Effectiveness of Vestibular Rehabilitation after Concussion: A Systematic Review of Randomised Controlled Trial
Abstract
1. Introduction
2. Methods
2.1. Standards
2.2. Search Strategy and Data Extraction (Information Sources)
2.3. Study Selection
2.4. Statistical Analysis and Narrative Synthesis
2.5. Risk of Bias
3. Results
3.1. Patient Demographics
3.2. Types of Interventions
3.3. Vestibular Rehabilitation
3.4. Outcomes
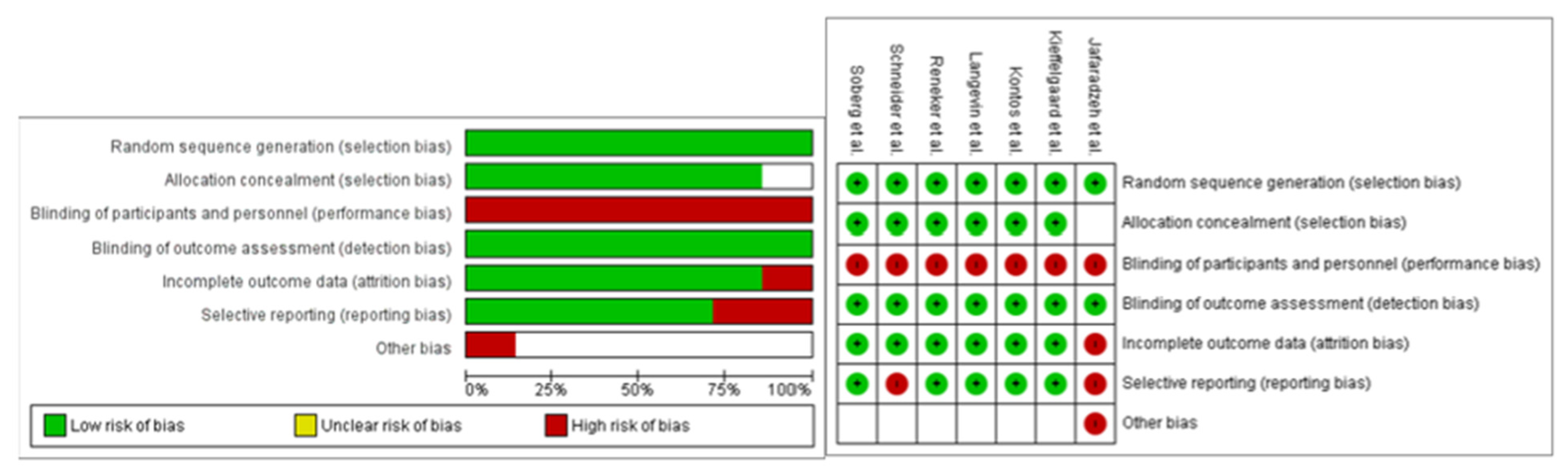
3.5. Risk of Bias
3.6. Efficacy of Intervention: Analysis (Synthesis of Results)
3.6.1. Return to Sport
3.6.2. Dizziness
3.6.3. Subjective Reports of Concussion Symptoms
3.6.4. Balance
3.6.5. Gait Impairment
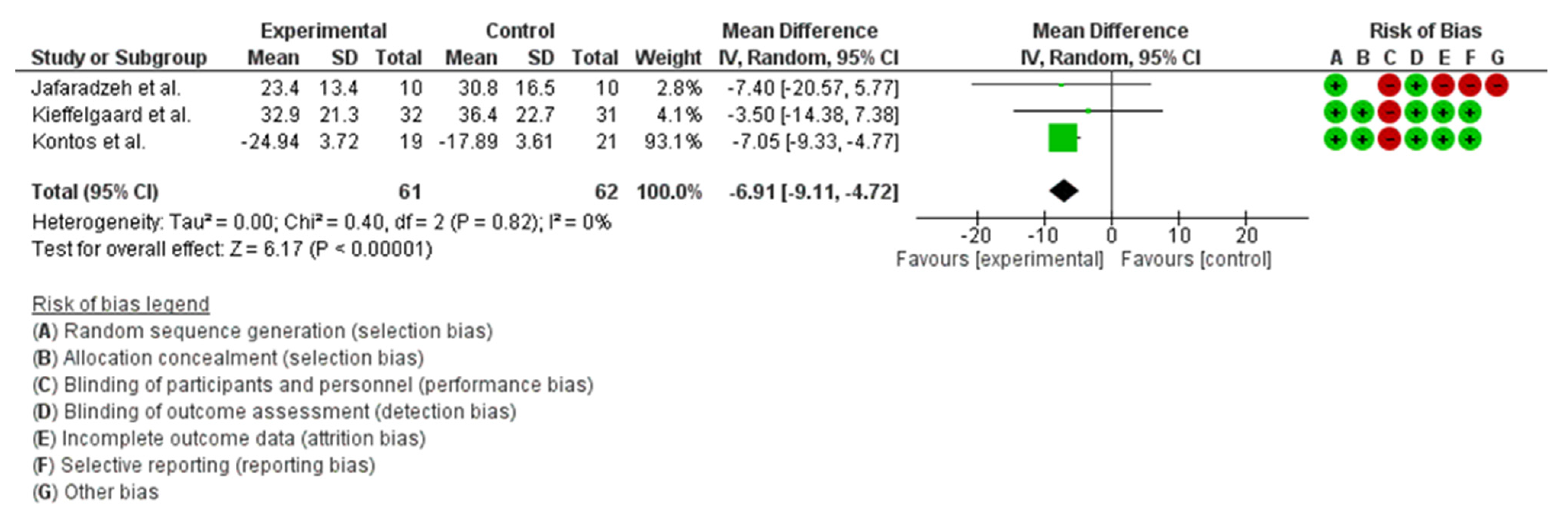
3.6.6. Quality of Life
3.6.7. Gaze Stabilisation
4. Discussion
4.1. Acuity and Return to Sport
4.2. Dizziness
4.3. Subjective Reports of Concussion Symptoms
4.4. Balance
4.5. Gait Impairments
4.6. Quality of Life
4.7. Gaze Stabilisation and VOR
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mucha, A.; Fedor, S.; DeMarco, D. Vestibular dysfunction and concussion. Handb. Clin. Neurol. 2018, 158, 135–144. [Google Scholar] [PubMed]
- Alsalaheen, B.A.; Mucha, A.; Morris, L.O.; Whitney, S.L.; Furman, J.M.; Camiolo-Reddy, C.E.; Collins, M.W.; Lovell, M.R.; Sparto, P.J. Vestibular rehabilitation for dizziness and balance disorders after concussion. J. Neurol. Phys. Ther. 2010, 34, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Broglio, S.P.; Collins, M.W.; Williams, R.M.; Mucha, A.; Kontos, A.P. Current and emerging rehabilitation for concussion: A review of the evidence. Clin. Sport. Med. 2015, 34, 213–231. [Google Scholar] [CrossRef] [PubMed]
- Han, B.I.; Song, H.S.; Kim, J.S. Vestibular rehabilitation therapy: Review of indications, mechanisms, and key exercises. J. Clin. Neurol. 2011, 7, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Murray, D.A.; Meldrum, D.; Lennon, O. Can vestibular rehabilitation exercises help patients with concussion? A systematic review of efficacy, prescription and progression patterns. Br. J. Sport. Med. 2017, 51, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Herdman, S. Vestibular Rehabilitation, 3rd ed.; F.A. Davis Company: Philadelphia, PA, USA, 2007. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 105906. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. Bmj 2015, 349, g7647. [Google Scholar] [CrossRef]
- Ardern, C.L.; Büttner, F.; Andrade, R.; Weir, A.; Ashe, M.C.; Holden, S.; Impellizzeri, F.M.; Delahunt, E.; Dijkstra, H.P.; Mathieson, S.; et al. Implementing the 27 PRISMA 2020 Statement items for systematic reviews in the sport and exercise medicine, musculoskeletal rehabilitation and sports science fields: The PERSiST (implementing Prisma in Exercise, Rehabilitation, Sport medicine and SporTs science) guidance. Br. J. Sport. Med. 2022, 56, 175–195. [Google Scholar]
- Varner, C.E.; Thompson, C.; de Wit, K.; Borgundvaag, B.; Houston, R.; McLeod, S. A randomized trial comparing prescribed light exercise to standard management for emergency department patients with acute mild traumatic brain injury. Acad. Emerg. Med. 2021, 28, 493–501. [Google Scholar] [CrossRef]
- Quatman-Yates, C.C.; Hunter-Giordano, A.; Shimamura, K.K.; Landel, R.; Alsalaheen, B.A.; Hanke, T.A.; McCulloch, K.L.; Altman, R.D.; Beattie, P.; Berz, K.E.; et al. Physical therapy evaluation and treatment after concussion/mild traumatic brain injury: Clinical practice guidelines linked to the international classification of functioning, disability and health from the academy of orthopaedic physical therapy, American Academy of sports physical therapy, academy of neurologic physical therapy, and academy of pediatric physical therapy of the American Physical therapy association. J. Orthop. Sport. Phys. Ther. 2020, 50, CPG1–CPG73. [Google Scholar]
- Bailey, C.; Meyer, J.; Briskin, S.; Tangen, C.; Hoffer, S.A.; Dundr, J.; Brennan, B.; Smith, P. Multidisciplinary concussion management: A model for outpatient concussion management in the acute and post-acute settings. J. Head Trauma Rehabil. 2019, 34, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Micay, R.; Richards, D.; Hutchison, M.G. Feasibility of a postacute structured aerobic exercise intervention following sport concussion in symptomatic adolescents: A randomised controlled study. BMJ Open Sport Exerc. Med. 2018, 4, e000404. [Google Scholar] [CrossRef] [PubMed]
- Dobney, D.M.; Grilli, L.; Beaulieu, C.; Straub, M.; Galli, C.; Saklas, M.; Friedman, D.; Dubrovsky, A.S.; Gagnon, I.J. Feasibility of early active rehabilitation for concussion recovery in youth: A randomized trial. Clin. J. Sport Med. 2020, 30, 519–525. [Google Scholar] [CrossRef]
- Kurowski, B.G.; Hugentobler, J.; Quatman-Yates, C.; Taylor, J.; Gubanich, P.J.; Altaye, M.; Wade, S.L. Aerobic exercise for adolescents with prolonged symptoms after mild traumatic brain injury: An exploratory randomized clinical trial. J. Head Trauma Rehabil. 2017, 32, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Chrisman, S.P.D.; Whitlock, K.B.; Mendoza, J.A.; Burton, M.S.; Somers, E.; Hsu, A.; Fay, L.; Palermo, T.M.; Rivara, F. P Pilot randomized controlled trial of an exercise program requiring minimal in-person visits for youth with persistent sport-related concussion. Front. Neurol. 2019, 10, 623. [Google Scholar] [CrossRef] [PubMed]
- Straudi, S.; Severini, G.; Sabbagh Charabati, A.; Pavarelli, C.; Gamberini, G.; Scotti, A.; Basaglia, N. The effects of video game therapy on balance and attention in chronic ambulatory traumatic brain injury: An exploratory study. BMC Neurol. 2017, 17, 86. [Google Scholar] [CrossRef] [PubMed]
- Vegt, C.B.V.; Hill-Pearson, C.A.; Hershaw, J.N.; Loftin, M.C.; Bobula, S.A.; Souvignier, A.R. A comparison of generalized and individualized vestibular rehabilitation therapy in a military TBI sample. J. Head Trauma Rehabil. 2022, 10, 1097. [Google Scholar]
- Tefertiller, C.; Hays, K.; Natale, A.; O’Dell, D.; Ketchum, J.; Sevigny, M.; Eagye, C.; Philippus, A.; Harrison-Felix, C. Results from a randomized controlled trial to address balance deficits after traumatic brain injury. Arch. Phys. Med. Rehabil. 2019, 100, 1409–1416. [Google Scholar] [CrossRef]
- Sisodia, V.; Hamid, J.; Guru, K. Efficacy of vestibular rehabilitation in management of balance deficit in Indian collegiate football players, with sport-related concussion–randomized clinical trial. Physiotherapy 2015, 101, e1403–e1404. [Google Scholar] [CrossRef][Green Version]
- Hays, K.; O’Dell, D.R.; Cuthbert, J.P.; Tefertiller, C.; Natale, A. Virtual-Reality Based Therapy for Balance Deficits during Traumatic Brain Injury Inpatient Rehabilitation. Arch. Phys. Med. Rehabil. 2015, 96, e44. [Google Scholar] [CrossRef]
- Cuff, S.; Rose, S.; Young, J. Early intervention in pediatric concussion patients with dizziness and balance problems. Clin. J. Sport Med. 2014, 24, 186–187. [Google Scholar]
- Kleffelgaard, I.; Soberg, H.L.; Tamber, A.L.; Bruusgaard, K.A.; Pripp, A.H.; Sandhaug, M.; Langhammer, B. The effects of vestibular rehabilitation on dizziness and balance problems in patients after traumatic brain injury: A randomized controlled trial. Clin. Rehabil. 2019, 33, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, S.; Pourbakht, A.; Bahrami, E.; Jalaie, S.; Bayat, A. Effect of early vestibular rehabilitation on vertigo and unsteadiness in patients with acute and sub-acute head trauma. Iran. J. Otorhinolaryngol. 2018, 30, 85. [Google Scholar] [PubMed]
- Reneker, J.C.; Hassen, A.; Phillips, R.S.; Moughiman, M.C.; Donaldson, M.; Moughiman, J. Feasibility of early physical therapy for dizziness after a sports-related concussion: A randomized clinical trial. Scand. J. Med. Sci. Sports 2017, 27, 2009–2018. [Google Scholar] [CrossRef] [PubMed]
- Soberg, H.L.; Andelic, N.; Langhammer, B.; Tamber, A.L.; Bruusgaard, K.A.; Kleffelgaard, I. Effect of vestibular rehabilitation on change in health-related quality of life in patients with dizziness and balance problems after traumatic brain injury: A randomized controlled trial. J. Rehabil. Med. 2021, 53, 2781. [Google Scholar] [PubMed]
- Kontos, A.P.; Eagle, S.R.; Mucha, A.; Kochick, V.; Reichard, J.; Moldolvan, C.; Holland, C.L.; Blaney, N.A.; Collins, M.W. A randomized controlled trial of precision vestibular rehabilitation in adolescents following concussion: Preliminary findings. J. Pediatr. 2021, 239, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Langevin, P.; Frémont, P.; Fait, P.; Dubé, M.O.; Bertrand-Charette, M.; Roy, J.S. Cervicovestibular Rehabilitation in Adults with Mild Traumatic Brain Injury: A Randomized Clinical Trial. J. Neurotrauma 2022, 39, 487–496. [Google Scholar] [CrossRef]
- Schneider, K.J.; Meeuwisse, W.H.; Nettel-Aguirre, A.; Barlow, K.; Boyd, L.; Kang, J.; Emery, C.A. Cervicovestibular rehabilitation in sport-related concussion: A randomised controlled trial. Br. J. Sport. Med. 2014, 48, 1294–1298. [Google Scholar] [CrossRef]
- Friscia, L.A.; Morgan, M.T.; Sparto, P.J.; Furman, J.M.; Whitney, S.L. Responsiveness of self-report measures in individuals with vertigo, dizziness and unsteadiness. Otol. Neurotol. 2014, 35, 884. [Google Scholar] [CrossRef]
- McDonnell, M.N.; Hillier, S.L. Vestibular rehabilitation for unilateral peripheral vestibular dysfunction. Cochrane Database Syst. Rev. 2015, 1, CD005397. [Google Scholar] [CrossRef]
- Langlois, J.A.; Rutland-Brown, W.; Wald, M.M. The epidemiology and impact of traumatic brain injury: A brief overview. J. Head Trauma Rehabil. 2006, 21, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Ponsford, J.; Willmott, C.; Rothwell, A.; Cameron, P.; Kelly, A.-M.; Nelms, R.; Curran, C.; Ng, K. Factors influencing outcome following mild traumatic brain injury in adults. J. Int. Neuropsychol. Soc. 2000, 6, 568–579. [Google Scholar] [CrossRef] [PubMed]

| Adaptation Exercise | process where nerve impulses in the brain are able to shift or “adapt” to the incorrect signals from the damaged vestibular system. This gradual shift allows your brain to recalibrate itself. |
| Substitution | recovery principle uses other body functions or strategies to replace the missing vestibular function. |
| Habituation | process allows you to gradually desensitize yourself to vestibular movement and stimulation if you are repeatedly exposed to it. |
| Replacement | different repositioning maneuvers can be performed to help resolve the spinning that occurs due to position changes. |
| Study Author/Year | Type of Study | Sample | Intervention | Outcome Measure | Results |
|---|---|---|---|---|---|
| Soberg H.L. et al. (2021) [26] | Single blind RCT | n = 64 (19 males, 45 females). Mean age was 39.4 (SD 13.0). There was a measure at the baseline (T0) then at the first follow-up (T1) at 2.7 (SD 0.8) months after the baseline. The second follow-up (T2) was 4.4 (SD 1.0) months after the baseline. | Both groups received the TAU Intervention Group: TAU combined with an individualised group-based VR programme, 16 sessions in 8 weeks. VR exercises were tailored and described in another study (27). Control group: only TAU. | QOLIBRI and HRQL were the main outcome measures. RPQ, VSS-SF, and HADS are the secondary outcome measures. | Significant group effect in favour of the intervention group in HRQL on the QOLIBRI. The score at T0 of the QOLIBRI was between 45.4 and 66.7 (SD between 19.2 and 22.7), while at T2, the score was between 55.3 and 66.6 (SD between 20.3 and 24.7). The p-value for the QOLIBRI was <0.02. |
| Reneker J.C. et al. (2017) [25] | Double-blind RCT | n = 41. The population included athletes, participating in sports aged 10–23 years with an acute concussion and dizziness diagnosed with PCS. The intervention group (n = 22) with a mean age of 16.5, control group (n = 19) with a mean age of 15.9. The follow-up was made after a 4-week period. | Group 1: The PT designed an individualised and progressive treatment plan. VR included different techniques (including habituation and adaptation), oculomotor control, neuromotor control (including proprioceptive and kinesthetic awareness), and balance exercises were added to each subject’s treatment regimen as indicated Generally, each intervention session lasted between 30 and 60 minutes. Group 2: The PT delivered interventions that ranged from sham, sub-therapeutic, and non-progressive therapeutic techniques to minimally progressive therapeutic techniques. | Primary outcomes: symptomatic recovery with PCS and medical clearance for RTP. | The median time for medical release was 10.5 days sooner in the experimental group than in the control group. The median time for PCS recovery was 3.5 days sooner in the experimental group than in the control group. Considering Cox proportional hazards regression for time until medical release for RTP, the experimental group demonstrated a hazard ratio of 2.91 compared to the control group. (95% CI: 1.01, 8.43). |
| Jafarzadeh, S, et al. (2018) [24] | RCT | n = 20 adult patients (aged 18–60 years). Patients had a mean age of 44.2 (SD 12.6). The follow-up was after 4 weeks of rehabilitation. | Participants were randomly divided into two groups. Control Group: received the usual medical therapy (Betaserc 8 mg pills; at least three pills per day). Intervention Group: received medical therapy and VR after a 4-week period. Different VR techniques were proposed considering the baseline condition of the patients. Different gaze stabilisation and adaptation exercises were used in all patients, although substitution exercises including standing and walking exercise were used only in patients with unsteadiness. More detailed data were summarized in the study. | DHI | Early vestibular rehabilitation programme can decrease vertigo symptoms and increase stability and balance performance. Medical therapy group at week one was 1.8 (SD = 10.9) while at week four was 0.2 (SD = 7.8). The medical therapy and vestibular rehabilitation group at week 1 was −2.0 (SD = 8.7) while at week four was 20.0 (SD = 11.0) with p = 0.000. |
| Kleffelgaard I. et al. (2019) [23] | RCT | n = 65 with TBI (45 females and 19 males). Intervention group (n = 32) with a mean age of 37.6 (SD 12.3) and control group (n = 31) with a mean age of 41.2 (SD 13.6). Baseline at 3.5 (mean) months after injury. First follow-up at a mean of 2.7 months. Second follow-up at two months after the end of the intervention. | Control group: (n = 32) did not receive any rehabilitation intervention. Intervention group: (n = 33) received a group-based vestibular rehabilitation. VR exercises were tailored and described in another study (27). The intervention was twice weekly for eight weeks. Both groups received usually multidisciplinary outpatient care. | Primary outcome: DHI Secondary Outcome: HiMAT, VSSV, VSSa, RP3, RPQ13, HADSa, HADSd, and BESS | First follow-up, statistically significant mean differences in favour of the intervention were found in DHI (−8.7 points, 95% CI: –16.6 to −0.9) and HiMAT (3.7 points, 95% CI: 1.4–6.0). The p-value was significant for first follow-up: the DHI p = 0.03 and the HiMAT p = 0.002. No significant difference in other outcomes. |
| Schneider M.J. et al. (2014) [29] | RCT | Treatment group (n = 15): 11 males, 4 females. Median age: 15 (SD 12–27). Control group (n = 16): 7 males, 9 females. Median age: 15 (SD 13–30). | Both groups performed non-provocative range of motion exercises, stretching, and postural education. Treatment group: in addition, received an individual designed vestibular rehabilitation and cervical spine physiotherapy. VR includes an individualised programme of habituation, gaze stabilisation, adaptation exercises, standing balance exercises, dynamic balance exercises, and canalith repositioning manoeuvres. | (1) Number of days until medical clearance to return to sport. (2) 11-point Numeric Pain Rating Scale score, ABC scale, DHI, SCAT2, DVA, head thrust test, modified motion sensitivity test, FGA, CFE, and JPE. | Return to Sport: OR 10.27, p < 0.001 for return to sport in 8 weeks for the intervention group. Intention to treat analysis: OR 3.91 (95% CI 1.34 to 11.34) for the treatment group to be medically cleared to return to sport compared with the control group, (p = 0.002). No between-group analyses for secondary outcomes were reported. |
| Kontos A.P. et al. (2021) [27] | RCT | Treatment group (n = 25): 16 females, 9 males. Median age: 15.3 (SD 1.6). Control group (n = 25): 15 females, 10 males. Median age: 15.3 (SD 1.7). The outcomes were recorded at 2 and 4 weeks post-intervention. The participants who were recovered by 2 or 4 weeks stopped the intervention and completed the clinical outcomes. | Both groups performed a behavioural management. Treatment group performed also individual VR and home VR exercises for 30 minutes per day. Control group: performed stretching and physical activity for 30 minutes per day. | VOMS: to assess the VOR, DHI, mBESS, and PCSS | There was a medium treatment effect size for horizontal VOR and VMS (0.09–0.11) and large for vertical VOR (0.16). The subscales of DHI-F demonstrated a medium treatment effect size (0.06–0.1), whereas all other secondary outcomes demonstrated a small treatment effect (0.01–0.06). Significant statistical difference was shown only for horizontal VOR (p = 0.04) and vertical VOR (p = 0.01). No other significantly differences were shown. |
| Langevin P. et al. (2022) [28] | RCT | Treatment group: (n = 30): 20 females, 10 males. Mean age: 38.9 (SD 14.56) Control group (n = 30): 21 females, 9 males. Mean age: 39.07 (SD 12.63). The outcomes were recorded at baseline, and after 3, 6, 12, and 26 weeks. | Both groups received education and advice about exercise tolerance and concussion. Control group received 8 sessions in 6 weeks of supervised cardiovascular exercise. Treatment group received the same treatment as control group +1 to 8 sessions of cervicovestibular treatment. The treatment consisted in manual and therapeutic exercises for cervical spine and repositioning manoeuvre, vestibular adaptation, ocular motor exercise, balance, and habituation exercise | PCSS, DHI, NPRS, clearance to return to function, VOMS, and head impulse test (HIT). | No group by time interaction difference was observed for PCSS, DHI, NPRS, and return to function. All the groups demonstrated a statistically significant difference from the baseline. A group by time interaction was observed for horizontal and vertical VOR in favour of the treatment group at 6 weeks (p < 0.01). A difference for group interactions was observed for HIT (p < 0.01). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galeno, E.; Pullano, E.; Mourad, F.; Galeoto, G.; Frontani, F. Effectiveness of Vestibular Rehabilitation after Concussion: A Systematic Review of Randomised Controlled Trial. Healthcare 2023, 11, 90. https://doi.org/10.3390/healthcare11010090
Galeno E, Pullano E, Mourad F, Galeoto G, Frontani F. Effectiveness of Vestibular Rehabilitation after Concussion: A Systematic Review of Randomised Controlled Trial. Healthcare. 2023; 11(1):90. https://doi.org/10.3390/healthcare11010090
Chicago/Turabian StyleGaleno, Erasmo, Edoardo Pullano, Firas Mourad, Giovanni Galeoto, and Francesco Frontani. 2023. "Effectiveness of Vestibular Rehabilitation after Concussion: A Systematic Review of Randomised Controlled Trial" Healthcare 11, no. 1: 90. https://doi.org/10.3390/healthcare11010090
APA StyleGaleno, E., Pullano, E., Mourad, F., Galeoto, G., & Frontani, F. (2023). Effectiveness of Vestibular Rehabilitation after Concussion: A Systematic Review of Randomised Controlled Trial. Healthcare, 11(1), 90. https://doi.org/10.3390/healthcare11010090









