Vignette Research Methodology: An Essential Tool for Quality Improvement Collaboratives
Abstract
:1. Introduction: How Can Vignette Research Methods Help Address Practice Variation and Support Quality Improvement?
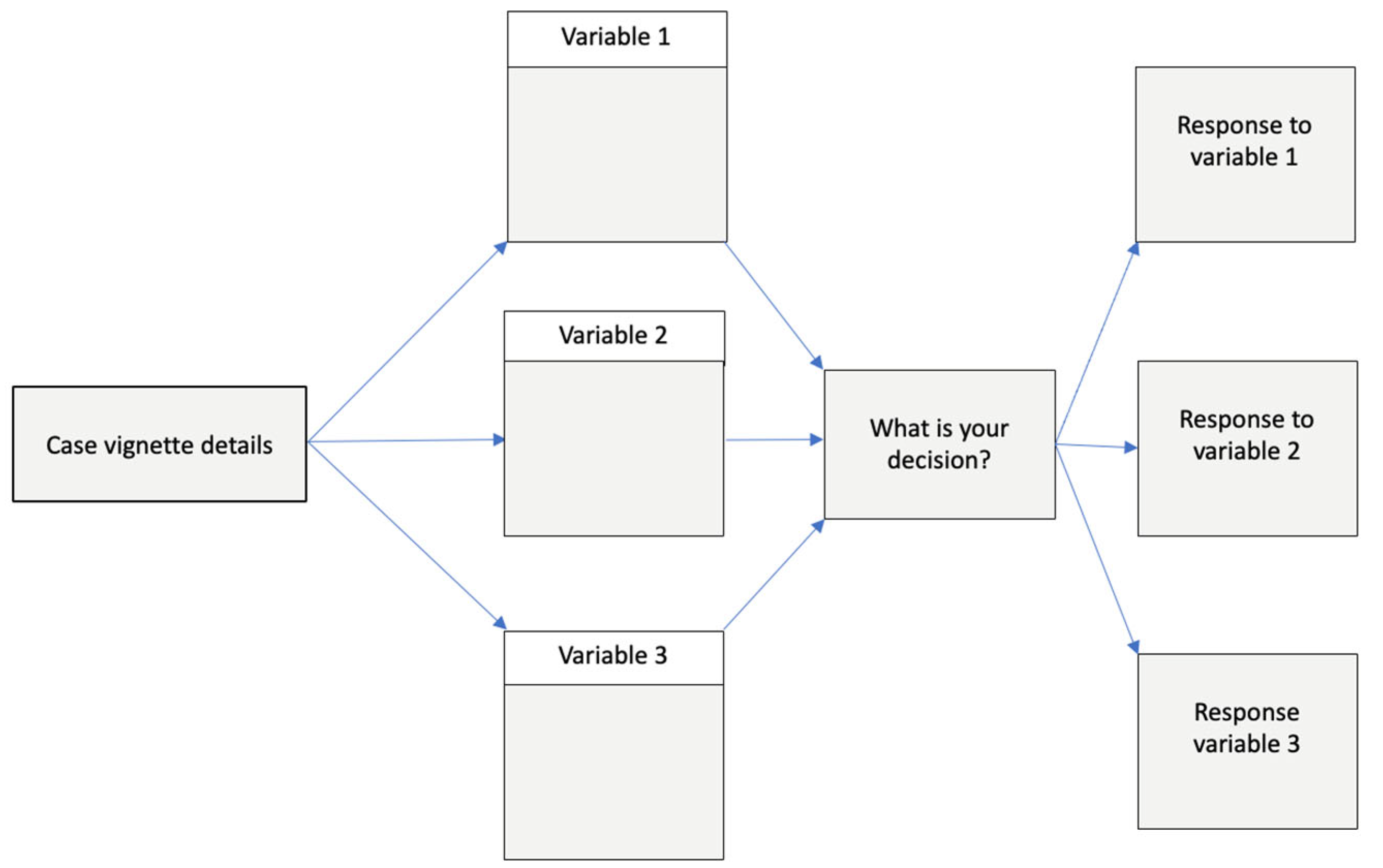
2. What Is Vignette Research Methodology?
3. What Evidence Supports the Validity and Utility of Vignette Methods in Healthcare?
4. How Does Vignette Design Impact the Accuracy and Validity of Results?
5. How Can Vignette Methods Support QICs?
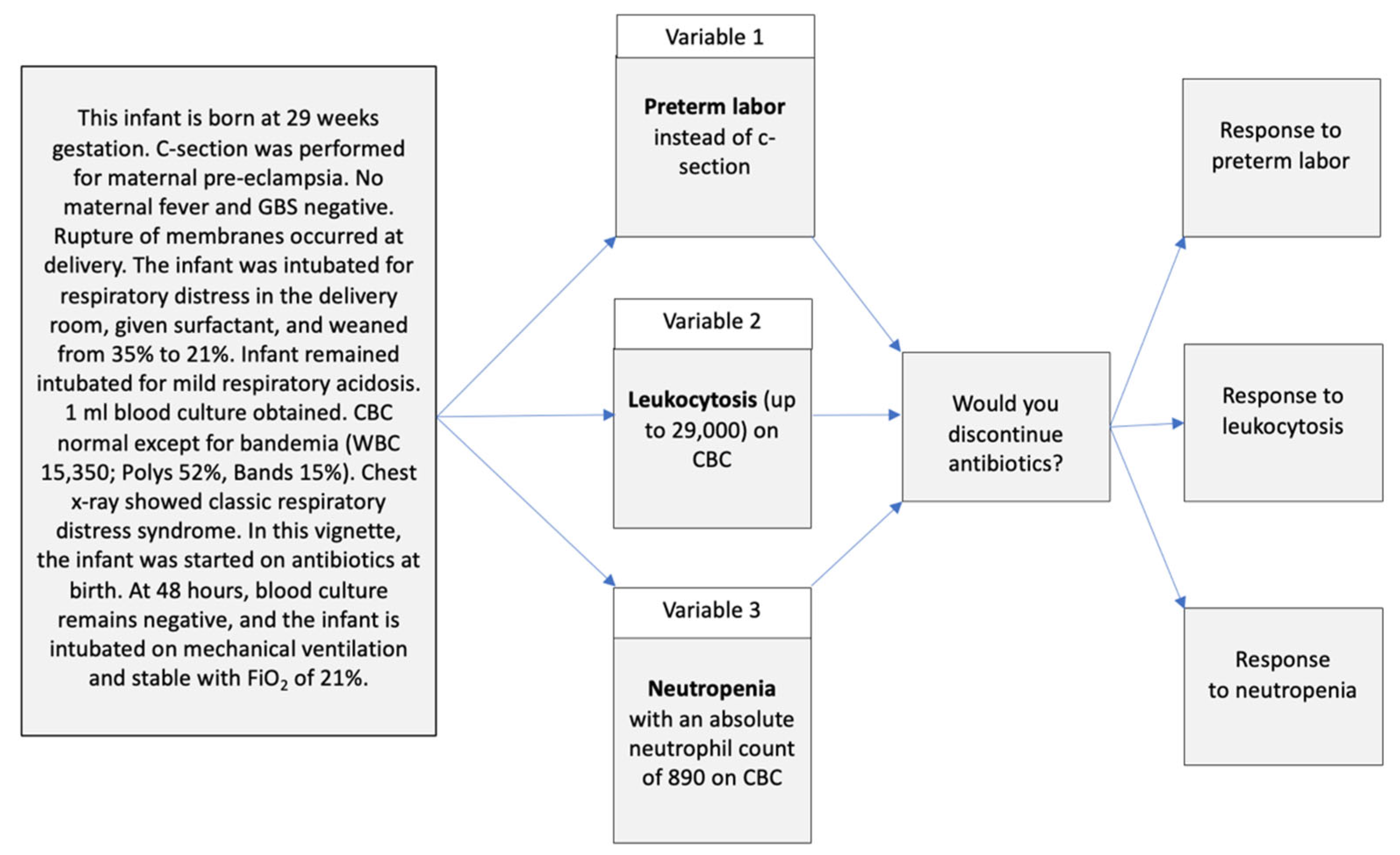
6. How and Why Did We Integrate Vignette Methods into the Antibiotic Stewardship QIC?
7. What Were the Vignette Results and How Did They Benefit the QIC?
8. Discussion: How Do the Vignettes Support the Implementation of Evidence-Based Medicine in QICs?
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pronovost, P.J. Enhancing physicians’ use of clinical guidelines. JAMA 2013, 310, 2501–2502. [Google Scholar] [CrossRef] [PubMed]
- Wennberg, J.E. Unwarranted variations in healthcare delivery: Implications for academic medical centres. BMJ 2002, 325, 961–964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabana, M.D.; Rand, C.S.; Powe, N.R.; Wu, A.W.; Wilson, M.H.; Abboud, P.A.; Rubin, H.R. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA 1999, 282, 1458–1465. [Google Scholar] [CrossRef] [PubMed]
- Committee on the Learning Health Care System in America; Institute of Medicine. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America; Smith, M., Saunders, R., Stuckhardt, L., McGinnis, J.M., Eds.; National Academies Press: Washington, DC, USA, 2013. [Google Scholar]
- Corallo, A.N.; Croxford, R.; Goodman, D.C.; Bryan, E.L.; Srivastava, D.; Stukel, T.A. A systematic review of medical practice variation in OECD countries. Health Policy 2014, 114, 5–14. [Google Scholar] [CrossRef]
- Institute of Medicine Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century; National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Djulbegovic, B. A Framework to Bridge the Gaps Between Evidence-Based Medicine, Health Outcomes, and Improvement and Implementation Science. J. Oncol. Pract. 2014, 10, 200–202. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, M.; Raghavan, A.; Suresh, G.K. Eliminating Undesirable Variation in Neonatal Practice. Clin. Perinatol. 2017, 44, 529–540. [Google Scholar] [CrossRef]
- McDonald, P. How Factorial Survey Analysis Improves Our Understanding of Employer Preferences. Swiss J. Sociol. 2019, 45, 237–260. [Google Scholar] [CrossRef] [Green Version]
- Converse, L.; Barrett, K.; Rich, E.; Reschovsky, J. Methods of Observing Variations in Physicians’ Decisions: The Opportunities of Clinical Vignettes. J. Gen. Intern. Med. 2015, 30, 586–594. [Google Scholar] [CrossRef] [Green Version]
- Veloski, J.; Tai, S.; Evans, A.S.; Nash, D.B. Clinical Vignette-Based Surveys: A Tool for Assessing Physician Practice Variation. Am. J. Med. Qual. 2005, 20, 151–157. [Google Scholar] [CrossRef]
- Sheringham, J.; Kuhn, I.; Burt, J. The use of experimental vignette studies to identify drivers of variations in the delivery of health care: A scoping review. BMC Med. Res. Methodol. 2021, 21, 81. [Google Scholar] [CrossRef]
- Renold, E. Using vignettes in qualitative research. Build. Res. Capacit. 2002, 3, 3–5. [Google Scholar]
- Green, P.E. On the design of choice experiments involving multifactor alternatives. J. Consum. Res. 1974, 1, 61–68. [Google Scholar] [CrossRef]
- Flach, S.D.; Diener, A. Eliciting patients’ preferences for cigarette and alcohol cessation: An application of conjoint analysis. Addict. Behav. 2004, 29, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Alexander, C.S.; Becker, H.J. The Use of Vignettes in Survey Research. Public Opin. Q. 1978, 42, 93–104. [Google Scholar] [CrossRef]
- Evans, S.C.; Roberts, M.C.; Keeley, J.W.; Blossom, J.B.; Amaro, C.M.; Garcia, A.M.; Stough, C.O.; Canter, K.S.; Robles, R.; Reed, G.M. Vignette methodologies for studying clinicians’ decision-making: Validity, utility, and application in ICD-11 field studies. Int. J. Clin. Health Psychol. 2015, 15, 160–170. [Google Scholar] [CrossRef] [Green Version]
- Gidengil, C.A.; Linder, J.A.; Beach, S.; Setodji, C.M.; Hunter, G.; Mehrotra, A. Using Clinical Vignettes to Assess Quality of Care for Acute Respiratory Infections. INQUIRY J. Health Care Organ. Provis. Financ. 2016, 53, 0046958016636531. [Google Scholar] [CrossRef] [Green Version]
- Peabody, J.W.; Luck, J.; Glassman, P.; Dresselhaus, T.R.; Lee, M. Comparison of vignettes, standardized patients, and chart abstraction: A prospective validation study of 3 methods for measuring quality. JAMA 2000, 283, 1715–1722. [Google Scholar] [CrossRef] [Green Version]
- Peabody, J.W.; Luck, J.; Glassman, P.; Jain, S.; Hansen, J.; Spell, M.; Lee, M. Measuring the quality of physician practice by using clinical vignettes: A prospective validation study. Ann. Intern. Med. 2004, 141, 771–780. [Google Scholar] [CrossRef] [Green Version]
- Peabody, J.W.; DeMaria, L.; Smith, O.; Hoth, A.; Dragoti, E.; Luck, J. Large-Scale Evaluation of Quality of Care in 6 Countries of Eastern Europe and Central Asia Using Clinical Performance and Value Vignettes. Glob. Health Sci. Pract. 2017, 5, 412. [Google Scholar] [CrossRef] [Green Version]
- Weems, L.; Strong, J.; Plummer, D.; Martin, J.; Zweng, T.N.; Lindsay, J.; Paculdo, D.; Tran, M.; Peabody, J. A Quality Collaboration in Heart Failure and Pneumonia Inpatient Care at Novant Health: Standardizing Hospitalist Practices to Improve Patient Care and System Performance. Jt. Comm. J. Qual. Patient Saf. Jt. Comm. Resour. 2019, 45, 199–206. [Google Scholar] [CrossRef]
- Burgon, T.; Casebeer, L.; Aasen, H.; Valdenor, C.; Tamondong-Lachica, D.; de Belen, E.; Paculdo, D.; Peabody, J. Measuring and Improving Evidence-Based Patient Care Using a Web-Based Gamified Approach in Primary Care (QualityIQ): Randomized Controlled Trial. J. Med. Internet Res. 2021, 23, e31042. [Google Scholar] [CrossRef]
- Su, D.; Steiner, P.M. An Evaluation of Experimental Designs for Constructing Vignette Sets in Factorial Surveys. Sociol. Methods Res. 2018, 49, 455–497. [Google Scholar] [CrossRef]
- Atzmüller, C.; Steiner, P.M. Experimental Vignette Studies in Survey Research. Methodology 2010, 6, 128–138. [Google Scholar] [CrossRef]
- Pham, T.; Roy, C.; Mariette, X.; Lioté, F.; Durieux, P.; Ravaud, P. Effect of response format for clinical vignettes on reporting quality of physician practice. BMC Health Serv. Res. 2009, 9, 128. [Google Scholar] [CrossRef] [Green Version]
- Bachmann, L.M.; Mühleisen, A.; Bock, A.; ter Riet, G.; Held, U.; Kessels, A.G. Vignette studies of medical choice and judgement to study caregivers’ medical decision behaviour: Systematic review. BMC Med. Res. Methodol. 2008, 8, 50. [Google Scholar] [CrossRef] [Green Version]
- Ellsbury, D.L.; Clark, R.H. Does quality improvement work in neonatology improve clinical outcomes? Curr. Opin. Pediatr. 2017, 29, 129–134. [Google Scholar] [CrossRef]
- Spitzer, A.R. Has Quality Improvement Really Improved Outcomes for Babies in the Neonatal Intensive Care Unit? Clin. Perinatol. 2017, 44, 469–483. [Google Scholar] [CrossRef]
- De la Perrelle, L.; Radisic, G.; Cations, M.; Kaambwa, B.; Barbery, G.; Laver, K. Costs and economic evaluations of Quality Improvement Collaboratives in healthcare: A systematic review. BMC Health Serv. Res. 2020, 20, 155. [Google Scholar] [CrossRef] [Green Version]
- Schouten, L.M.; Hulscher, M.E.; van Everdingen, J.J.; Huijsman, R.; Grol, R.P. Evidence for the impact of quality improvement collaboratives: Systematic review. BMJ 2008, 336, 1491–1494. [Google Scholar] [CrossRef] [Green Version]
- Wells, S.; Tamir, O.; Gray, J.; Naidoo, D.; Bekhit, M.; Goldmann, D. Are quality improvement collaboratives effective? A systematic review. BMJ Qual. Saf. 2018, 27, 226–240. [Google Scholar] [CrossRef]
- Lee, H.C.; Bennett, M.V.; Crockett, M.; Crowe, R.; Gwiazdowski, S.G.; Keller, H.; Kurtin, P.; Kuzniewicz, M.; Mazzeo, A.M.; Schulman, J.; et al. Comparison of Collaborative Versus Single-Site Quality Improvement to Reduce NICU Length of Stay. Pediatrics 2018, 142, e20171395. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Kurtin, P.S.; Wight, N.E.; Chance, K.; Cucinotta-Fobes, T.; Hanson-Timpson, T.A.; Nisbet, C.C.; Rhine, W.D.; Risingsun, K.; Wood, M.; et al. A quality improvement project to increase breast milk use in very low birth weight infants. Pediatrics 2012, 130, e1679–e1687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.C.; Powers, R.J.; Bennett, M.V.; Finer, N.N.; Halamek, L.P.; Nisbet, C.; Crockett, M.; Chance, K.; Blackney, D.; von Köhler, C.; et al. Implementation Methods for Delivery Room Management: A Quality Improvement Comparison Study. Pediatrics 2014, 134, e1378–e1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wirtschafter, D.D.; Powers, R.J.; Pettit, J.S.; Lee, H.C.; Boscardin, W.J.; Ahmad Subeh, M.; Gould, J.B. Nosocomial infection reduction in VLBW infants with a statewide quality-improvement model. Pediatrics 2011, 127, 419–426. [Google Scholar] [CrossRef] [Green Version]
- Gould, J.B. Building the First Statewide Quality Improvement Collaborative, the CPQCC: A Historic Perspective. Children 2020, 7, 177. [Google Scholar] [CrossRef]
- Wirtschafter, D.D.; Danielsen, B.H.; Main, E.K.; Korst, L.M.; Gregory, K.D.; Wertz, A.; Stevenson, D.K.; Gould, J.B.; California Perinatal Quality Care Collaborative. Promoting antenatal steroid use for fetal maturation: Results from the California Perinatal Quality Care Collaborative. J. Pediatr. 2006, 148, 606–612. [Google Scholar] [CrossRef]
- Institute for Healthcare Improvement. The Breakthrough Series: IHI’s Collaborative Model for Achieving Breakthrough Improvement; IHI Innovation Series white paper; Institute for Healthcare Improvement: Boston, MA, USA, 2003. [Google Scholar]
- Shah, V.; Warre, R.; Lee, S.K. Quality Improvement Initiatives in Neonatal Intensive Care Unit Networks: Achievements and Challenges. Acad. Pediatr. 2013, 13, S75–S83. [Google Scholar] [CrossRef]
- Schulman, J.; Benitz, W.E.; Profit, J.; Lee, H.C.; Duenas, G.; Bennett, M.V.; Jocson, M.A.L.; Schutzengel, R.; Gould, J.B. Newborn Antibiotic Exposures and Association With Proven Bloodstream Infection. Pediatrics 2019, 144, e20191105. [Google Scholar] [CrossRef]
- Schulman, J.; Dimand, R.J.; Lee, H.C.; Duenas, G.V.; Bennett, M.V.; Gould, J.B. Neonatal Intensive Care Unit Antibiotic Use. Pediatrics 2015, 135, 826–833. [Google Scholar] [CrossRef] [Green Version]
- Schulman, J.; Profit, J.; Lee, H.C.; Dueñas, G.; Bennett, M.V.; Parucha, J.; Jocson, M.A.L.; Gould, J.B. Variations in Neonatal Antibiotic Use. Pediatrics 2018, 142, e20180115. [Google Scholar] [CrossRef] [Green Version]
- Joshi, N.S.; Huynh, K.; Lu, T.; Lee, H.C.; Frymoyer, A. Epidemiology and trends in neonatal early onset sepsis in California, 2010–2017. J. Perinatol. 2022, 42, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Schrag, S.J.; Farley, M.M.; Petit, S.; Reingold, A.; Weston, E.J.; Pondo, T.; Hudson Jain, J.; Lynfield, R. Epidemiology of Invasive Early-Onset Neonatal Sepsis, 2005 to 2014. Pediatrics 2016, 138, e20162013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Payton, K.S.E.; Wirtschafter, D.; Bennett, M.V.; Benitz, W.E.; Lee, H.C.; Kristensen-Cabrera, A.; Nisbet, C.C.; Gould, J.; Parker, C.; Sharek, P.J. Vignettes Identify Variation in Antibiotic Use for Suspected Early Onset Sepsis. Hosp. Pediatr. 2021, 11, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Meeker, D.; Linder, J.A.; Fox, C.R.; Friedberg, M.W.; Persell, S.D.; Goldstein, N.J.; Knight, T.K.; Hay, J.W.; Doctor, J.N. Effect of Behavioral Interventions on Inappropriate Antibiotic Prescribing Among Primary Care Practices. JAMA 2016, 315, 562. [Google Scholar] [CrossRef] [Green Version]
- Harrison, R.; Hinchcliff, R.A.; Manias, E.; Mears, S.; Heslop, D.; Walton, V.; Kwedza, R. Can feedback approaches reduce unwarranted clinical variation? A systematic rapid evidence synthesis. BMC Health Serv. Res. 2020, 20, 40. [Google Scholar] [CrossRef] [Green Version]
- Mjelle, A.B.; Guthe, H.J.T.; Reigstad, H.; Bjørke Monsen, A.L.; Markestad, T. Serum concentrations of C-reactive protein in healthy term-born Norwegian infants 48–72 hours after birth. Acta Paediatr. 2018, 108, 849–854. [Google Scholar] [CrossRef]
- Perrone, S.; Lotti, F.; Longini, M.; Rossetti, A.; Bindi, I.; Bazzini, F.; Belvisi, E.; Sarnacchiaro, P.; Scapellato, C.; Buonocore, G. Creactive protein in healthy term newborns during the first 48 hours of life. Arch. Dis. Child. Fetal Neonatal Ed. 2017, 103, F163–F166. [Google Scholar] [CrossRef]
- Puopolo, K.M.; Benitz, W.E.; Zaoutis, T.E. Management of Neonates Born at ≥35 0/7 Weeks’ Gestation With Suspected or Proven Early-Onset Bacterial Sepsis. Pediatrics 2018, 142, e20182894. [Google Scholar] [CrossRef] [Green Version]
- Puopolo, K.M.; Benitz, W.E.; Zaoutis, T.E. Management of Neonates Born at ≤34 6/7 Weeks’ Gestation With Suspected or Proven Early-Onset Bacterial Sepsis. Pediatrics 2018, 142, e20182896. [Google Scholar] [CrossRef] [Green Version]
- Donabedian, A. Evaluating the quality of medical care. 1966. Milbank Q. 2005, 83, 691–729. [Google Scholar] [CrossRef] [Green Version]
- Johnson, M.J.; May, C.R. Promoting professional behaviour change in healthcare: What interventions work, and why? A theory-led overview of systematic reviews. BMJ Open 2015, 5, e008592. [Google Scholar] [CrossRef]
- Rynkiewich, K. Finding “What’s Wrong with Us”: Antibiotic Prescribing Practice Among Physicians in the United States. Front. Sociol. 2020, 5, 5. [Google Scholar] [CrossRef] [Green Version]
- Zamboni, K.; Baker, U.; Tyagi, M.; Schellenberg, J.; Hill, Z.; Hanson, C. How and under what circumstances do quality improvement collaboratives lead to better outcomes? A systematic review. Implement Sci. 2020, 15, 27. (In English) [Google Scholar] [CrossRef]



| How QICs Influence Individuals to Follow Evidence-Based Practice Guidelines | Vignette Results |
|---|---|
| Increased commitment and confidence in using data to prioritize problems that they can impact | Provide objective data on variation and decisions; Data visualizations of variation can guide discussions about priority areas to target |
| Increased accountability by making optimal clinical approach very clear | Display range of variation allowing constructive discourse on optimal approaches to improvement |
| Provide opportunity for peer reflection and group problem solving | Graphical displays comparing proportions of individuals making each decision provide a concrete visualization of opportunities |
| Bottom-up, inclusive team-oriented shared responsibility; Culture of joint problem solving | Vignettes help reach individuals that may feel distant from the QI work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Payton, K.S.E.; Gould, J.B. Vignette Research Methodology: An Essential Tool for Quality Improvement Collaboratives. Healthcare 2023, 11, 7. https://doi.org/10.3390/healthcare11010007
Payton KSE, Gould JB. Vignette Research Methodology: An Essential Tool for Quality Improvement Collaboratives. Healthcare. 2023; 11(1):7. https://doi.org/10.3390/healthcare11010007
Chicago/Turabian StylePayton, Kurlen S. E., and Jeffrey B. Gould. 2023. "Vignette Research Methodology: An Essential Tool for Quality Improvement Collaboratives" Healthcare 11, no. 1: 7. https://doi.org/10.3390/healthcare11010007





