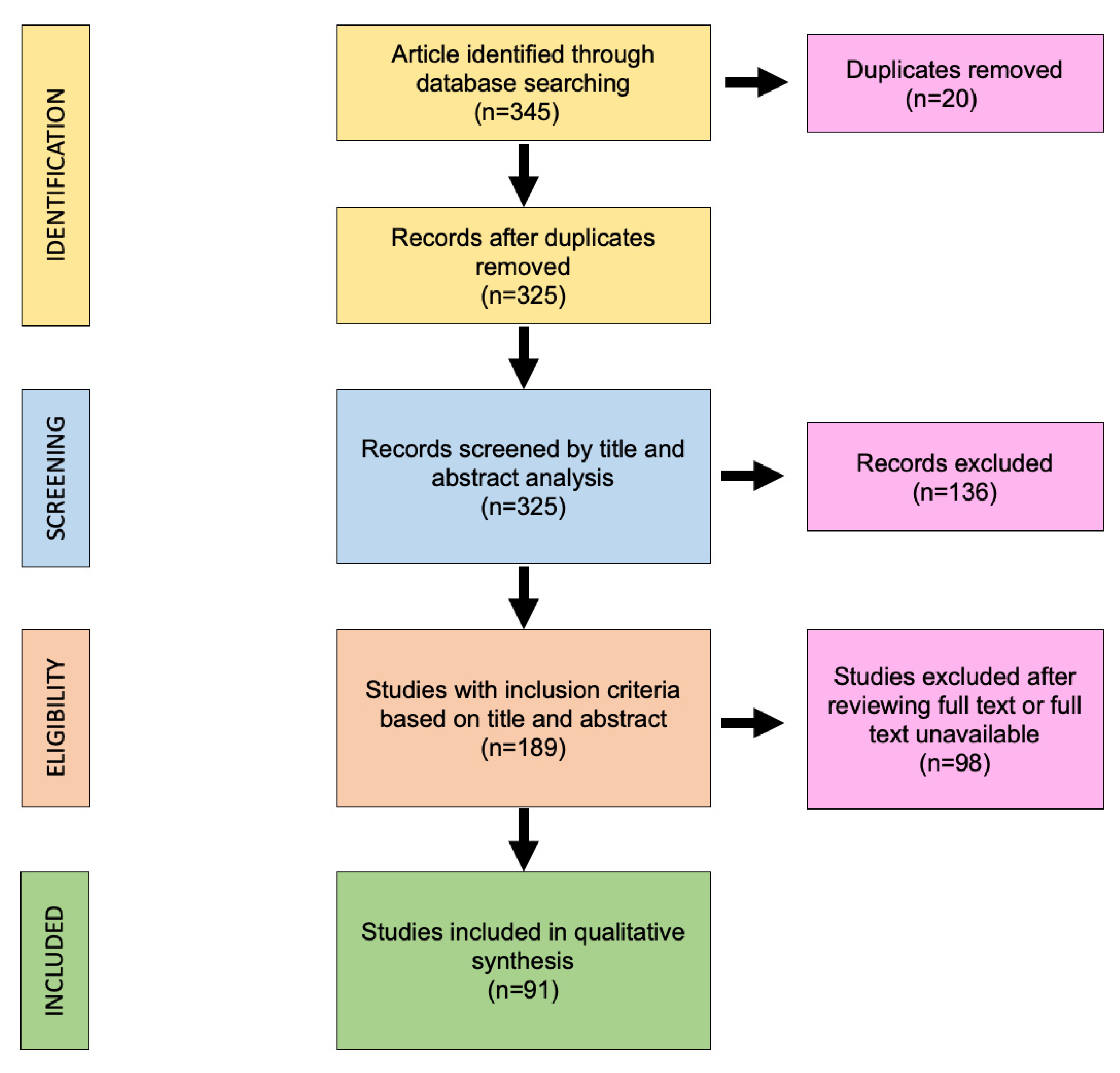
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Study Selection
2.3. Data Extraction
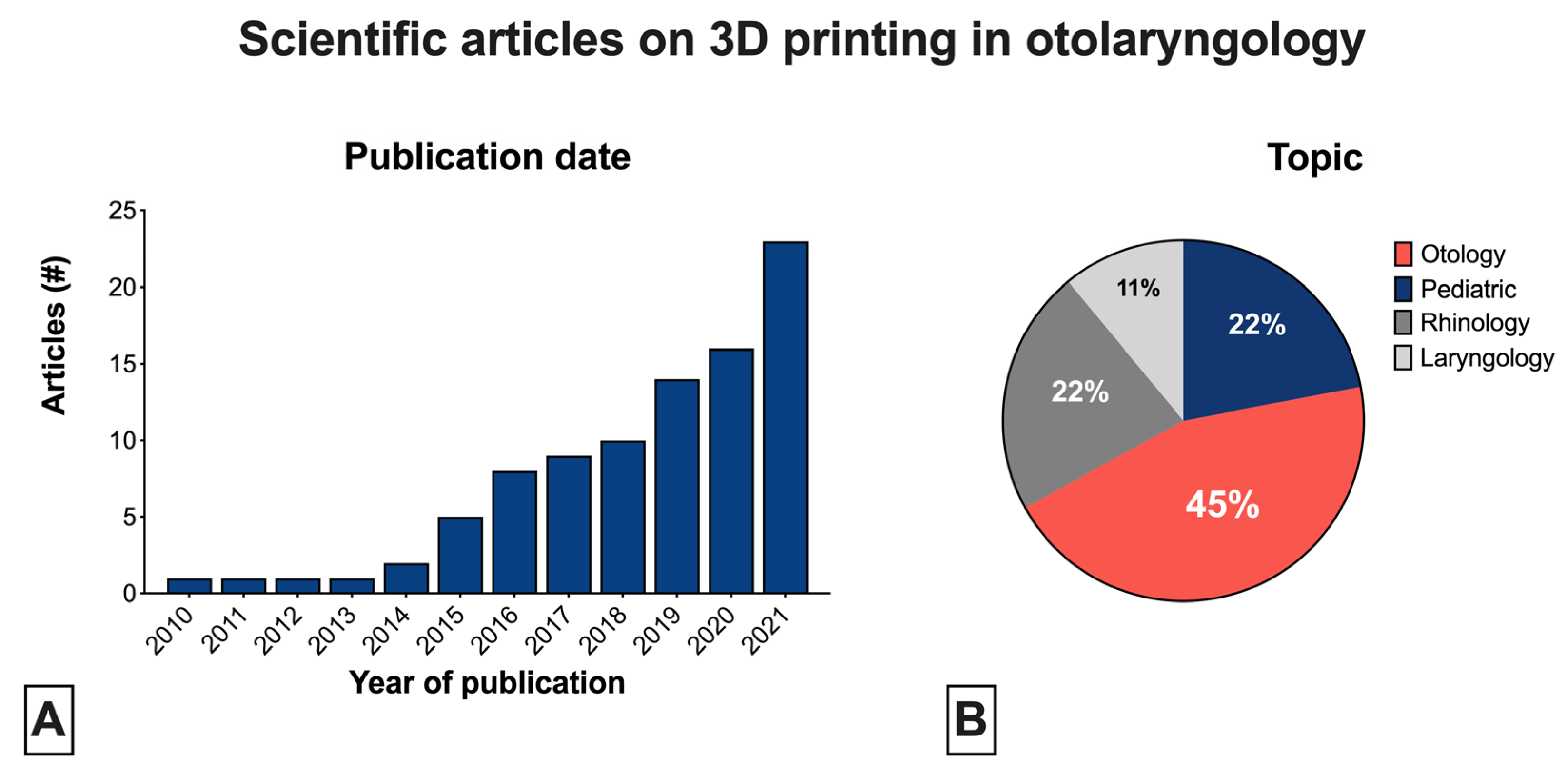
3. Results
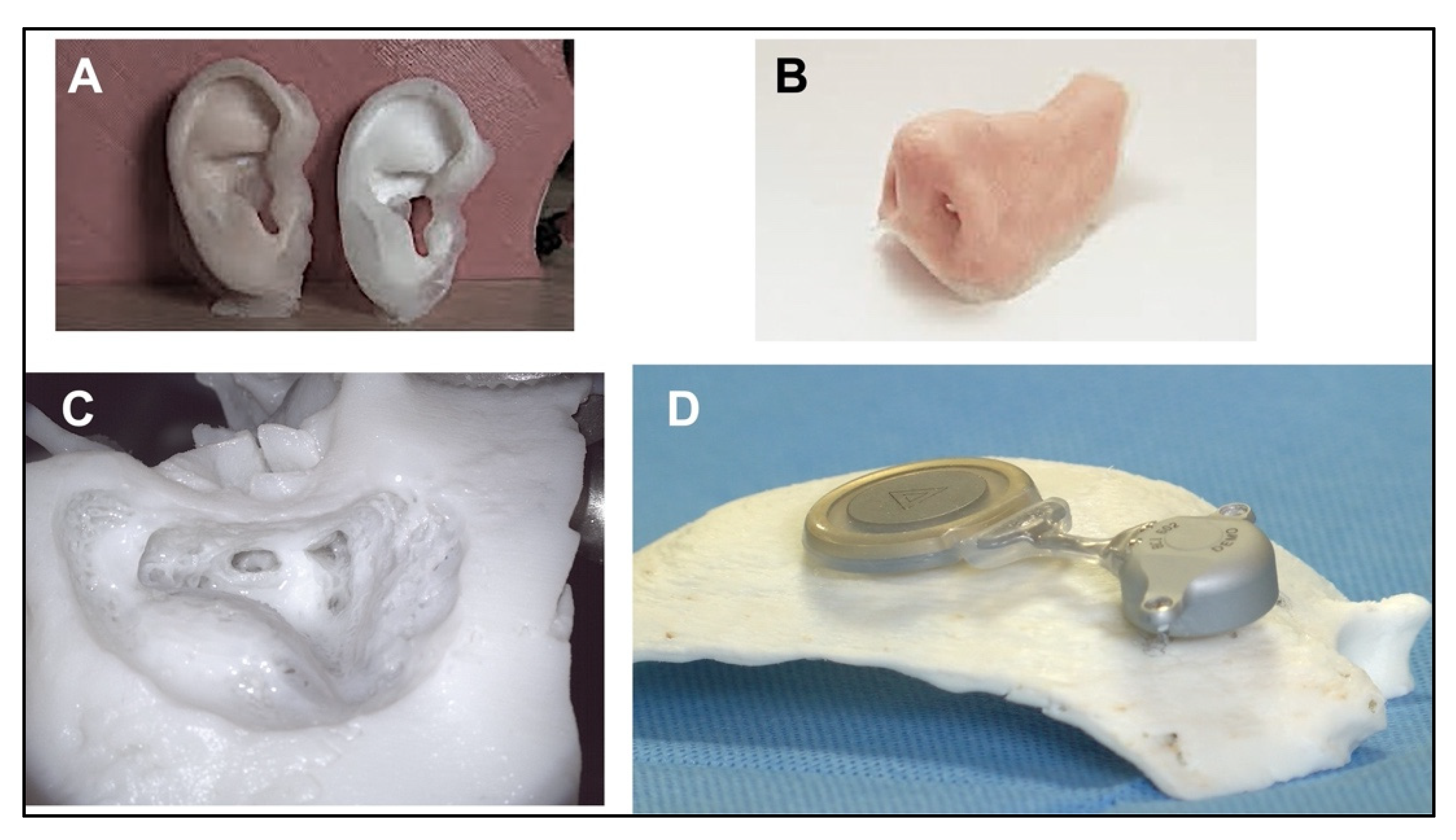
3.1. Otologic Applications
3.2. Rhinology Applications
3.3. Laryngology Applications
3.4. Pediatric Applications
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hull, C.W. Apparatus for Production of Three-Dimensional Objects by Stereolithography. U.S. Patent 6027324-A, 8 August 1984. [Google Scholar]
- Canzi, P.; Magnetto, M.; Marconi, S.; Morbini, P.; Mauramati, S.; Aprile, F.; Avato, I.; Auricchio, F.; Benazzo, M. New frontiers and emerging applications of 3D printing in ENT surgery: A systematic review of the literature. Acta Otorhinolaryngol. Ital. 2018, 38, 286–303. [Google Scholar] [CrossRef] [PubMed]
- Crafts, T.D.; Ellsperman, S.E.; Wannemuehler, T.J.; Bellicchi, T.D.; Shipchandler, T.Z.; Mantravadi, A.V. Three-Dimensional Printing and Its Applications in Otorhinolaryngology-Head and Neck Surgery. Otolaryngol. Head Neck Surg. 2017, 156, 999–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, C.J.; Giannopoulos, A.A.; Hong, B.Y.; Witterick, I.J.; Irish, J.C.; Lee, J.; Vescan, A.; Mitsouras, D.; Dang, W.; Campisi, P.; et al. Clinical applications of three-dimensional printing in otolaryngology-head and neck surgery: A systematic review. Laryngoscope 2019, 129, 2045–2052. [Google Scholar] [CrossRef] [PubMed]
- Di Gesu, R.; Acharya, A.P.; Jacobs, I.; Gottardi, R. 3D printing for tissue engineering in otolaryngology. Connect Tissue Res. 2020, 61, 117–136. [Google Scholar] [CrossRef] [PubMed]
- Kaye, R.; Goldstein, T.; Zeltsman, D.; Grande, D.A.; Smith, L.P. Three dimensional printing: A review on the utility within medicine and otolaryngology. Int. J. Pediatr. Otorhinolaryngol. 2016, 89, 145–148. [Google Scholar] [CrossRef]
- Zhong, N.; Zhao, X. 3D printing for clinical application in otorhinolaryngology. Eur. Arch. Otorhinolaryngol. 2017, 274, 4079–4089. [Google Scholar] [CrossRef]
- Della Volpe, A.; De Lucia, A.; Ippolito, V.; Pastore, V.; Iuppariello, L.; Formisano, M.; Clemente, F.; Di Stadio, A. Use of a 3D reconstruction model in a patient with severe atresia auris for optimal placement of Bonebridge transcutaneous bone conduction implant. Eur. Arch. Otorhinolaryngol. 2021, 278, 3559–3564. [Google Scholar] [CrossRef]
- Da Cruz, M.J.; Francis, H.W. Face and content validation of a novel three-dimensional printed temporal bone for surgical skills development. J. Laryngol. Otol. 2015, 129 (Suppl. 3), S23–S29. [Google Scholar] [CrossRef]
- Rose, A.S.; Webster, C.E.; Harrysson, O.L.; Formeister, E.J.; Rawal, R.B.; Iseli, C.E. Pre-operative simulation of pediatric mastoid surgery with 3D-printed temporal bone models. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 740–744. [Google Scholar] [CrossRef]
- Rose, A.S.; Kimbell, J.S.; Webster, C.E.; Harrysson, O.L.; Formeister, E.J.; Buchman, C.A. Multi-material 3D Models for Temporal Bone Surgical Simulation. Ann. Otol. Rhinol. Laryngol. 2015, 124, 528–536. [Google Scholar] [CrossRef]
- Kozin, E.D.; Remenschneider, A.K.; Cheng, S.; Nakajima, H.H.; Lee, D.J. Three-Dimensional Printed Prosthesis for Repair of Superior Canal Dehiscence. Otolaryngol. Head Neck Surg. 2015, 153, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.; Hatamleh, M.M. Complete integration of technology for improved reproduction of auricular prostheses. J. Prosthet. Dent. 2014, 111, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Kuru, I.; Maier, H.; Muller, M.; Lenarz, T.; Lueth, T.C. A 3D-printed functioning anatomical human middle ear model. Hear Res. 2016, 340, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Cheng, K.; Flanagan, S.; Greenberg, S. Utility of 3D printed temporal bones in pre-surgical planning for complex BoneBridge cases. Eur. Arch. Otorhinolaryngol. 2017, 274, 3021–3028. [Google Scholar] [CrossRef]
- Zhuo, C.; Lei, L.; Zhao, Y.; Liu, W.; Wu, S.; Wang, C.; Zhang, Y.; Huang, S.; Dong, D. Creation and validation of three-dimensional printed models for basic nasal endoscopic training. Int. Forum Allergy Rhinol. 2019, 9, 695–701. [Google Scholar] [CrossRef]
- Alrasheed, A.S.; Nguyen, L.H.P.; Mongeau, L.; Funnell, W.R.J.; Tewfik, M.A. Development and validation of a 3D-printed model of the ostiomeatal complex and frontal sinus for endoscopic sinus surgery training. Int. Forum Allergy Rhinol. 2017, 7, 837–841. [Google Scholar] [CrossRef] [Green Version]
- Muelleman, T.J.; Peterson, J.; Chowdhury, N.I.; Gorup, J.; Camarata, P.; Lin, J. Individualized Surgical Approach Planning for Petroclival Tumors Using a 3D Printer. J. Neurol. Surg. B Skull. Base 2016, 77, 243–248. [Google Scholar] [CrossRef] [Green Version]
- Khan, G.; Choi, Y.S.; Park, E.S.; Choi, Y.D. The Application of Three-Dimensional Simulation Program and Three-Dimensional Printing in Secondary Rhinoplasty. J. Craniofac. Surg. 2018, 29, e774–e777. [Google Scholar] [CrossRef]
- Daniel, M.; Watson, J.; Hoskison, E.; Sama, A. Frontal sinus models and onlay templates in osteoplastic flap surgery. J. Laryngol. Otol. 2011, 125, 82–85. [Google Scholar] [CrossRef]
- Onerci Altunay, Z.; Bly, J.A.; Edwards, P.K.; Holmes, D.R.; Hamilton, G.S.; O’Brien, E.K.; Carr, A.B.; Camp, J.J.; Stokken, J.K.; Pallanch, J.F. Three-dimensional printing of large nasal septal perforations for optimal prosthetic closure. Am. J. Rhinol. Allergy 2016, 30, 287–293. [Google Scholar] [CrossRef]
- Mallon, A.; Farnan, T. A case report detailing the use of 3D printing technology in surgical planning and decision making in ENT surgery-an axial 3D first in Northern Ireland. Int. J. Surg. Case Rep. 2021, 87, 106407. [Google Scholar] [CrossRef] [PubMed]
- Gillett, D.; Bashari, W.; Senanayake, R.; Marsden, D.; Koulouri, O.; MacFarlane, J.; van der Meulen, M.; Powlson, A.S.; Mendichovszky, I.A.; Cheow, H.; et al. Methods of 3D printing models of pituitary tumors. 3D Print Med. 2021, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Hertegard, S. Tissue engineering in the larynx and airway. Curr. Opin. Otolaryngol. Head Neck Surg. 2016, 24, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, A.L.; Haddad, G.; Haydar, A.; Hamade, R. The 3D Printing of the Paralyzed Vocal Fold: Added Value in Injection Laryngoplasty. J. Voice 2018, 32, 499–501. [Google Scholar] [CrossRef]
- Chen, J.; Shen, Y.; Shen, Z.; Cheng, L.; Zhou, S. Tissue engineering of the larynx: A contemporary review. J. Clin. Lab. Anal. 2021, 35, e23646. [Google Scholar] [CrossRef]
- Maguire, S.K.; Razavi, C.; Sevimli, Y.; Akst, L.M. Three-dimensional printing of a low-cost, high-fidelity laryngeal dissection station. Laryngoscope 2018, 128, 944–947. [Google Scholar] [CrossRef]
- Kimijima, T.; Edanaga, M.; Yamakage, M. Superior sealing effect of a three-dimensional printed modified supraglottic airway compared with the i-gel in a three-dimensional printed airway model. J. Anesth. 2018, 32, 655–662. [Google Scholar] [CrossRef]
- Cates, D.J.; Magnetta, M.J.; Smith, L.J.; Rosen, C.A. Novel, anatomically appropriate balloon dilation technique of the glottis to treat posterior glottic stenosis in a 3D-printed model. Laryngoscope 2019, 129, 2239–2243. [Google Scholar] [CrossRef]
- Romero, R.G.T.; Colton, M.B.; Thomson, S.L. 3D-Printed Synthetic Vocal Fold Models. J. Voice 2021, 35, 685–694. [Google Scholar] [CrossRef]
- Villegas, M.C.; Chamorro, M.V.; Fandino-Reyes, A.; Jimenez-Fandino, L.H. 3D Printed Larynx as a Novel Simulation Tool for Window Elaboration in Medialization Laryngoplasty. J. Voice 2021. [Google Scholar] [CrossRef]
- Tian, H.; Gao, S.; Yu, J.; Zhou, X.; Chen, X.; Zuo, L.; Cai, X.; Song, B.; Yu, K. Application of digital modeling and three-dimensional printing of titanium mesh for reconstruction of thyroid cartilage in partial laryngectomy. Acta Otolaryngol. 2022, 142, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Torsello, M.; Salvati, A.; Borro, L.; Meucci, D.; Tropiano, M.L.; Cialente, F.; Secinaro, A.; Del Fattore, A.; Emiliana, C.M.; Francalanci, P.; et al. 3D bioprinting in airway reconstructive surgery: A pilot study. Int. J. Pediatr. Otorhinolaryngol. 2022, 161, 111253. [Google Scholar] [CrossRef] [PubMed]
- Richard, Z.; Jackson, E.; Jung, J.P.; Kanotra, S.P. Feasibility and potential of three-dimensional printing in laryngotracheal stenosis. J. Laryngol. Otol. 2019, 133, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, K.R.; Cote, V.; Tsui, Y.; Kudernatsch, S.; Peterson, D.R.; Valdez, T.A. Pediatric laryngeal simulator using 3D printed models: A novel technique. Laryngoscope 2017, 127, E132–E137. [Google Scholar] [CrossRef]
- Grolman, W.; Schouwenburg, P.F.; Verbeeten, B., Jr.; de Boer, M.F.; Meeuwis, C.A. Three-dimensional models of the tracheostoma using stereolithography. ORL J. Otorhinolaryngol. Relat. Spec. 1995, 57, 338–342. [Google Scholar] [CrossRef]
- Sood, V.; Green, G.E.; Les, A.; Ohye, R.G. Advanced Therapies for Severe Tracheobronchomalacia: A Review of the Use of 3D-Printed, Patient-Specific, Externally Implanted, Bioresorbable Airway Splints. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card Surg. Annu. 2021, 24, 37–43. [Google Scholar] [CrossRef]
- Les, A.S.; Ohye, R.G.; Filbrun, A.G.; Ghadimi Mahani, M.; Flanagan, C.L.; Daniels, R.C.; Kidwell, K.M.; Zopf, D.A.; Hollister, S.J.; Green, G.E. 3D-printed, externally-implanted, bioresorbable airway splints for severe tracheobronchomalacia. Laryngoscope 2019, 129, 1763–1771. [Google Scholar] [CrossRef]
- Zopf, D.A.; Hollister, S.J.; Nelson, M.E.; Ohye, R.G.; Green, G.E. Bioresorbable airway splint created with a three-dimensional printer. N. Engl. J. Med. 2013, 368, 2043–2045. [Google Scholar] [CrossRef]
- Morrison, R.J.; Hollister, S.J.; Niedner, M.F.; Mahani, M.G.; Park, A.H.; Mehta, D.K.; Ohye, R.G.; Green, G.E. Mitigation of tracheobronchomalacia with 3D-printed personalized medical devices in pediatric patients. Sci. Transl. Med. 2015, 7, 285ra264. [Google Scholar] [CrossRef] [Green Version]
- Carlos, C.; Parkes, W.; James, A.L. Application of 3-dimensional Modeling to Plan Totally Endoscopic Per-Meatal Drainage of Petrous Apex Cholesterol Granuloma. Otolaryngol. Head Neck Surg. 2015, 153, 1074–1075. [Google Scholar] [CrossRef]
- Stramiello, J.A.; Saddawi-Konefka, R.; Ryan, J.; Brigger, M.T. The role of 3D printing in pediatric airway obstruction: A systematic review. Int. J. Pediatr. Otorhinolaryngol. 2020, 132, 109923. [Google Scholar] [CrossRef] [PubMed]
- Simorgh, S.; Alasvand, N.; Khodadadi, M.; Ghobadi, F.; Malekzadeh Kebria, M.; Brouki Milan, P.; Kargozar, S.; Baino, F.; Mobasheri, A.; Mozafari, M. Additive manufacturing of bioactive glass biomaterials. Methods 2022, 208, 75–91. [Google Scholar] [CrossRef] [PubMed]
- Ozbolat, I.T.; Yu, Y. Bioprinting toward organ fabrication: Challenges and future trends. IEEE Trans. Biomed. Eng. 2013, 60, 691–699. [Google Scholar] [CrossRef] [PubMed]
- VanKoevering, K.K.; Zopf, D.A.; Hollister, S.J. Tissue Engineering and 3-Dimensional Modeling for Facial Reconstruction. Facial. Plast. Surg. Clin. N. Am. 2019, 27, 151–161. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zoccali, F.; Colizza, A.; Cialente, F.; Di Stadio, A.; La Mantia, I.; Hanna, C.; Minni, A.; Ralli, M.; Greco, A.; de Vincentiis, M. 3D Printing in Otolaryngology Surgery: Descriptive Review of Literature to Define the State of the Art. Healthcare 2023, 11, 108. https://doi.org/10.3390/healthcare11010108
Zoccali F, Colizza A, Cialente F, Di Stadio A, La Mantia I, Hanna C, Minni A, Ralli M, Greco A, de Vincentiis M. 3D Printing in Otolaryngology Surgery: Descriptive Review of Literature to Define the State of the Art. Healthcare. 2023; 11(1):108. https://doi.org/10.3390/healthcare11010108
Chicago/Turabian StyleZoccali, Federica, Andrea Colizza, Fabrizio Cialente, Arianna Di Stadio, Ignazio La Mantia, Charlie Hanna, Antonio Minni, Massimo Ralli, Antonio Greco, and Marco de Vincentiis. 2023. "3D Printing in Otolaryngology Surgery: Descriptive Review of Literature to Define the State of the Art" Healthcare 11, no. 1: 108. https://doi.org/10.3390/healthcare11010108
APA StyleZoccali, F., Colizza, A., Cialente, F., Di Stadio, A., La Mantia, I., Hanna, C., Minni, A., Ralli, M., Greco, A., & de Vincentiis, M. (2023). 3D Printing in Otolaryngology Surgery: Descriptive Review of Literature to Define the State of the Art. Healthcare, 11(1), 108. https://doi.org/10.3390/healthcare11010108









