Description of Stone Morphology and Crystalluria Improve Diagnosis and Care of Kidney Stone Formers
Abstract
1. Introduction to Stone Morphological Analyses
2. Classification of Kidney Stones According to Stone Composition and Morphology
2.1. Calcium Oxalate Stones
2.1.1. Type I Stones: COM
Type Ia
Type Ib and Id
Type Ic
Type Ie
2.1.2. Type II Stones: COD
Type IIa
Type IIb
Type IIc
2.1.3. Mixed COM and COD Stones (Type I + II)
2.1.4. Caoxite Stones
2.2. Uric Acid and Urate Stones (Type III)
2.2.1. Type IIIa
2.2.2. Type IIIb
2.2.3. Type IIIc
2.2.4. Type IIId
2.3. Phosphate Stones (Type IV)
2.3.1. Type IVa1
2.3.2. The Type IVa2 Carbapatite Subtype and Distal Renal Tubular Acidosis
2.3.3. Type IVb
2.3.4. Type IVc
2.3.5. Type IVd
2.3.6. Scanning Electron Microscopy in CaP Stones
2.3.7. Mixed CaOx and CaP Stones
2.4. Other Stones
2.4.1. Type V Stones
2.4.2. Type VI Stones
2.4.3. Other Types
3. Morpho(-Constitutional) Analysis of Kidney Stones: Clinical Relevance
4. Introduction to Crystalluria
- (i)
- leads to the diagnosis of severe monogenic diseases (cystinuria, primary hyperoxaluria, adenine phosphoribosyltransferase deficiency)
- (ii)
- identifies crystals due to drugs, which may be responsible for acute or chronic kidney injury
- (iii)
- allows the assessment of metabolic disorders associated with stone formation
- (iv)
- may predict the risk of stone recurrence.
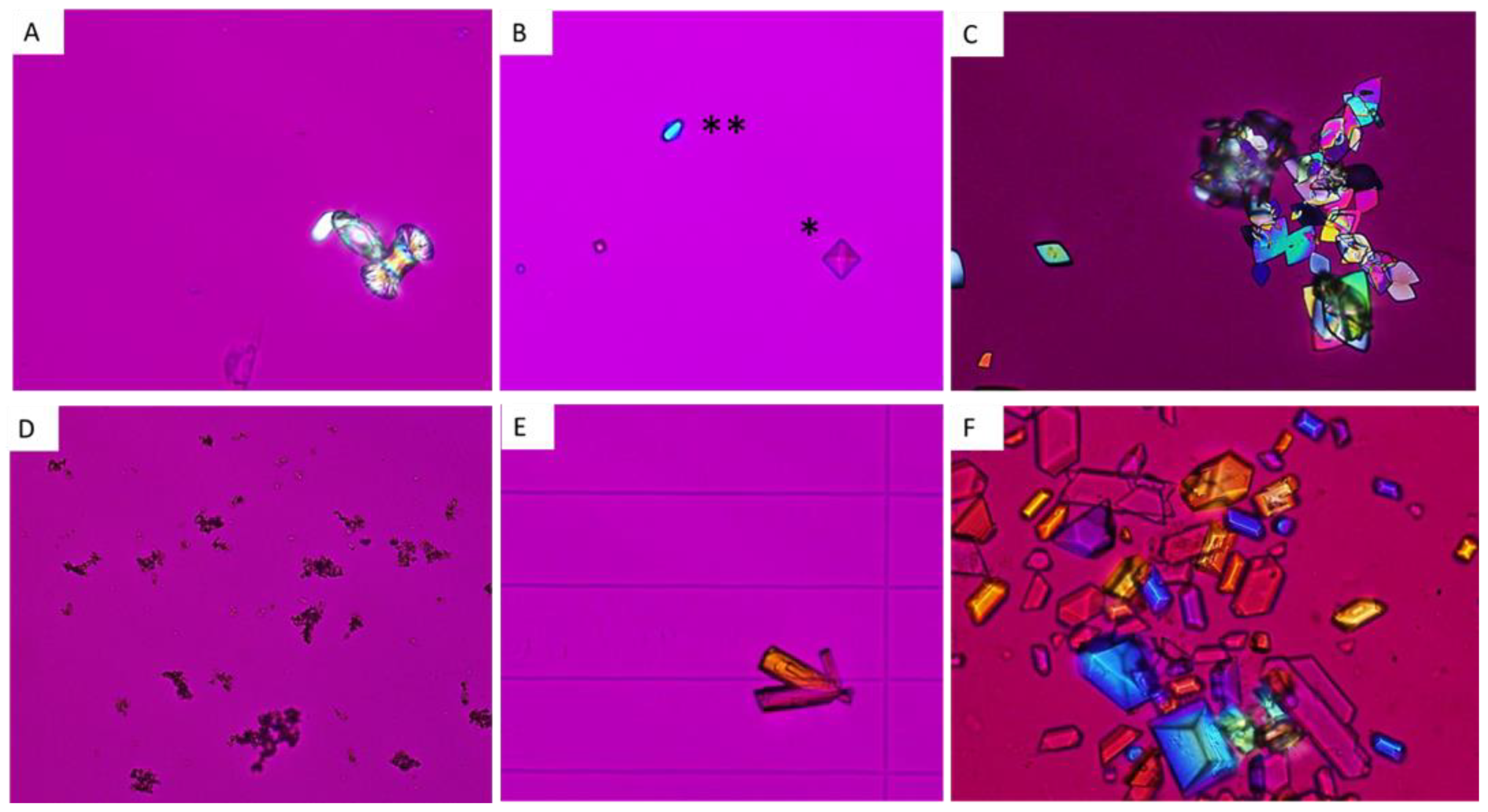
5. Classification of Urinary Crystals
5.1. Calcium Oxalate Monohydrate and Dihydrate: COM and COD
5.1.1. COM or Whewellite
5.1.2. COD or Weddellite
5.2. Uric Acid and Urate Salts
5.2.1. Uric acid
5.2.2. Urate salts
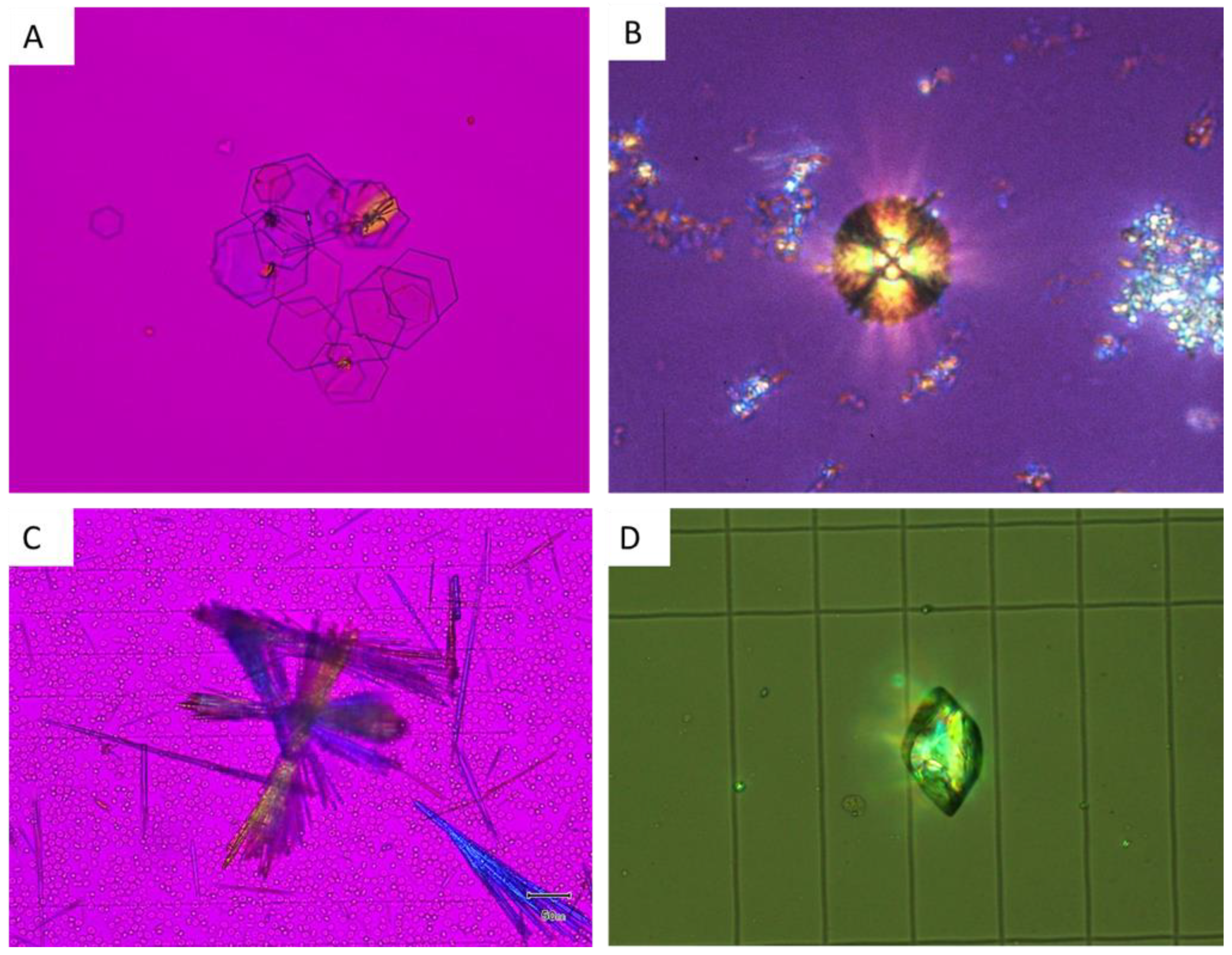
5.3. CaP Crystals and Struvite
5.3.1. CaP
5.3.2. Struvite
5.4. Crystals Due to Monogenic Diseases
5.4.1. Cystine
5.4.2. 2,8-Dihydroxyadenine
5.4.3. Xanthine
5.4.4. COM
5.4.5. Others
5.5. Drug-Induced Crystals
6. Crystalluria Study Reveals Lithogenic Activity and Predicts Stone Recurrence
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Daudon, M.; Bader, C.A.; Jungers, P. Urinary Calculi: Review of Classification Methods and Correlations with Etiology. Scanning Microsc. 1993, 7, 1081–1104; discussion 1104–1106. [Google Scholar]
- Daudon, M.; Dessombz, A.; Frochot, V.; Letavernier, E.; Haymann, J.-P.; Jungers, P.; Bazin, D. Comprehensive Morpho-Constitutional Analysis of Urinary Stones Improves Etiological Diagnosis and Therapeutic Strategy of Nephrolithiasis. Comptes Rendus Chim. 2016, 19, 1470–1491. [Google Scholar] [CrossRef]
- Worcester, E.M.; Coe, F.L. Clinical Practice. Calcium Kidney Stones. N. Engl. J. Med. 2010, 363, 954–963. [Google Scholar] [CrossRef]
- Sakhaee, K.; Maalouf, N.M.; Sinnott, B. Clinical Review. Kidney Stones 2012: Pathogenesis, Diagnosis, and Management. J. Clin. Endocrinol. Metab. 2012, 97, 1847–1860. [Google Scholar] [CrossRef]
- Coe, F.L.; Worcester, E.M.; Evan, A.P. Idiopathic Hypercalciuria and Formation of Calcium Renal Stones. Nat. Rev. Nephrol. 2016, 12, 519–533. [Google Scholar] [CrossRef]
- Williams, J.C.; Gambaro, G.; Rodgers, A.; Asplin, J.; Bonny, O.; Costa-Bauzá, A.; Ferraro, P.M.; Fogazzi, G.; Fuster, D.G.; Goldfarb, D.S.; et al. Urine and Stone Analysis for the Investigation of the Renal Stone Former: A Consensus Conference. Urolithiasis 2021, 49, 1–16. [Google Scholar] [CrossRef]
- Daudon, M. Epidemiology of nephrolithiasis in France. Ann. Urol. 2005, 39, 209–231. [Google Scholar] [CrossRef]
- Bazin, D.; Leroy, C.; Tielens, F.; Bonhomme, C.; Bonhomme-Coury, L.; Damay, F.; Denmat, D.L.; Sadoine, J.; Rode, J.; Frochot, V.; et al. Hyperoxaluria Is Related to Whewellite and Hypercalciuria to Weddellite: What Happens When Crystalline Conversion Occurs? Comptes Rendus Chim. 2016, 19, 1492–1503. [Google Scholar] [CrossRef]
- de Perre, E.V.; Bazin, D.; Estrade, V.; Bouderlique, E.; Wissing, K.M.; Daudon, M.; Letavernier, E. Randall’s Plaque as the Origin of Idiopathic Calcium Oxalate Stone Formation: An Update. Comptes Rendus Chim. 2022, 25, 373–391. [Google Scholar] [CrossRef]
- Letavernier, E.; Vandermeersch, S.; Traxer, O.; Tligui, M.; Baud, L.; Ronco, P.; Haymann, J.-P.; Daudon, M. Demographics and Characterization of 10,282 Randall Plaque-Related Kidney Stones: A New Epidemic? Medicine 2015, 94, e566. [Google Scholar] [CrossRef]
- Shee, K.; Stoller, M.L. Perspectives in Primary Hyperoxaluria—Historical, Current and Future Clinical Interventions. Nat. Rev. Urol. 2022, 19, 137–146. [Google Scholar] [CrossRef]
- Daudon, M.; Jungers, P.; Bazin, D. Peculiar Morphology of Stones in Primary Hyperoxaluria. N. Engl. J. Med. 2008, 359, 100–102. [Google Scholar] [CrossRef]
- Maalouf, N.M.; Tondapu, P.; Guth, E.S.; Livingston, E.H.; Sakhaee, K. Hypocitraturia and Hyperoxaluria after Roux-En-Y Gastric Bypass Surgery. J. Urol. 2010, 183, 1026–1030. [Google Scholar] [CrossRef]
- Witting, C.; Langman, C.B.; Assimos, D.; Baum, M.A.; Kausz, A.; Milliner, D.; Tasian, G.; Worcester, E.; Allain, M.; West, M.; et al. Pathophysiology and Treatment of Enteric Hyperoxaluria. Clin. J. Am. Soc. Nephrol. CJASN 2021, 16, 487–495. [Google Scholar] [CrossRef]
- Lange, J.N.; Wood, K.D.; Knight, J.; Assimos, D.G.; Holmes, R.P. Glyoxal Formation and Its Role in Endogenous Oxalate Synthesis. Adv. Urol. 2012, 2012, 819202. [Google Scholar] [CrossRef] [PubMed]
- Sakhaee, K.; Maalouf, N.M. Metabolic Syndrome and Uric Acid Nephrolithiasis. Semin. Nephrol. 2008, 28, 174–180. [Google Scholar] [CrossRef]
- Daudon, M.; Traxer, O.; Conort, P.; Lacour, B.; Jungers, P. Type 2 Diabetes Increases the Risk for Uric Acid Stones. J. Am. Soc. Nephrol. JASN 2006, 17, 2026–2033. [Google Scholar] [CrossRef]
- Clarke, A.M.; McKenzie, R.G. Ileostomy and the Risk of Urinary Uric Acid Stones. Lancet Lond. Engl. 1969, 2, 395–397. [Google Scholar] [CrossRef]
- Meiouet, F.; El Kabbaj, S.; Daudon, M. Pediatric Urolithiasis in Morocco: Composition of 432 Urinary Calculi Analyzed by Infrared Spectroscopy. Progres Urol. J. Assoc. Fr. Urol. Soc. Fr. Urol. 2019, 29, 173–182. [Google Scholar] [CrossRef]
- Dessombz, A.; Letavernier, E.; Haymann, J.-P.; Bazin, D.; Daudon, M. Calcium Phosphate Stone Morphology Can Reliably Predict Distal Renal Tubular Acidosis. J. Urol. 2015, 193, 1564–1569. [Google Scholar] [CrossRef]
- Carpentier, X.; Daudon, M.; Traxer, O.; Jungers, P.; Mazouyes, A.; Matzen, G.; Véron, E.; Bazin, D. Relationships between Carbonation Rate of Carbapatite and Morphologic Characteristics of Calcium Phosphate Stones and Etiology. Urology 2009, 73, 968–975. [Google Scholar] [CrossRef]
- Daudon, M.; Bouzidi, H.; Bazin, D. Composition and Morphology of Phosphate Stones and Their Relation with Etiology. Urol. Res. 2010, 38, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Bollée, G.; Dollinger, C.; Boutaud, L.; Guillemot, D.; Bensman, A.; Harambat, J.; Deteix, P.; Daudon, M.; Knebelmann, B.; Ceballos-Picot, I. Phenotype and Genotype Characterization of Adenine Phosphoribosyltransferase Deficiency. J. Am. Soc. Nephrol. JASN 2010, 21, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Daudon, M.; Frochot, V.; Bazin, D.; Haymann, J.-P.; Letavernier, E. Medullary Sponge Kidney: What Kind of Stones? Comptes Rendus Chim. 2022, 25, 269–279. [Google Scholar] [CrossRef]
- Daudon, M.; Jungers, P.; Lacour, B. Clinical value of crystalluria study. Ann. Biol. Clin. 2004, 62, 379–393. [Google Scholar]
- Daudon, M.; Frochot, V.; Bazin, D.; Jungers, P. Crystalluria Analysis Improves Significantly Etiologic Diagnosis and Therapeutic Monitoring of Nephrolithiasis. Comptes Rendus Chim. 2016, 19, 1514–1526. [Google Scholar] [CrossRef]
- Daudon, M.; Hennequin, C.; Bader, C.; Jungers, P.; Lacour, B.; Drüecke, T. Inhibitors of Crystallization. Adv. Nephrol. Necker Hosp. 1995, 24, 167–216. [Google Scholar]
- Jouvet, P.; Priqueler, L.; Gagnadoux, M.F.; Jan, D.; Beringer, A.; Lacaille, F.; Revillon, Y.; Broyer, M.; Daudon, M. Crystalluria: A Clinically Useful Investigation in Children with Primary Hyperoxaluria Post-Transplantation. Kidney Int. 1998, 53, 1412–1416. [Google Scholar] [CrossRef]
- Demotier, S.; Limelette, A.; Charmillon, A.; Baux, E.; Parent, X.; Mestrallet, S.; Pavel, S.; Servettaz, A.; Dramé, M.; Muggeo, A.; et al. Incidence, Associated Factors, and Effect on Renal Function of Amoxicillin Crystalluria in Patients Receiving High Doses of Intravenous Amoxicillin (The CRISTAMOX Study): A Cohort Study. EClinicalMedicine 2022, 45, 101340. [Google Scholar] [CrossRef]
- Jamme, M.; Oliver, L.; Ternacle, J.; Lepeule, R.; Moussafeur, A.; Haymann, J.-P.; San, S.; Fiore, A.; Mongardon, N.; Daudon, M.; et al. Amoxicillin Crystalluria Is Associated with Acute Kidney Injury in Patients Treated for Acute Infective Endocarditis. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.—Eur. Ren. Assoc. 2021, 36, 1955–1958. [Google Scholar] [CrossRef]
- de Montmollin, N.; Letavernier, E.; Fartoukh, M.; Labbe, V. Acute Sulfamethoxazole-Induced Crystal Nephropathy. Intensive Care Med. 2018, 44, 1575–1576. [Google Scholar] [CrossRef] [PubMed]
- Zaworski, J.; Bouderlique, E.; Anglicheau, D.; Duong Van Huyen, J.-P.; Gnemmi, V.; Gibier, J.-B.; Neugebauer, Y.; Haymann, J.-P.; Bazin, D.; Frochot, V.; et al. 1-Methyluric Acid Nephropathy. Kidney Int. Rep. 2020, 5, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Rafat, C.; Zaworski, J.; Luque, Y.; Daudon, M.; Doreille, A.; Mesnard, L.; Letavernier, E. Noninvasive Screening of Vancomycin-Associated Cast Nephropathy. Kidney Int. 2022, 101, 425. [Google Scholar] [CrossRef] [PubMed]
- Daudon, M.; Hennequin, C.; Boujelben, G.; Lacour, B.; Jungers, P. Serial Crystalluria Determination and the Risk of Recurrence in Calcium Stone Formers. Kidney Int. 2005, 67, 1934–1943. [Google Scholar] [CrossRef] [PubMed]
- Daudon, M.; Cohen-Solal, F.; Barbey, F.; Gagnadoux, M.-F.; Knebelmann, B.; Jungers, P. Cystine Crystal Volume Determination: A Useful Tool in the Management of Cystinuric Patients. Urol. Res. 2003, 31, 207–211. [Google Scholar] [CrossRef] [PubMed]
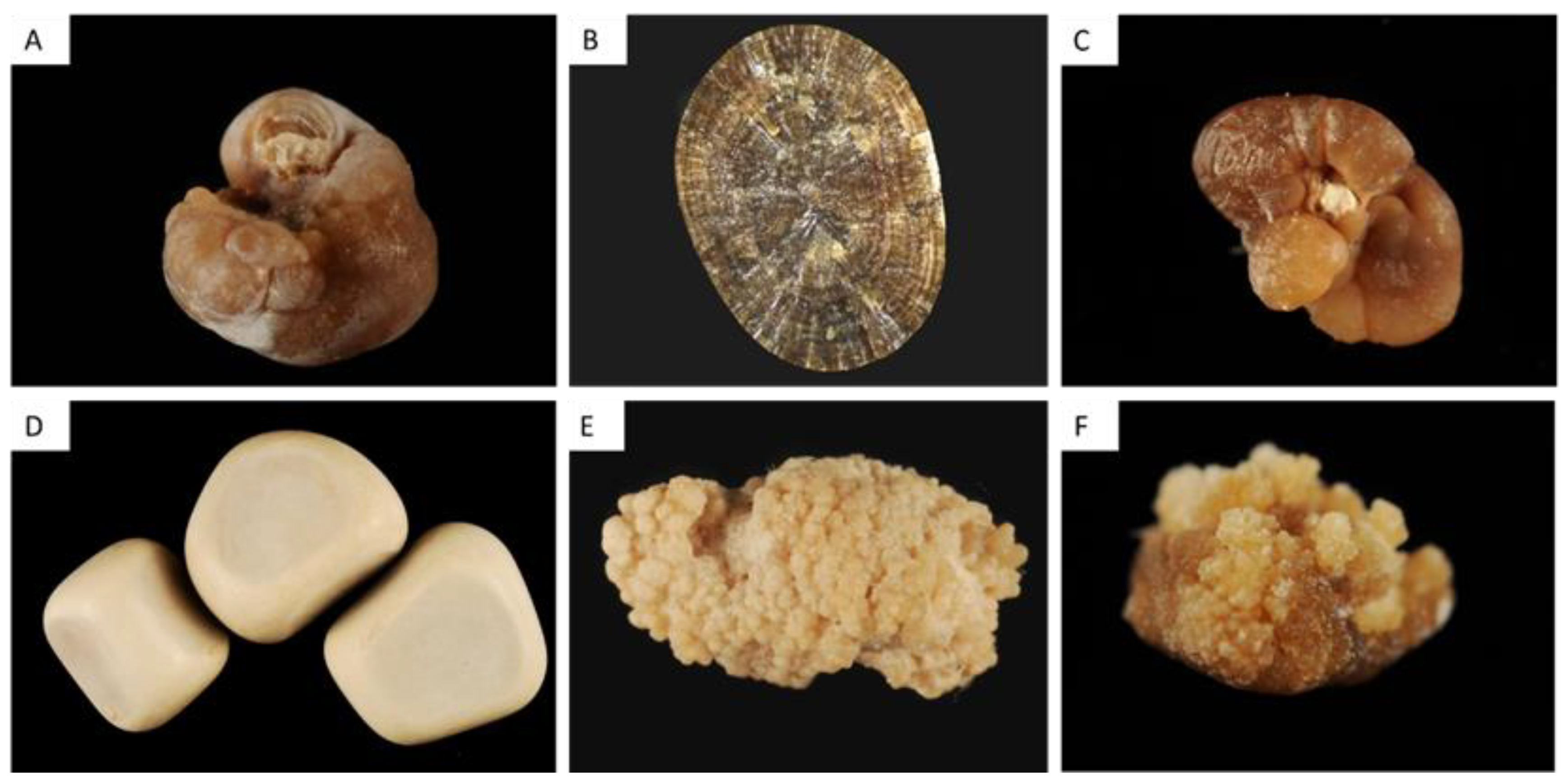
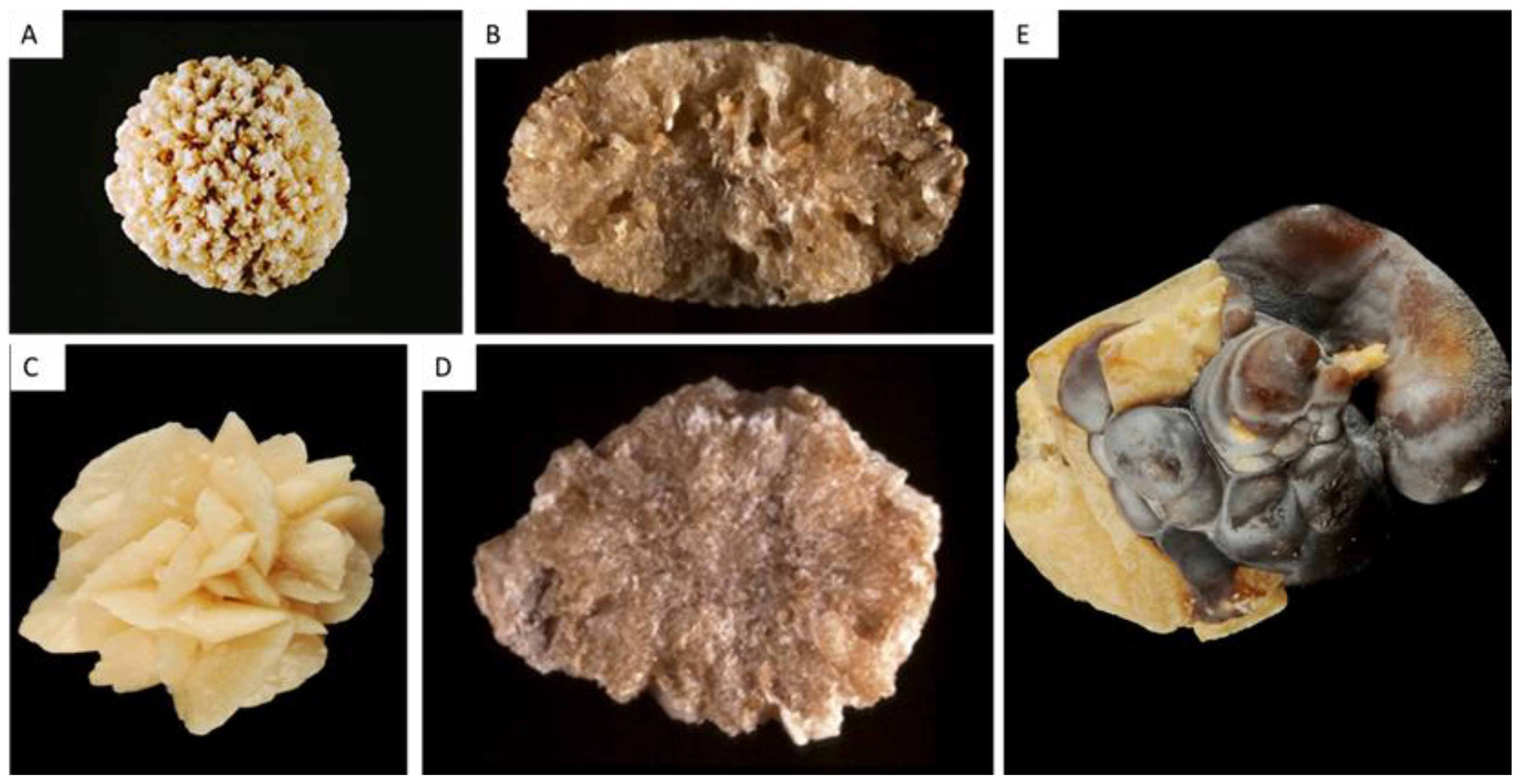
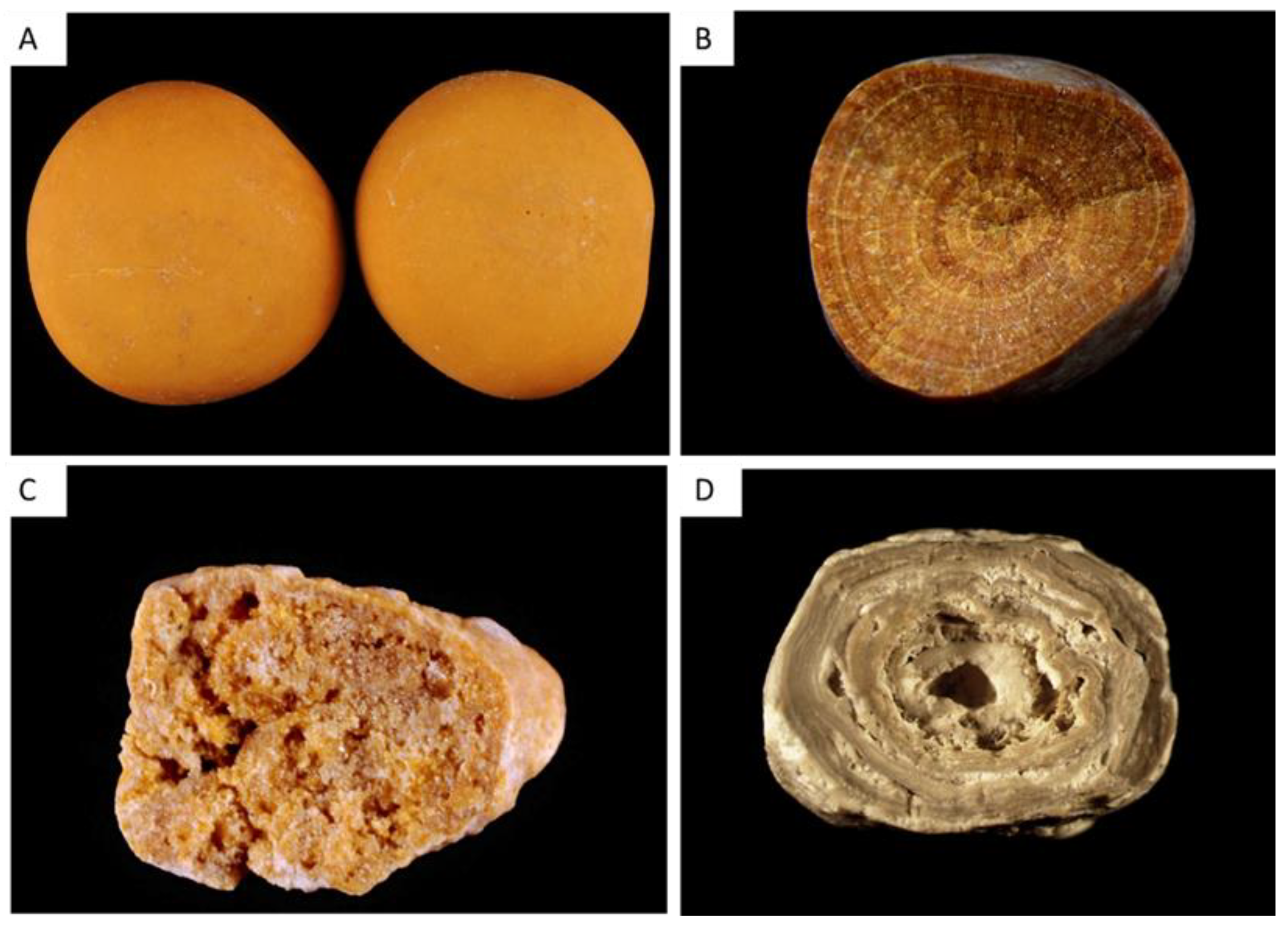
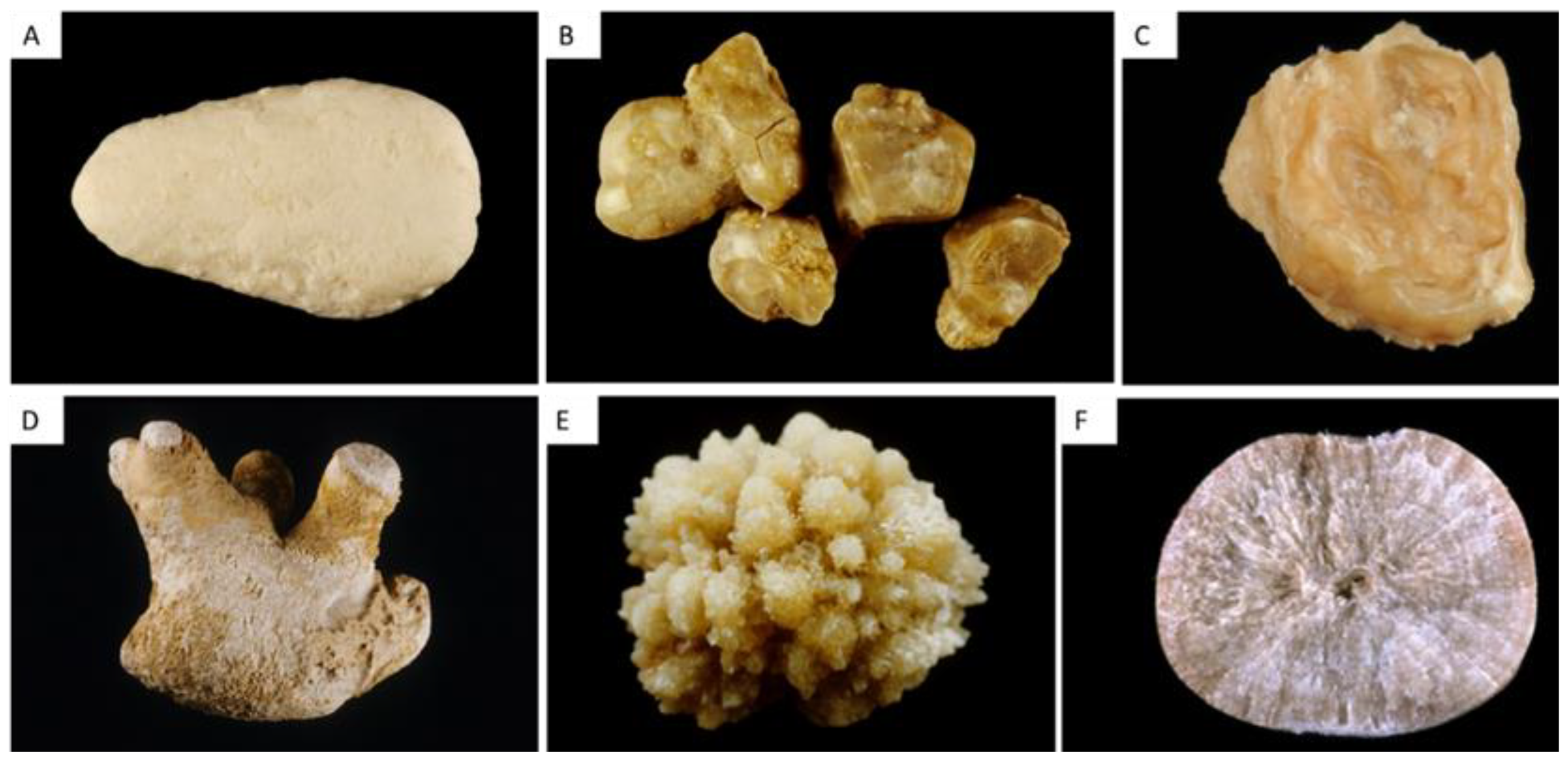
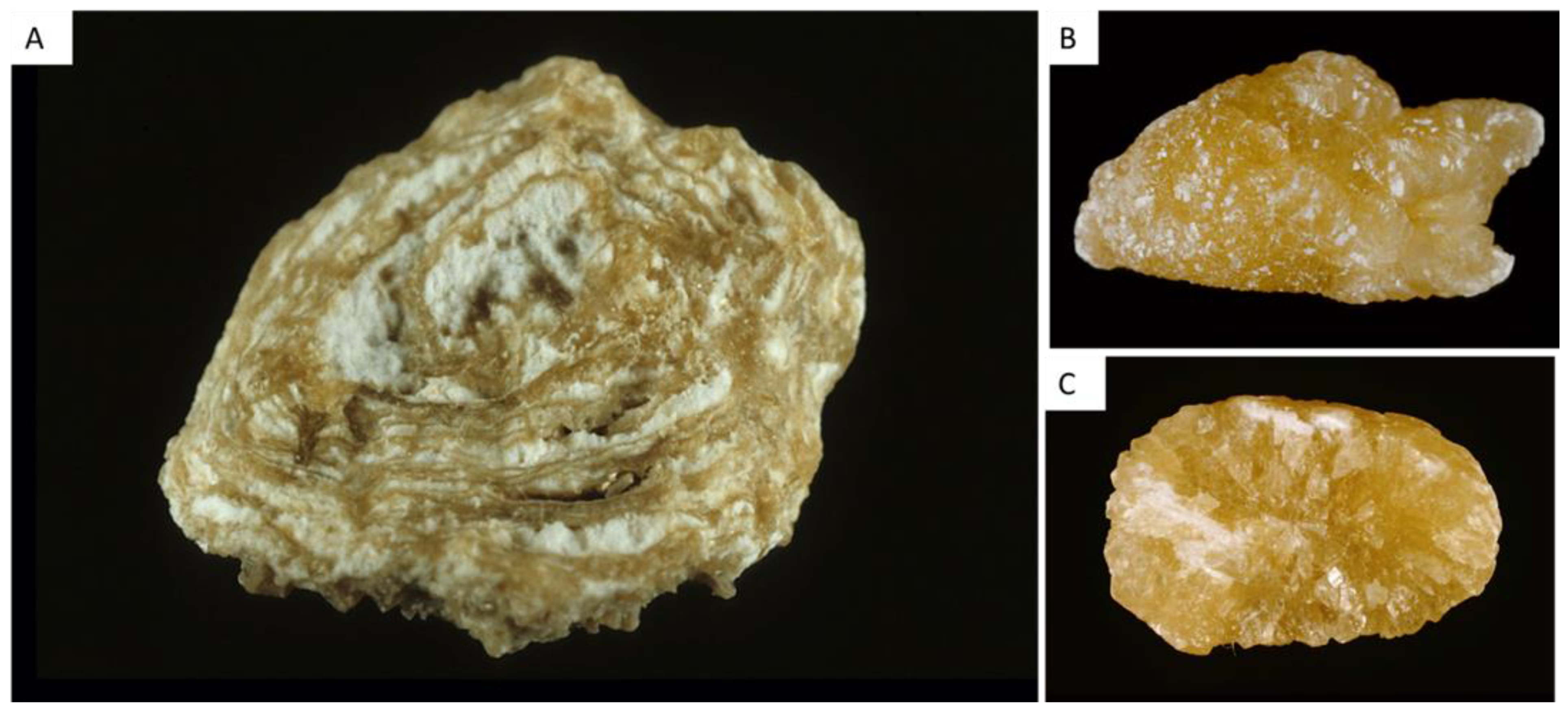
| Type | Main Component | Surface | Section |
|---|---|---|---|
| Ia | Whewellite | Mammillary surface. Frequent umbilication and Randall’s plaque indicative of papillary origin. Color: brown. | Section made of concentric and compact layers with a radiating organization. Color: brown. |
| Ib | Whewellite | Mammillary and rough surface. No umbilication. Color: brown to dark brown. | Compact unorganized section. Sometimes, the presence of gaps. Color: brown to dark brown. |
| Ic | Whewellite | Budding surface. Light color, cream to pale yellow-brown, sometimes whitish in children. | Finely granular and poorly organized section. Light color, cream to pale yellow-brown. |
| Id | Whewellite | Smooth surface. Color: homogeneous, beige or pale brown. | Compact section made of thin concentric layers. Color: pale brown or beige. |
| Ie | Whewellite | Locally budding, mammillary or rough surface. Color: often heterogeneous, pale yellow-brown to brown. | Locally unorganized and loose structure, locally more compact radiating structure. Color: heterogeneous, pale yellow-brown to brown. |
| IIa | Weddellite | Spiculated surface showing aggregated bipyramidal crystals with right angles and sharp edges. Color: pale yellow-brown. | Section showing loose radial crystallization. Color: pale yellow-brown. |
| IIb | Weddellite | Spiculated surface showing aggregated bipyramidal crystals with blunt angles and ridges. Color: pale yellow-brown. | Section showing compact unorganized crystallization. Color: pale yellow-brown. |
| IIc | Weddellite | Rough surface. Color: gray-beige to dark yellow-brown. | Unorganized core with a diffuse concentric compact structure at the periphery. Color: gray-beige to dark yellow-brown. |
| IIIa | Uric acid anhydrous | Homogeneous smooth surface. Color: homogeneous, typically orange, sometimes cream, ochre or yellowish. | Homogeneous compact, concentric structure with a radiating organization. Color: typically orange. |
| IIIb | Uric acid dihydrate ± uric acid anhydrous | Heterogeneous embossed, rough and porous surface. Heterogeneous color from beige to brown-orange. | Poorly organized section with frequent porous areas. Color: orange. |
| IIIc | Urate salts, including ammonium hydrogen urate | Homogeneous or slightly heterogeneous rough and locally porous surface. Color: homogeneous, cream to grayish. | Unorganized porous section. Color: whitish to grayish. |
| IIId | Ammonium hydrogen urate | Heterogeneous embossed, rough and porous surface. Heterogeneous color: grayish to dark brown. | Section made of alternated layers, thick and brownish or thin and whitish to grayish, locally porous. Sometimes, locally purplish. |
| IVa1 | Carbapatite | Homogeneous rough surface. Color: whitish to beige. | Section: poorly organized, or diffuse concentric layers. Color: whitish to beige. |
| IVa2 | Carbapatite | Embossed and varnished surface with small cracks. Glazed appearance. Color: homogeneous, pale brown-yellow to pale brown. | Section made of compact alternated layers, thick brown-yellow and thin beige. Often, multiple nuclei (from collecting duct origin). |
| IVb | Carbapatite + other calcium phosphates (±struvite) | Heterogeneous, both embossed and rough surface with confluent superficial deposits. Heterogeneous color: cream to dark brown. | Section made of irregularly alternating thick, whitish, and thin, brown-yellow layers. |
| IVc | Struvite | Homogenous surface made of amalgamate crystals with blunt angles and edges. | Section: crude radial crystallization. Color: whitish. |
| IVd | Brushite | Finely rough or dappled surface. Color: whitish to beige. | Radial crystallization with more or less visible concentric layers. Color: whitish to beige. |
| Va | Cystine | Rough surface. Color: yellowish. | Section: poorly organized, sometimes a radiating organization. Color: yellowish. |
| Vb | Cystine | Smooth surface. Color: homogeneous, cream to yellowish. | Concentric layers at the periphery, an unorganized core. Color: heterogeneous, cream (periphery) to yellowish (core). |
| VIa | Proteins | Matrix soft calculi. Homogeneous surface. Color: cream to pale brown. | Unorganized section. Color: cream to pale brown. |
| VIb | Proteins ± drugs or metabolic compounds | Heterogeneous, irregularly rough surface. Locally scaled. Color: dark brown to black. | Crude and diffuse foliated structure. Color: dark brown to black. Other components often present in these stones may alter the structure and the color. |
| VIc | Proteins + whewellite | Homogeneous, smooth surface with clefts and scales. Color: dark brown. | Section made of a dark brown protein shield surrounding a loose, unorganized light core containing whewellite crystals mixed with proteins. |
| VII | Miscellaneous | Various morphologies and colors according to the stone composition (infrequent purines and drugs). | Variable organization and color according to the stone composition. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Letavernier, E.; Bazin, D.; Daudon, M. Description of Stone Morphology and Crystalluria Improve Diagnosis and Care of Kidney Stone Formers. Healthcare 2023, 11, 2. https://doi.org/10.3390/healthcare11010002
Letavernier E, Bazin D, Daudon M. Description of Stone Morphology and Crystalluria Improve Diagnosis and Care of Kidney Stone Formers. Healthcare. 2023; 11(1):2. https://doi.org/10.3390/healthcare11010002
Chicago/Turabian StyleLetavernier, Emmanuel, Dominique Bazin, and Michel Daudon. 2023. "Description of Stone Morphology and Crystalluria Improve Diagnosis and Care of Kidney Stone Formers" Healthcare 11, no. 1: 2. https://doi.org/10.3390/healthcare11010002
APA StyleLetavernier, E., Bazin, D., & Daudon, M. (2023). Description of Stone Morphology and Crystalluria Improve Diagnosis and Care of Kidney Stone Formers. Healthcare, 11(1), 2. https://doi.org/10.3390/healthcare11010002





