What Is Known about Midazolam? A Bibliometric Approach of the Literature
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Search and Selection Strategy
2.2. Eligibility Criteria
2.3. Data Extraction
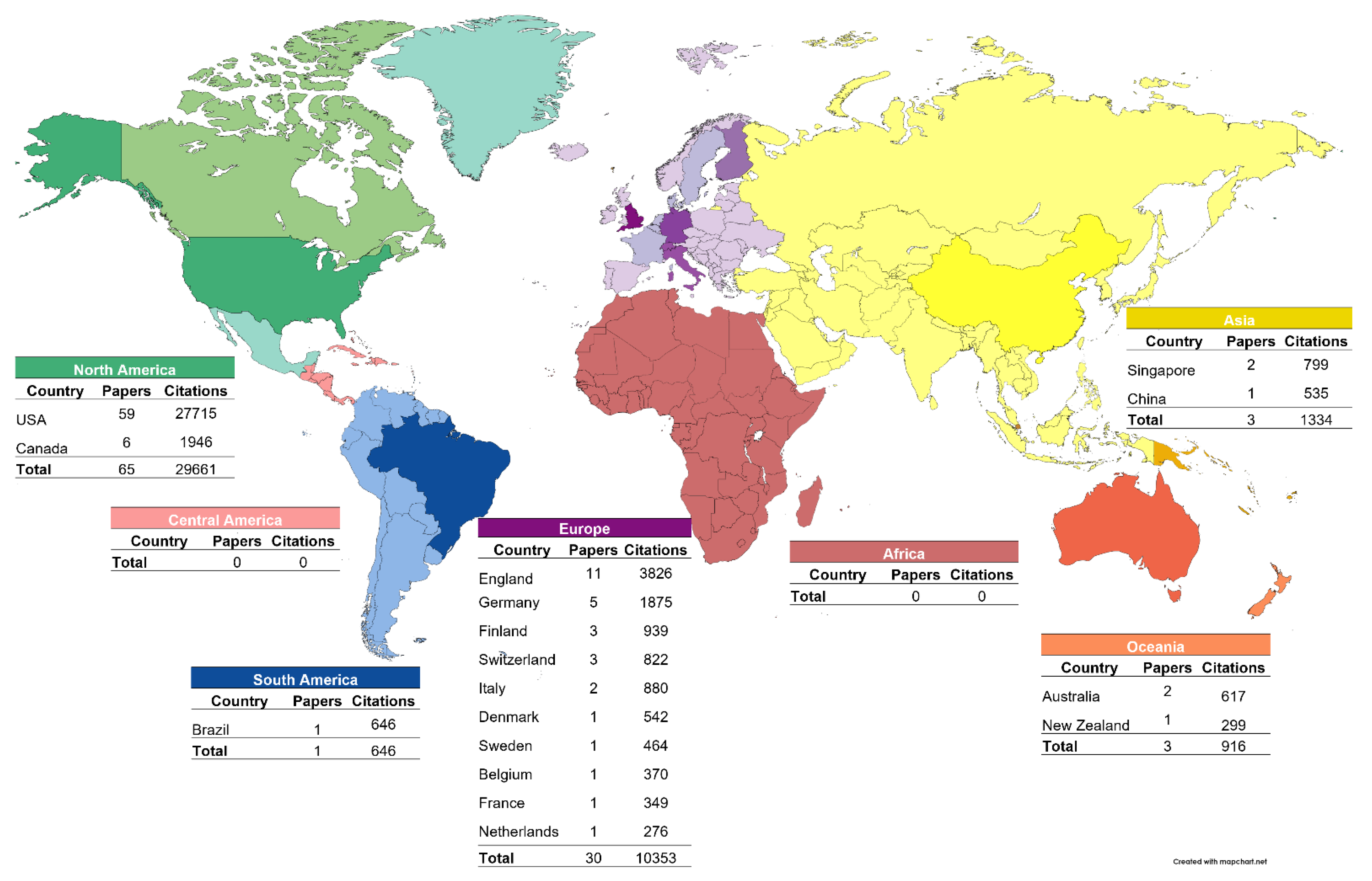
2.4. Worldwide Distribution Analysis and Network Visualization
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nordt, S.P.; Clark, R.F. Midazolam: A review of therapeutic uses and toxicity. J. Emerg. Med. 1997, 15, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Garnock-Jones, K.P. Oromucosal midazolam: A review of its use in pediatric patients with prolonged acute convulsive seizures. Pediatr. Drugs 2012, 14, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.C.; Besag, F.M.; Neville, B.G. Buccal midazolam and rectal diazepam for treatment of prolonged seizures in childhood and adolescence: A randomised trial. Lancet 1999, 353, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Tomlin, S. Medicines tailored for children—The introduction of buccal midazolam. Pharm. J. 2011, 287, 161–162. [Google Scholar]
- Olkkola, K.T.; Ahonen, J. Midazolam and other benzodiazepines. Handb. Exp. Pharmacol. 2008, 182, 335–360. [Google Scholar] [CrossRef]
- Gorski, J.C.; Vannaprasaht, S.; Hamman, M.A.; Ambrosius, W.T.; Bruce, M.A.; Haehner-Daniels, B.; Hall, S.D. The effect of age, sex, and rifampin administration on intestinal and hepatic cytochrome P450 3A activity. Clin. Pharmacol. Ther. 2003, 74, 275–287. [Google Scholar] [CrossRef]
- Dundee, J.W.; Halliday, N.J.; Harper, K.W.; Brogden, R.N. Midazolam: A Review of its Pharmacological Properties and Therapeutic Use. Drugs 1984, 28, 519–543. [Google Scholar] [CrossRef]
- Armijo, J.A.; Herranz, J.L.; Pardo, M.A.P.; Adin, J. Intranasal and buccal midazolam in the treatment of acute seizures. Rev. Neurol. 2004, 38, 458–468. [Google Scholar]
- Zhao, Z.Y.; Wang, H.; Wen, B.; Yang, Z.; Feng, K.; Fan, J. A Comparison of Midazolam, Lorazepam, and Diazepam for the Treatment of Status Epilepticus in Children: A Network Meta-analysis. J. Child. Neurol. 2016, 31, 1093–1107. [Google Scholar] [CrossRef]
- Ahmad, P.; Dummer, P.M.H.; Noorani, T.Y.; Asif, J.A. The top 50 most-cited articles published in the International Endodon-tic Journal. Int. Endod. J. 2019, 52, 803–818. [Google Scholar] [CrossRef] [PubMed]
- Alderson, P.; Green, S.; Higgins, J. Glossary of Terms in the Cochrane Collaboration. Cochrane Handb. Syst. Rev. 2005. Available online: http://community-archive.cochrane.org/sites/default/files/uploads/glossary.pdf%0Ahttp://scholar.google.com/scholar?http://community.cochrane.org/sites/default/files/uploads/glossary.pdfhl=en&btnG=Search&q=intitle:Glossary+of+Terms+in+the+Cochrane+Collabor (accessed on 11 June 2022).
- Van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef]
- Glauser, T.; Shinnar, S.; Gloss, D.; Alldredge, B.; Arya, R.; Bainbridge, J.; Bare, M.; Bleck, T.; Dodson, W.E.; Garrity, L.; et al. Evidence-based guideline: Treatment of convulsive status epilepticus in children and adults: Report of the guideline com-mittee of the American epilepsy society. Epilepsy Curr. 2016, 16, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Kress, J.P.; Pohlman, A.S.; O′Connor, M.F.; Hall, J.B. Daily interruption of sedative infusions in critically ill patients under-going mechanical ventilation. N. Engl. J. Med. 2000, 342, 1471–1477. [Google Scholar] [CrossRef] [PubMed]
- Murrough, J.W.; Iosifescu, D.V.; Chang, L.C.; Al Jurdi, R.K.; Green, C.E.; Perez, A.M.; Iqbal, S.; Pillemer, S.; Foulkes, A.; Shah, A.; et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: A two-site randomized controlled trial. Am. J. Psychiatry 2013, 170, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Riker, R.R.; Shehabi, Y.; Bokesch, P.M. Dexmedetomidine vs midazolam for sedation of critically Ill patients A randomized trial. J. Am. Med. Assoc. 2009, 301, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Jevtovic-Todorovic, V.; Hartman, R.E.; Izumi, Y.; Benshoff, N.D.; Dikranian, K.; Zorumski, C.F.; Olney, J.W.; Wozniak, D.F. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persis-tent learning deficits. J. Neurosci. 2003, 23, 876–882. [Google Scholar] [CrossRef]
- Ferguson, N.D.; Cook, D.J.; Guyatt, G.H.; Mehta, S.; Hand, L.; Austin, P.; Zhou, Q.; Matte, A.; Walter, S.D.; Lamontagne, F.; et al. High-frequency oscillation in early acute respiratory distress syndrome. N. Engl. J. Med. 2013, 368, 795–805. [Google Scholar] [CrossRef]
- Casali, A.G.; Gosseries, O.; Rosanova, M.; Boly, M.; Sarasso, S.; Casali, K.R.; Casarotto, S.; Bruno, M.-A.; Laureys, S.; Tononi, G.; et al. A theoretically based index of consciousness independent of sensory processing and behavior. Sci. Transl. Med. 2013, 5, 3006294. [Google Scholar] [CrossRef]
- Jacobi, J.; Fraser, G.L.; Coursin, D.B.; Riker, R.R.; Fontaine, D.; Wittbrodt, E.T.; Chalfin, D.B.; Masica, M.F.; Bjerke, H.S.; Co-plin, W.M.; et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit. Care Med. 2002, 30, 119–141. [Google Scholar] [CrossRef]
- Jakob, S.M.; Ruokonen, E.; Grounds, R.M.; Sarapohja, T.; Garratt, C.; Pocock, S.J.; Bratty, J.R.; Takala, J. Dexmedetomidine vs. midazolam or propofol for sedation during prolonged mechanical ventilation: Two randomized controlled trials. J. Am. Med. Assoc. 2012, 307, 1151–1160. [Google Scholar] [CrossRef]
- Strøm, T.; Martinussen, T.; Toft, P. A protocol of no sedation for critically ill patients receiving mechanical ventilation: A randomised trial. Lancet 2010, 375, 475–480. [Google Scholar] [CrossRef]
- Silbergleit, R.; Durkalski, V.; Lowenstein, D.; Conwit, R.; Pancioli, A.; Palesch, Y.; Barsan, W. Intramuscular versus Intrave-nous Therapy for Prehospital Status Epilepticus. N. Engl. J. Med. 2012, 366, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Needham, D.M.; Korupolu, R.; Zanni, J.M.; Pradhan, P.; Colantuoni, E.; Palmer, J.B.; Brower, R.G.; Fan, E. Early Physical Medicine and Rehabilitation for Patients with Acute Respiratory Failure: A Quality Improvement Project. Arch. Phys. Med. Rehabil. 2010, 91, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Lamba, J.K.; Lin, Y.S.; Schuetz, E.G.; Thummel, K. Genetic contribution to variable human CYP3A-mediated metabolism. Adv. Drug. Deliv. Rev. 2002, 54, 1271–1294. [Google Scholar] [CrossRef]
- Glass, P.S.; Bloom, M.; Kearse, L.; Rosow, C.; Sebel, P.; Manberg, P. Bispectral analysis measures sedation and memory ef-fects of propofol, midazolam, isoflurane, and alfentanil in healthy volunteers. Anesthesiology 1997, 86, 836–847. [Google Scholar] [CrossRef]
- Klotz, U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab. Rev. 2000, 41, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Izzo, A.A.; Ernst, E. Interactions between herbal medicines and prescribed drugs: An updated systematic review. Drugs 2009, 69, 1777–1798. [Google Scholar] [CrossRef]
- Mehta, S.; Burry, L.; Cook, D.; Fergusson, D.; Steinberg, M.; Granton, J.; Herridge, M.; Ferguson, N.; Devlin, J.; Tanios, M.; et al. Daily sedation interruption in mechanically ventilated critically ill patients cared for with a sedation protocol: A randomized controlled trial. J. Am. Med. Assoc. 2012, 308, 1985–1992. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, L.A.; Grillo, J.; Liu, Q.; Bullock, J.M.; Moon, Y.J.; Song, P.; Brar, S.S.; Madabushi, R.; Wu, T.C.; et al. Applica-tions of physiologically based pharmacokinetic (PBPK) modeling and simulation during regulatory review. Clin. Pharmacol. Ther. 2011, 89, 259–267. [Google Scholar] [CrossRef]
- Dresser, G.K. Pharmacokinetic-pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition. Clin. Pharmacokinet. 2000, 38, 41–57. [Google Scholar] [CrossRef]
- Cherenik, D.A.; Gillings, D.; Laine, H.; Hendler, J.; Silver, J.M.; Davidson, A.B.; Schwam, E.M.; Siegel, J.L. Validity and Reli-ability of the Observers. J. Clin. Psychopharmacol. 1990, 10, 244–251. [Google Scholar] [CrossRef]
- Greicius, M.D.; Kiviniemi, V.; Tervonen, O.; Vainionpää, V.; Alahuhta, S.; Reiss, A.L.; Menon, V. Persistent default-mode network connectivity during light sedation. Hum. Brain Mapp. 2008, 29, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Thummel, K.E.; Wilkinson, G.R. In vitro and in vivo drug interactions involving human CYP3A. Annu. Rev. Pharmacol. Toxicol. 1998, 38, 389–430. [Google Scholar] [CrossRef] [PubMed]
- Shorvon, S.; Ferlisi, M. The outcome of therapies in refractory and super-refractory convulsive status epilepticus and rec-ommendations for therapy. Brain 2012, 135, 2314–2328. [Google Scholar] [CrossRef] [PubMed]
- Verbeeck, R.K. Pharmacokinetics and dosage adjustment in patients with hepatic dysfunction. Eur. J. Clin. Pharmacol. 2008, 64, 1147–1161. [Google Scholar] [CrossRef]
- Pandharipande, P.; Cotton, B.A.; Shintani, A.; Thompson, J.; Pun, B.T.; Morris, J.A.; Dittus, R.; Ely, E.W. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J. Trauma Inj. Infect. Crit. Care 2008, 65, 34–41. [Google Scholar] [CrossRef]
- Ferrarelli, F.; Massimini, M.; Sarasso, S.; Casali, A.; Riedner, B.A.; Angelini, G.; Tononi, G.; Pearce, R.A. Breakdown in corti-cal effective connectivity during midazolam-induced loss of consciousness. Proc. Natl. Acad. Sci. USA 2010, 107, 2681–2686. [Google Scholar] [CrossRef]
- Hu, Z.; Yang, X.; Ho, P.C.L.; Chan, S.Y.; Heng, P.W.S.; Chan, E.; Duan, W.; Koh, H.L.; Zhou, S. Herb-drug interactions: A literature review. Drugs 2005, 65, 1239–1282. [Google Scholar] [CrossRef]
- Bowles, C.J.A.; Leicester, R.; Romaya, C.; Swarbrick, E.; Williams, C.B.; Epstein, O. A prospective study of colonoscopy prac-tice in the UK today: Are we adequately prepared for national colorectal cancer screening tomorrow? Gut 2004, 53, 277–283. [Google Scholar] [CrossRef]
- Li, X.Q.; Andersson, T.B.; Ahlström, M.; Weidolf, L. Comparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole on human cytochrome P450 activities. Drug Metab. Dispos. 2004, 32, 821–827. [Google Scholar] [CrossRef]
- Paine, M.F.; Khalighi, M.; Fisher, J.M.; Shen, D.D.; Kunze, K.L.; Marsh, C.; Perkins, J.D.E.; Thummel, K. Characterization of interintestinal and intraintestinal variations in human CYP3A-dependent metabolism. J. Pharmacol. Exp. Ther. 1997, 283, 1552–1562. [Google Scholar] [PubMed]
- Niemi, M.; Backman, J.T.; Fromm, M.F.; Neuvonen, P.J.; Kivistö, K.T. Pharmacokinetic interactions with rifampicin: Clinical relevance. Clin. Pharmacokinet. 2003, 42, 819–850. [Google Scholar] [CrossRef] [PubMed]
- Shehabi, Y. Delirium duration and mortality in lightly sedated, mechanically ventilated intensive care patients. Crit. Care Med. 2010, 38, 2311–2318. [Google Scholar] [CrossRef] [PubMed]
- McQuaid, K.R. A systematic review and meta-analysis of randomized, controlled trials of moderate sedation for routine endoscopic procedures. Gastrointest. Endosc. 2008, 67, 910–923. [Google Scholar] [CrossRef]
- Stevens, R.D.; Dowdy, D.W.; Michaels, R.K.; Mendez-Tellez, P.A.; Pronovost, P.J.; Needham, D.M. Neuromuscular dysfunc-tion acquired in critical illness: A systematic review. Intensive Care Med. 2007, 33, 1876–1891. [Google Scholar] [CrossRef]
- Payen, F.; Chanques, G.; Mantz, J.; Hercule, C.; Auriant, I.; Leguillou, J.-L.; Binhas, M.; Genty, C.; Rolland, C.; Bosson, J.-L.; et al. Current practices in sedation and analgesia for mechanically ventilated critically ill patients: A prospective multicenter patient-based study. Anesthesiology 2007, 106, 687–695. [Google Scholar] [CrossRef]
- Meierkord, H.; Boon, P.; Engelsen, B.; Göcke, K.; Shorvon, S.; Tinuper, P.; Holtkamp, M. EFNS guideline on the management of status epilepticus in adults. Eur. J. Neurol. 2010, 17, 348–355. [Google Scholar] [CrossRef]
- Kollef, M.H.; Levy, N.T.; Ahrens, T.S.; Schaiff, R.; Prentice, D.; Sherman, G. The use of continuous IV sedation is associated with prolongation of mechanical ventilation. Chest 1998, 114, 541–548. [Google Scholar] [CrossRef]
- Cruz, A.P.M.; Frei, F.; Graeff, F. Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacol. Biochem. Behav. 1994, 49, 171–176. [Google Scholar] [CrossRef]
- Johnson, T.N.; Rostami-Hodjegan, A.; Tucker, G.T. Prediction of the clearance of eleven drugs and associated variability in neonates, infants and children. Clin. Pharmacokinet 2006, 45, 931–956. [Google Scholar] [CrossRef]
- Bailey, D.G.; Malcolm, J.; Arnold, O.; Spence, J.D. Grapefruit juice-drug interactions. Br. J. Clin. Pharmacol. 1998, 46, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Riss, J.; Cloyd, J.; Gates, J.; Collins, S. Benzodiazepines in epilepsy: Pharmacology and pharmacokinetics. Acta Neurol. Scand. 2008, 118, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Walsky, R.L.; Obach, R.S. Validated assays for human cytochrome P450 activities. Drug Metab. Dispos. 2004, 32, 647–660. [Google Scholar] [CrossRef]
- Zhou, S.; Chan, S.Y.; Goh, B.C.; Chan, E.; Duan, W.; Huang, M.; McLeod, H.L. Mechanism-based inhibition of cytochrome P450 3A4 by therapeutic drugs. Clin. Pharmacokinet. 2005, 44, 279–304. [Google Scholar] [CrossRef]
- Lichtenstein, D.R.; Jagannath, S.; Baron, T.H.; Anderson, M.A.; Banerjee, S.; Dominitz, J.A.; Fanelli, R.D.; Gan, S.I.; Harrison, M.E.; Ikenberry, S.O.; et al. Sedation and anesthesia in GI endoscopy. Gastrointest. Endosc. 2008, 68, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, J.R.; Wysong, A.; van der Starre, P.J.; Block, T.; Miller, C.; Reitz, B.A. Dexmedetomidine and the reduction of postoperative delirium after cardiac surgery. Psychosomatics 2009, 50, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, R.E.; Tieves, K.S.; Hoffman, G.M.; Ghanayem, N.S.; Amlie-Lefond, C.M.; Schwabe, M.J.; Chusid, M.J.; Rupprecht, C.E. Survival after Treatment of Rabies with Induction of Coma. N. Engl. J. Med. 2005, 352, 2508–2514. [Google Scholar] [CrossRef]
- Lowenstein, D.H.; Alldredge, B.K. Status epilepticus. N. Engl. J. Med. 1998, 338, 970–976. [Google Scholar] [CrossRef]
- Krauss, B.; Green, S.M. Procedural sedation and analgesia in children. Lancet 2006, 367, 766–780. [Google Scholar] [CrossRef]
- Yon, J.H.; Daniel-Johnson, J.; Carter, L.; Jevtovic-Todorovic, V. Anesthesia induces neuronal cell death in the developing rat brain via the intrinsic and extrinsic apoptotic pathways. Neuroscience 2005, 135, 815–827. [Google Scholar] [CrossRef]
- De Graeff, A.; Dean, M. Palliative sedation therapy in the last weeks of life: A literature review and recommendations for standards. J. Palliat. Med. 2006, 10, 67–85. [Google Scholar] [CrossRef]
- Claassen, J. Treatment of refractory status epilepticus with pentobarbital, propofol, or midazolam: A systematic review. Epilepsia 2002, 43, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Venkatakrishnan, K. Effects of the antifungal agents on oxidative drug metabolism. Clinical relevance. Clin. Pharmacokinet. 2000, 38, 111–180. [Google Scholar] [CrossRef]
- Young, C.; Jevtovic-Todorovic, V.; Qin, Y.-Q.; Tenkova, T.; Wang, H.; Labruyere, J.; Olney, J.W. Potential of ketamine and midazolam, individually or in combination, to induce apoptotic neurodegeneration in the infant mouse brain. Br. J. Pharmacol. 2005, 146, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Thummel, K.E.; O′Shea, D.; Paine, M.F.; Shen, D.D.; Kunze, K.L.; Perkins, J.D.; Wilkinson, G.R. Oral first-pass elimination of midazolam involves both gastrointestinal and hepatic CYP3A-mediated metabolism. Clin. Pharmacol. Ther. 1996, 59, 491–502. [Google Scholar] [CrossRef]
- Reves, J.G.; Fragen, R.J.; Vinik, H.R.; Greenblatt, D.J. Midazolam: Pharmacology and uses. Anesthesiology 1985, 62, 310–324. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, R.E.; Banner, W.; Berlin, C.M.; Blumer, J.L.; Gorman, R.L.; Lambert, G.H.; Wilson, G.S.; Bennett, D.R.; Cordero, J.F.; Cote, C.J.; et al. Guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures. Pediatrics 1992, 1110–1115. [Google Scholar]
- Lin, Y.S.; Dowling, A.L.S.; Quigley, S.D.; Farin, F.M.; Zhang, J.; Lamba, J.; Schuetz, E.G.; Thummel, K.E. Co-regulation of CYP3A4 and CYP3A5 and contribution to hepatic and intestinal midazolam metabolism. Mol. Pharmacol. 2002, 62, 162–172. [Google Scholar] [CrossRef]
- Kim, R.B.; Wandel, C.; Leake, B.; Cvetkovic, M.; Fromm, M.F.; Dempsey, P.J.; Roden, M.M.; Belas, F.; Chaudhary, A.K.; Ro-den, D.M.; et al. Interrelationship between substrates and inhibitors of human CYP3A and P-glycoprotein. Pharm. Res. 1999, 16, 408–414. [Google Scholar] [CrossRef]
- Putensen, C.; Zech, S.; Wrigge, H.; Zinserling, J.; Stüber, F.; VON Spiegel, T.; Mutz, N. Long-term effects of spontaneous breathing during ventilatory support in patients with acute lung injury. Am. J. Respir. Crit. Care Med. 2001, 164, 43–49. [Google Scholar] [CrossRef]
- Kronbach, T.; Mathys, D.; Umeno, M.; Gonzalez, F.J.A.; Meyer, U. Oxidation of midazolam and triazolam by human liver cytochrome P450IIIA4. Mol. Pharmacol. 1989, 36, 89–96. [Google Scholar] [PubMed]
- Schuetz, E.G. Modulators and substrates of P-glycoprotein and cytochrome P4503A coordinately up-regulate these proteins in human colon carcinoma cells. Mol. Pharmacol. 1996, 49, 311–318. [Google Scholar] [PubMed]
- Streetman, D.S.; Bertino, J.S.; Nafziger, A.N. Phenotyping of drug-metabolizing enzymes in adults: A review of in-vivo cytochrome P450 phenotyping probes. Pharmacogenetics 2000, 10, 187–216. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, J.; Robertson, S.; Norris, E.; Appleton, R.; Whitehouse, W.P.; Phillips, B.; Martland, T.; Berry, K.; Collier, J.; Smith, S.; et al. Safety and efficacy of buccal midazolam versus rectal diazepam for emergency treatment of seizures in children: A randomised controlled trial. Lancet 2005, 366, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Dielenberg, R.A.; McGregor, I.S. Defensive behavior in rats towards predatory odors: A review. Neurosci. Biobehav. Rev. 2001, 25, 597–609. [Google Scholar] [CrossRef]
- Paine, M.F.; Shen, D.D.; Kunze, K.L.; Perkins, J.D.; Marsh, C.; McVicar, J.P.; Barr, D.M.; Gillies, B.S.; Thummel, K.E. First-pass metabolism of midazolam by the human intestine. Clin. Pharmacol. Ther. 1996, 60, 14–24. [Google Scholar] [CrossRef]
- Özdemir, V.; Kalow, W.; Tang, B.K.; Paterson, A.D.; Walker, S.E.; Endrenyi, L.; Kashuba, A.D. Evaluation of the genetic component of variability in CYP3A4 activity: A repeated drug administration method. Pharmacogenetics 2000, 10, 373–388. [Google Scholar] [CrossRef]
- Gorski, J.C.; Jones, D.R.; Haehner-Daniels, B.D.; Hamman, M.A.; O’Mara, E.M., Jr.; Hall, S.D. The contribution of intestinal and hepatic CYP3A to the interaction between midazolam and clarithromycin. Clin. Pharmacol. Ther. 1998, 64, 133–143. [Google Scholar] [CrossRef]
- Andrew Williams, J.; Ring, B.J.; Cantrell, V.E.; Jones, D.R.; Eckstein, J.; Ruterbories, K.; Hamman, M.A.; Hall, S.D.; Wrighton, S.A. Comparative metabolic capabilities of CYP3A4, CYP3A5, and CYP3A7. Drug Metab. Dispos. 2002, 30, 883–891. [Google Scholar] [CrossRef]
- Venn, R.M.; Bradshaw, C.J.; Spencer, R.; Brealey, D.; Caudwell, E.; Naughton, C.; Vedio, A.; Singer, M.; Feneck, R.; Treacher, D.; et al. Preliminary UK experience of dexmedetomidine, a novel agent for postoperative sedation in the intensive care unit. Anaesthesia 2011, 54, 1136–1142. [Google Scholar] [CrossRef]
- Bai, D.; Zhu, G.; Pennefather, P.; Jackson, M.F.; MacDonald, J.F.; Orser, B.A. Distinct functional and pharmacological proper-ties of tonic and quantal inhibitory postsynaptic currents mediated by γ-aminobutyric acidA receptors in hippocampal neurons. Mol. Pharmacol. 2001, 59, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.; Madani, S.; Wei, X.-X.; Reynolds, K.; Huang, S.-M. Evaluation of cytochrome p450 probe substrates commonly used by the pharmaceutical industry to study in vitro drug interactions. Drug Metab. Dispos. 2002, 30, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Lang, E.V.; Benotsch, E.G.; Fick, L.J.; Lutgendorf, S.; Berbaum, M.L.; Berbaum, K.; Logan, H.; Spiegel, D. Adjunctive non-pharmacological analgesia for invasive medical procedures: A randomised trial. Lancet 1999, 355, 1486–1490. [Google Scholar] [CrossRef] [PubMed]
- Gurley, B.J.; Gardner, S.F.; Hubbard, M.A.; Williams, D.K.; Gentry, W.B.; Cui, Y.; Ang, C.Y.W. Cytochrome P450 phenotypic ratios for predicting herb-drug interactions in humans. Clin. Pharmacol. Ther. 2002, 72, 276–287. [Google Scholar] [CrossRef]
- Schmiedlin-Ren, P.; Edwards, D.J.; Fitzsimmons, M.E.; He, K.; Lown, K.S.; Woster, P.M.; Rahman, A.; Thummel, K.E.; Fisher, J.M.; Hollenberg, P.F.; et al. Mechanisms of enhanced oral availability of CYP3A4 substrates by grapefruit constituents - Decreased enterocyte CYP3A4 concentration and mechanism-based inactivation by furanocoumarins. Mol. Pharmacol. 1997, 51, 741–754. [Google Scholar] [CrossRef]
- Olkkola, K.T.; Backman, J.T.; Neuvonen, P.J. Midazolam should be avoided in patients receiving the systemic antimycotics ketoconazole or itraconazole. Clin. Pharmacol. Ther. 1994, 55, 481–485. [Google Scholar] [CrossRef]
- Pelkonen, O.; Mäeenpäeä, J.; Taavitsainen, P.; Rautio, A.; Raunio, H. Inhibition and induction of human cytochrome P450 (CYP) enzymes. Xenobiotica 1998, 28, 1203–1253. [Google Scholar] [CrossRef]
- Kenworthy, K.E. CYP3A4 drug interactions: Correlation of 10 in vitro probe substrates. Br. J. Clin. Pharmacol. 1999, 48, 716–727. [Google Scholar] [CrossRef]
- Wang, Z.; Gorski, J.C.; Hamman, M.A.; Huang, S.; Lesko, L.J.; Hall, S.D. The effects of St John’s wort (Hypericum perforatum) on human cytochrome P450 activity. Clin. Pharmacol. Ther. 2001, 70, 317–326. [Google Scholar] [CrossRef]
- Krauss, B.; Green, S.M. Sedation and Analgesia for Procedures in Children. N. Engl. J. Med. 2000, 342, 938–945. [Google Scholar] [CrossRef]
- Christopher Gorski, J.; Hall, S.D.; Jones, D.R.; VandenBranden, M. Regioselective biotransformation of midazolam by members of the human cytochrome P450 3A (CYP3A) subfamily. Biochem. Pharmacol. 1994, 47, 1643–1653. [Google Scholar] [CrossRef] [PubMed]
- Arrowsmith, J.B.; Gerstman, B.B.; Fleischer, D.E.; Benjamin, S.B. Results from the American Society for Gastrointestinal En-doscopy/U.S. Food and Drug Administration collaborative study on complication rates and drug use during gastrointestinal endoscopy. Gastrointest. Endosc. 1991, 37, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Parke, T.J.; Stevens, J.E.; Rice, A.S.; Greenaway, C.L.; Bray, R.J.; Smith, P.J.; Waldmann, C.S.; Verghese, C. Metabolic acidosis and fatal myocardial failure after propofol infusion in children: Five case reports. Br. Med. J. 1992, 305, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Ostermann, M.E.; Keenan, S.P.; Seiferling, R.A.; Sibbald, W.J. Sedation in the intensive care unit: A systematic review. J. Am. Med. Assoc. 2000, 283, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Lown, K.S.; Kolars, J.C.E.; Thummel, K.; Barnett, J.L.; Kunze, K.L.A.; Wrighton, S.; Watkins, P.B. Interpatient heterogeneity in expression of CYP3A4 and CYP3A5 in small bowel: Lack of prediction by the erythromycin breath test. Drug Metab. Dispos. 1994, 22, 947–955. [Google Scholar] [PubMed]
- Kupferschmidt, H.R.; Ha, H.R.; Ziegler, W.H.; Meier, P.J.; Krähenbühl, S. Interaction between grapefruit juice and midazo-lam in humans. Clin. Pharmacol. Ther. 1995, 58, 20–28. [Google Scholar] [CrossRef]
- Bailey, P.L.; Pace, N.L.; Ashburn, M.A.; Moll, J.W.B.; East, K.A.; Stanley, T.H. Frequent hypoxemia and apnea after sedation with midazolam and fentanyl. Anesthesiology 1990, 73, 826–830. [Google Scholar] [CrossRef]
- Greenblatt, D.J.; Abernethy, D.R.; Locniskar, A.; Harmatz, J.S.; Limjuco, R.A.; Shader, R.I. Effect of age, gender, and obesity on midazolam kinetics. Anesthesiology 1984, 61, 27–35. [Google Scholar] [CrossRef]
- Quine, M.A. Prospective audit of upper gastrointestinal endoscopy in two regions of England: Safety, staffing, and sedation methods. Gut 1995, 36, 462–467. [Google Scholar] [CrossRef]
- Schmiedlin-Ren, P.; Thummel, K.E.; Fisher, J.M.; Paine, M.F.; Lown, K.S.; Watkins, P.B. Expression of enzymatically active CYP3A4 by Caco-2 cells grown on extracellular matrix-coated permeable supports in the presence of 1 al-pha,25-dihydroxyvitamin D-3. Mol. Pharmacol. 1997, 51, 741–754. [Google Scholar] [CrossRef]
- Thummel, K.E.; Shen, D.D.; Podoll, T.D.; Kunze, K.L.; Trager, W.F.; Hartwell, P.S.A.; Raisys, V.; Marsh, C.; McVicar, J.P.; Barr, D.M. Use of midazolam as a human cytochrome P450 3A probe: I. In vitro-in vivo correlations in liver transplant patients. J. Pharmacol. Exp. Ther. 1994, 271, 549–556. Available online: https://pubmed.ncbi.nlm.nih.gov/7965755/ (accessed on 11 June 2022).
- Cheng, D.C.H.; Karski, J.; Peniston, C.; Asokumar, B.; Raveendran, G.; Carroll, J.; Nierenberg, H.; Roger, S.; Mickle, D.; Tong, J.; et al. Morbidity outcome in early versus conventional tracheal extubation after coronary artery bypass grafting: A pro-spective randomized controlled trial. J. Thorac. Cardiovasc. Surg. 1996, 112, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Hunt, C.M.; Westerkam, W.R.; Stave, G.M. Effect of age and gender on the activity of human hepatic CYP3A. Biochem. Pharmacol. 1992, 44, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Ashton, H. Guidelines for the Rational Use of Benzodiazepines: When and What to Use. Drugs 1994, 48, 25–40. [Google Scholar] [CrossRef]
- Heinrichs, S.C.; Pich, E.M.; Miczek, K.A.; Britton, K.T.; Koob, G.F. Corticotropin-releasing factor antagonist reduces emo-tionality in socially defeated rats via direct neurotropic action. Brain Res. 1992, 581, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Olkkola, K.T.; Aranko, K.; Luurila, H.; Hiller, A.; Saarnivaara, L.; Himberg, J.-J.; Neuvonen, P.J. A potentially hazardous interaction between erythromycin and midazolam. Clin. Pharmacol. Ther. 1993, 53, 298–305. [Google Scholar] [CrossRef]
- Magorian, T.; Flannery, K.B.; Miller, R.D. Comparison of rocuronium, succinylcholine, and vecuronium for rapid- sequence induction of anesthesia in adult patients. Anesthesiology 1993, 79, 913–918. [Google Scholar] [CrossRef]
- Mangano, D.T.; Siliciano, D.; Hollenberg, M.; Leung, J.M.; Browner, W.S.; Goehner, P.; Merrick, S.; Verrier, E. Postoperative myocardial ischemia: Therapeutic trials using intensive analgesia following surgery. Anesthesiology 1992, 76, 342–353. [Google Scholar] [CrossRef]
- Allonen, H.; Ziegler, G.; Klotz, U. Midazolam kinetics. Clin. Pharmacol. Ther. 1981, 30, 653–661. [Google Scholar] [CrossRef]
- Moura, L.K.B.; De Mesquita, R.F.; Mobin, M.; Matos, F.T.C.; Monte, T.L.; Lago, E.C.; Falcão, C.A.M.; Ferraz, M.D.A.L.; Santos, T.C.; Sousa, L.R.M. Uses of bibliometric techniques in public health research. Iran. J. Public Health 2017, 46, 1435–1436. Available online: https://pubmed.ncbi.nlm.nih.gov/29308389/ (accessed on 11 June 2022).
- Ullah, R.; Ullah, R. Top-cited Articles in Regenerative Endodontics: A Bibliometric Analysis. J. Endod. 2018, 44, 1650–1664. [Google Scholar] [CrossRef]
- Goho, C. Oral midazolam-grapefruit juice drug interaction. Pediatr. Dent. 2001, 23, 365–366. [Google Scholar] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Moher, D. Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. J. Clin. Epidemiol. 2021, 134, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Osawa, M.; Aihara, M.; Izumi, T.; Ohtsuka, Y.; Haginoya, K.; Kato, I.; Kaneko, K.; Sugai, K.; Takahashi, T.; et al. Efficacy of Intravenous Midazolam for Status Epilepticus in Childhood. Pediatr. Neurol. 2007, 36, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Clementino, L.C.; Souza, K.S.C.; Castelo-Branco, M.; Perazzo, M.F.; Ramos-Jorge, M.L.; Mattos, F.F.; Paiva, S.M.; Mar-tins-Júnior, P.A. Top 100 most-cited oral health-related quality of life papers: Bibliometric analysis. Community Dent. Oral Epidemiol. 2022, 50, 199–205. [Google Scholar] [CrossRef]
- Lahat, E.; Goldman, M.; Barr, J.; Bistritzer, T.; Berkovitch, M. Comparison of intranasal midazolam with intravenous diaze-pam for treating febrile seizures in children: Prospective randomised study. Br. Med. J. 2000, 321, 83–86. [Google Scholar] [CrossRef]
- Bakkalbasi, N.; Bauer, K.; Glover, J.; Wang, L. Three options for citation tracking: Google Scholar, Scopus and Web of Science. Biomed. Digit. Libr. 2006, 3, 7. [Google Scholar] [CrossRef]
- Falagas, M.; Pitsouni, E.I.; Malietzis, G.; Pappas, G. Comparison of PubMed, Scopus, Web of Science, and Google Scholar: Strengths and weaknesses. FASEB J. 2008, 22, 338–342. [Google Scholar] [CrossRef]
- Keighobadi, M.; Nakhaei, M.; Sharifpour, A.; Khasseh, A.A.; Safanavaei, S.; Tabaripour, R.; Fakhar, M. A Bibliometric Analysis of Global Research on Lophomonas Sin Scopus (1933–2019). Infect. Disord. Drug Targets 2020, 21, 230–237. [Google Scholar] [CrossRef]
- Alryalat, S.A.S.; Malkawi, L.W.; Momani, S.M. Comparing bibliometric analysis using pubmed, scopus, and web of science databases. J. Vis. Exp. 2019, 2019, 58494. [Google Scholar] [CrossRef]
- Barends, C.R.M. Dexmedetomidine versus midazolam in procedural sedation. A systematic review of efficacy and safety. PLoS ONE 2017, 12, e0169525. [Google Scholar] [CrossRef]
- Garcia, R.; Salluh, J.I.; Andrade, T.R.; Farah, D.; da Silva, P.S.; Bastos, D.F.; Fonseca, M.C. A systematic review and me-ta-analysis of propofol versus midazolam sedation in adult intensive care (ICU) patients. J. Crit. Care 2021, 64, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Barr, J.; Fraser, G.; Puntillo, K.; Ely, E.W.; Gélinas, C.; Dasta, J.F.; Davidson, J.E.; Devlin, J.W.; Kress, J.P.; Joffe, A.M.; et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit. Care Med. 2013, 41, 263–306. [Google Scholar] [CrossRef] [PubMed]
- Swart, E.L.; Plotz, F.B. Growing up with Midazolam in the Neonatal and Pediatric Intensive Care. Curr. Drug Metab. 2012, 13, 760–766. [Google Scholar] [CrossRef] [PubMed]


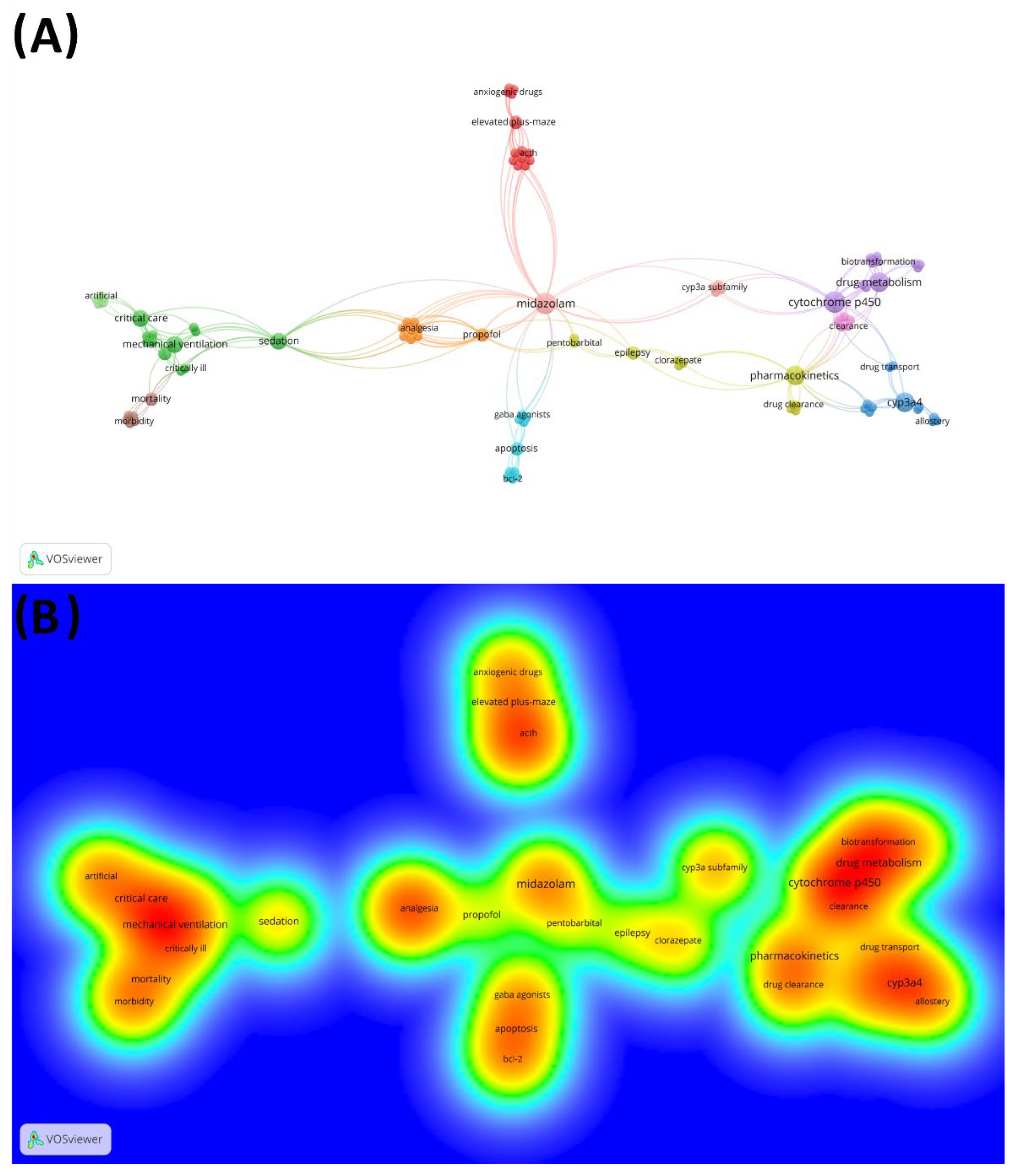
| Rankings Density of Citation/No Citation, WoS-CC a | Author/Year | Density of Citation | No Citation, WoS-CC a | No. Citation, Scopus | No. Citation, Google Scholar | Study Object | DOI/URL |
|---|---|---|---|---|---|---|---|
| 01/24 | Glauser et al., 2016 [13] | 92.20 | 476 | 519 | 837 | Epilepsy treatment | https://doi.org/10.5698/1535-7597-16.1.48 |
| 02/10 | Murrough et al., 2013 [15] | 84.75 | 678 | 727 | 1031 | Depression use | https://doi.org/10.1176/appi.ajp.2013.13030392 |
| 03/01 | Kress et al., 2000 [14] | 83.05 | 1744 | 311 | 3494 | Sedative effects | https://doi.org/10.1056/NEJM200005183422002 |
| 04/04 | Riker et al., 2009 [16] | 80.83 | 970 | 1163 | 1720 | Sedation aspects | https://doi.org/10.1001/jama.2009.56 |
| 05/02 | Jevtovic-Todorovic et al., 2003 [17] | 75.94 | 1367 | 1553 | 2147 | Anesthesia effects | https://doi.org/10.1523/jneurosci.23-03-00876.2003 |
| 06/20 | Ferguson et al., 2013 [18] | 64.38 | 515 | 595 | 890 | Sedation aspects | https://doi.org/10.1056/NEJMoa1215554 |
| 07/22 | Casali et al., 2013 [19] | 60.88 | 487 | 532 | 787 | Sedation aspects | https://doi.org/10.1126/scitranslmed.3006294 |
| 08/03 | Jacobi et al., 2002 [20] | 59.95 | 1139 | 1483 | 2321 | Sedation and analgesia aspects | https://doi.org/10.1097/00003246-200201000-00020 |
| 09/19 | Jakob et al., 2012 [21] | 57.67 | 519 | 582 | 958 | Sedation aspects | https://doi.org/10.1001/jama.2012.304 |
| 10/17 | Strøm et al., 2010 [22] | 49.27 | 542 | 610 | 1006 | Sedation aspects | https://doi.org/10.1016/S0140-6736(09)62072-9 |
| 11/39 | Silbergleit et al., 2012 [23] | 43.00 | 387 | 460 | 674 | Epilepsy treatment | https://doi.org/10.1056/NEJMoa1107494 |
| 12/28 | Needham et al., 2010 [24] | 40.91 | 450 | 476 | 817 | Sedation aspects | https://doi.org/10.1016/j.apmr.2010.01.002 |
| 13/07 | Lamba et al., 2002 [25] | 38.84 | 738 | 806 | 1152 | Cytochrome P450 approach | https://doi.org/10.1016/S0169-409X(02)00066-2 |
| 14/06 | Glass et al., 1997 [26] | 36.86 | 884 | 1085 | 1850 | Sedation aspects | https://doi.org/10.1097/00000542-199704000-00014 |
| 15/31 | Klotz, 2009 [27] | 35.17 | 422 | 466 | 761 | Historical aspects | https://doi.org/10.1080/03602530902722679 |
| 16/37 | Izzo & Ernst, 2009 [28] | 32.75 | 393 | 460 | 982 | Drug interactions | https://doi.org/10.2165/11317010-000000000-00000 |
| 17/79 | Mehta et al., 2012 [29] | 32.44 | 292 | 330 | 519 | Sedation aspects | https://doi.org/10.1001/jama.2012.13872 |
| 18/64 | Zhao et al., 2011 [30] | 32.30 | 323 | 341 | 451 | Pharmacology | https://doi.org/10.1038/clpt.2010.298 |
| 19/11 | Dresser et al., 2000 [31] | 31.33 | 658 | 807 | 1115 | Cytochrome P450 | https://doi.org/10.2165/00003088-200038010-00003 |
| 20/05 | Chernik et al., 1990 [32] | 30.77 | 954 | 1071 | 1741 | Sedation aspects | https://doi.org/10.1097/00004714-199008000-00003 |
| 21/36 | Greicius et al., 2008 [33] | 30.54 | 397 | 421 | 600 | Sedation aspects | https://doi.org/10.1002/hbm.20537 |
| 22/08 | Thummel & Wilkinson, 1998 [34] | 30.26 | 696 | 128 | 1071 | Cytochrome P450 approach | https://doi.org/10.1146/annurev.pharmtox.38.1.389 |
| 23/95 | Shorvon & Ferlisi, 2012 [35] | 29.33 | 264 | 310 | 451 | Epilepsy treatment | https://doi.org/10.1093/brain/aws091 |
| 24/42 | Verbeeck, 2008 [36] | 28.46 | 370 | 466 | 599 | Liver evaluation | https://doi.org/10.1007/s00228-008-0553-z |
| 25/43 | Pan-dharipande et al., 2008 [37] | 28.31 | 368 | 406 | 668 | Risk factors | https://doi.org/10.1097/TA.0b013e31814b2c4d |
| 26/81 | Ferrarelli et al., 2010 [38] | 27.73 | 305 | 334 | 493 | Anesthesia aspects | https://doi.org/10.1073/pnas.0913008107 |
| 27/29 | Hu et al., 2005 [39] | 27.50 | 440 | 769 | 283 | Pharmacology | https://doi.org/10.2165/00003495-200565090-00005 |
| 28/26 | Bowles et al., 2004 [40] | 27.29 | 464 | 558 | 863 | Sedation aspects | https://doi.org/10.1136/gut.2003.016436 |
| 29/27 | Li et al., 2004 [41] | 27.29 | 464 | 508 | 775 | Cytochrome P450 approach | https://doi.org/10.1124/dmd.32.8.821 |
| 30/12 | Paine et al., 1997 [42] | 27.00 | 648 | 694 | 974 | Cytochrome P450 approach | https://jpet.aspetjour-nals.org/content/283/3/1552.long |
| 31/25 | Niemi et al., 2003 [43] | 26.33 | 474 | 530 | 761 | Pharmacology | https://doi.org/10.2165/00003088-200342090-00003 |
| 32/82 | Shehabi et al., 2010 [44] | 26.27 | 289 | 327 | 445 | Cytochrome P450 approach | https://doi.org/10.1097/CCM.0b013e3181f85759 |
| 33/58 | McQuaid & Laine, 2008 [45] | 25.92 | 337 | 348 | 524 | Sedation aspects | https://doi.org/10.1016/j.gie.2007.12.046 |
| 34/49 | Stevens et al., 2007 [46] | 25.57 | 358 | 417 | 692 | Risk factors | https://doi.org/10.1007/s00134-007-0772-2 |
| 35/53 | Payen et al., 2007 [47] | 24.93 | 349 | 402 | 689 | Analgesia and sedation aspects | https://doi.org/10.1097/01.anes.0000264747.09017.da |
| 36/90 | Meierkord et al., 2010 [48] | 24.64 | 271 | 327 | 488 | Epilepsy treatment | https://doi.org/10.1111/j.1468-1331.2009.02917.x |
| 37/15 | Kollef et al., 1998 [49] | 24.00 | 552 | 713 | 1165 | Sedation aspects | https://doi.org/10.1378/chest.114.2.541 |
| 38/13 | Cruz et al., 1994 [50] | 23.93 | 646 | 676 | 929 | Anxiety use | https://doi.org/10.1016/0091-3057(94)90472-3 |
| 39/50 | Johnson et al., 2006 [51] | 23.73 | 356 | 380 | 509 | Cytochrome P450 approach | https://doi.org/10.2165/00003088-200645090-00005 |
| 40/18 | Bailey et al., 1998 [52] | 23.26 | 535 | 658 | 938 | Food interaction | https://doi.org/10.1046/j.1365-2125.1998.00764.x |
| 41/74 | Riss et al., 2008 [53] | 23.00 | 299 | 345 | 541 | Pharmacology | https://doi.org/10.1111/j.1600-0404.2008.01004.x |
| 42/38 | Walsky & Obach, 2004 [54] | 22.88 | 389 | 429 | 561 | Cytochrome P450 approach | https://doi.org/10.1124/dmd.32.6.647 |
| 43/48 | Zhou et al., 2005 [55] | 22.44 | 359 | 424 | 545 | Cytochrome P450 approach | https://doi.org/10.2165/00003088-200544030-00005 |
| 44/84 | Lichtenstein et al., 2008 [56] | 21.85 | 284 | 373 | 538 | Guideline | https://doi.org/10.1016/j.gie.2008.09.029 |
| 45/96 | Maldonado et al., 2009 [57] | 21.83 | 262 | 309 | 560 | Sedation aspects | https://doi.org/10.1176/appi.psy.50.3.206 |
| 46/57 | Willoughby et al., 2005 [58] | 21.50 | 344 | 432 | 675 | Sedation aspects | https://doi.org/10.1056/NEJMoa050382 |
| 47/23 | Lowenstein & Alldredge, 1998 [59] | 21.09 | 485 | 20 | 35 | Epilepsy treatment | https://doi.org/10.1056/NEJM199804023381407 |
| 48/68 | Krauss & Green, 2006 [60] | 21.07 | 316 | 365 | 607 | Sedation and analgesia aspects | https://doi.org/10.1016/S0140-6736(06)68230-5 |
| 49/60 | Yon et al., 2005 [61] | 20.63 | 330 | 378 | 508 | Apoptotic pathways investigation | https://doi.org/10.1016/j.neuroscience.2005.03.064 |
| 50/85 | De Graeff & Dean, 2007 [62] | 20.21 | 283 | 327 | 555 | Clinical use | https://doi.org/10.1089/jpm.2006.0139 |
| 51/41 | Claassen et al., 2002 [63] | 19.95 | 379 | 466 | 717 | Epilepsy treatment | https://doi.org/10.1046/j.1528-1157.2002.28501.x |
| 52/32 | Venkata-krishnan et al., 2000 [64] | 19.90 | 418 | 474 | 642 | Cytochrome P450 approach | https://doi.org/10.2165/00003088-200038020-00002 |
| 53/67 | Young et al., 2005 [65] | 19.88 | 318 | 371 | 519 | Neurodegeneration | https://doi.org/10.1038/sj.bjp.0706301 |
| 54/21 | Thummel et al., 1996 [66] | 19.84 | 496 | 550 | 703 | Cytochrome P450 approach | https://doi.org/10.1016/S0009-9236(96)90177-0 |
| 55/09 | Reves et al., 1985 [67] | 19.00 | 684 | 750 | 1243 | Midazolam pharmacology | https://doi.org/10.1097/00000542-198503000-00017 |
| 56/16 | Kauffman et al., 1992 [68] | 19.72 | 543 | 501 | 645 | Sedation aspects | https://pennstate.pure.elsevier.com/en/publications/guidelines-for-monitoring-and-management-of-pediatric-patients-du-2 |
| 57/51 | Lin et al., 2002 [69] | 19.68 | 355 | 377 | 537 | Cytochrome P450 approach | https://doi.org/10.1124/mol.62.1.162 |
| 58/34 | Kim et al., 1999 [70] | 18.36 | 404 | 452 | 599 | Cytochrome P450 approach | https://doi.org/10.1023/a:1018877803319 |
| 59/44 | Putensen et al., 2001 [71] | 18.30 | 366 | 442 | 692 | Acute respiratory | https://doi.org/10.1164/ajrccm.164.1.2001078 |
| 60/14 | Kronbach et al., 1989 [72] | 17.47 | 559 | 529 | 688 | Cytochrome P450 approach | https://citeseerx.ist.psu.edu/view-doc/download?doi=10.1.1.989.1642&rep=rep1&type=pdf |
| 61/30 | Schuetz et al., 1996 [73] | 17.36 | 434 | 495 | 641 | Cytochrome P450 approach | https://molpharm.aspetjournals.org/content/49/2/311.long |
| 62/45 | Streetman et al., 2000 [74] | 17.29 | 363 | 404 | 536 | Cytochrome P450 approach | https://doi.org/10.1097/00008571-200004000-00001 |
| 63/88 | McIntyre et al., 2005 [75] | 17.06 | 273 | 330 | 505 | Epilepsy treatment | https://doi.org/10.1016/S0140-6736(05)66909-7 |
| 64/61 | Dielenberg & McGregor, 2001 [76] | 16.40 | 328 | 338 | 481 | Behavioral and anxiety evaluation | https://doi.org/10.1016/S0149-7634(01)00044-6 |
| 65/35 | Paine et al., 1996 [77] | 16.12 | 403 | 437 | 591 | Cytochrome P450 approach | https://doi.org/10.1016/S0009-9236(96)90162-9 |
| 66/66 | Özdemir et al., 2000 [78] | 15.19 | 319 | 350 | 487 | Cytochrome P450 approach | https://doi.org/10.1097/00008571-200007000-00001 |
| 67/54 | Gorski et al., 1998 [79] | 15.17 | 349 | 381 | 471 | Cytochrome P450 approach | https://doi.org/10.1016/S0009-9236(98)90146-1 |
| 68/83 | Andrew Williams et al., 2002 [80] | 14.95 | 284 | 322 | 551 | Sedation aspects | https://doi.org/10.1124/dmd.30.8.883 |
| 69/63 | Venn et al., 1999 [81] | 14.68 | 323 | 417 | 702 | Comparison with other drugs | https://doi.org/10.1046/j.1365-2044.1999.01114.x |
| 70/80 | Bai et al., 2001 [82] | 14.55 | 291 | 303 | 445 | GABA receptor | https://doi.org/10.1124/mol.59.4.814 |
| 71/93 | Yuan et al., 2002 [83] | 14.11 | 268 | 303 | 429 | Cytochrome P450 approach | https://doi.org/10.1124/dmd.30.12.1311 |
| 72/77 | Lang et al., 2000 [84] | 14.10 | 296 | 390 | 660 | Anesthesia and sedation aspects | https://doi.org/10.1016/S0140-6736(00)02162-0 |
| 73/94 | Gurley et al., 2002 [85] | 13.89 | 264 | 293 | 397 | Cytochrome P450 approach | https://doi.org/10.1067/mcp.2002.126913 |
| 74/59 | Schmiedlin-Ren et al., 1997 [86] | 13.83 | 332 | 366 | 466 | Cytochrome P450 approach | https://molpharm.aspetjournals.org/content/51/5/741.long |
| 75/87 | Olkkola et al., 1994 [87] | 13.33 | 360 | 498 | 786 | Cytochrome P450 approach | https://doi.org/10.1038/clpt.1994.60 |
| 76/71 | Pelkonen et al., 1998 [88] | 13.22 | 304 | 312 | 393 | Cytochrome P450 approach | https://doi.org/10.1080/004982598238886 |
| 77/13,18 | Kenworthy et al., 1999 [89] | 13.18 | 290 | 309 | 415 | Cytochrome P450 approach | https://doi.org/10.1046/j.1365-2125.1999.00073.x |
| 78/81 | Wang et al., 2001 [90] | 13.10 | 262 | 328 | 452 | Cytochrome P450 approach | https://doi.org/10.1067/mcp.2001.118522 |
| 79/89 | Krauss & Green, 2000 [91] | 12.95 | 272 | 333 | 481 | Sedation and analgesia aspects | https://doi.org/10.1056/NEJM200003303421306 |
| 80/55 | Christopher Gorski et al., 1994 [92] | 12.81 | 346 | 368 | 479 | Cytochrome P450 approach | https://doi.org/10.1016/0006-2952(94)90543-6 |
| 81/40 | Arrowsmith et al., 1991 [93] | 12.77 | 383 | 450 | 595 | Adverse effects | https://doi.org/10.1016/S0016-5107(91)70773-6 |
| 82/52 | Parke et al., 1992 [94] | 12.14 | 352 | 475 | 697 | Sedation aspects | https://doi.org/10.1136/bmj.305.6854.613 |
| 83/100 | Ostermann et al., 2000 [95] | 12.05 | 253 | 323 | 552 | Sedation aspects | https://doi.org/10.1001/jama.283.11.1451 |
| 84/62 | Lown et al., 1994 [96] | 12.00 | 324 | 353 | 452 | Cytochrome P450 approach | https://dmd.aspetjour-nals.org/content/22/6/947.long |
| 85/73 | Kup-ferschmidt et al., 1995 [97] | 11.65 | 303 | 345 | 439 | Pharmacology | https://doi.org/10.1016/0009-9236(95)90068-3 |
| 86/98 | Scott et al., 1999 [3] | 11.64 | 256 | 307 | 463 | Epilepsy treatment | https://doi.org/10.1016/S0140-6736(98)06425-3 |
| 87/47 | Bailey et al., 1990 [98] | 11.61 | 360 | 382 | 443 | Respiratory and adverse effects | https://doi.org/10.1097/00000542-199011000-00005 |
| 88/33 | Greenblatt et al., 1984 [99] | 11.27 | 417 | 448 | 653 | Pharmacology | https://doi.org/10.1097/00000542-198461010-00006 |
| 89/78 | Quine et al., 1995 [100] | 11.27 | 293 | 303 | 464 | Sedation aspects | https://doi.org/10.1136/gut.36.3.462 |
| 90/91 | Schmiedlin-Ren et al., 1997 [101] | 11.25 | 270 | 295 | 398 | Cytochrome P450 approach | https://doi.org/10.1124/mol.51.5.741 |
| 91/72 | Thummel et al., 1994 [102] | 11.22 | 303 | 314 | 405 | Cytochrome P450 approach | https://jpet.aspetjournals.orgcontent/271/1549.long |
| 92/86 | Cheng et al., 1996 [103] | 11.04 | 276 | 300 | 446 | Sedation aspects | https://doi.org/10.1016/S0022-5223(96)70062-4 |
| 93/65 | Hunt et al., 1992 [104] | 11.03 | 320 | 355 | 528 | Cytochrome P450 approach | https://doi.org/10.1016/0006-2952(92)90010-G |
| 94/76 | Ashton, 1994 [105] | 11.00 | 297 | 358 | 563 | Advantages and disadvantages | https://doi.org/10.2165/00003495-199448010-00004 |
| 95/70 | Heinrichs et al., 1992 [106] | 10.48 | 304 | 338 | 498 | Behavioral evaluation | https://doi.org/10.1016/0006-8993(92)90708-H |
| 96/87 | Olkkola et al., 1993 [107] | 9.82 | 275 | 310 | 383 | Pharmacology | https://doi.org/10.1038/clpt.1993.25 |
| 97/92 | Magorian et al., 1993 [108] | 9.61 | 269 | 323 | 602 | Anesthesia aspects | https://doi.org/10.1097/00000542-199311000-00007 |
| 98/99 | Mangano et al., 1992 [109] | 8.83 | 256 | 311 | 489 | Analgesia aspects | https://doi.org/10.1097/00000542-199203000-00004 |
| 99/56 | Allonen et al., 1981 [110] | 8.63 | 345 | 300 | 433 | Effects on the body | https://doi.org/10.1038/clpt.1981.217 |
| 100/75 | Dundee et al., 1984 [7] | 8.08 | 299 | 296 | 337 | Pharmacology | https://doi.org/10.2165/00003495-198428060-00002 |
| Characteristics | Number of Papers | Number of Citations in WoS-CC a | Citation Ratio b |
|---|---|---|---|
| Publication period | |||
| 1981–1991 | 8 | 4001 | 500, 13 |
| 1992–2002 | 52 | 22,125 | 425, 48 |
| 2003–2013 | 39 | 16,308 | 418, 15 |
| 2014–2016 | 1 | 476 | 476, 00 |
| Journal (Impact factor c) | |||
| Clinical Pharmacology & Therapeutics (7.051) | 10 | 3380 | 338, 00 |
| Anesthesiology (9.198) | 7 | 3219 | 459, 86 |
| New England Journal of Medicine (176.082) | 6 | 3747 | 624, 50 |
| Drug Metabolism and Disposition (3.579) | 6 | 2061 | 343, 50 |
| Clinical Pharmacokinetics (5.577) | 5 | 2265 | 453, 00 |
| Molecular Pharmacology (4.058) | 5 | 1909 | 381, 80 |
| Lancet (202.731) | 5 | 1683 | 336, 60 |
| Drugs (11.431) | 4 | 1429 | 357, 25 |
| Jama-Journal of the American Medical Association (157.375) | 4 | 2034 | 508, 50 |
| Gastrointestinal Endoscopy (10.396) | 3 | 1004 | 334, 67 |
| Critical Care Medicine (9296) | 2 | 1428 | 714, 00 |
| Journal of Pharmacology and Experimental Therapeutics (4404) | 2 | 951 | 475, 50 |
| British Journal of Clinical Pharmacology (3716) | 2 | 825 | 412, 5 |
| Gut (31,795) | 2 | 757 | 378, 5 |
| Pharmacogenetics (7221) d | 2 | 682 | 341 |
| Biochemical Pharmacology (6100) | 2 | 666 | 333 |
| Other journals with only one article | 33 | 1487 | 450, 60 |
| Experimental design | |||
| Non-systematic review | 21 | 7898 | 376, 09 |
| Cohort | 14 | 6261 | 447, 21 |
| Randomized clinical trial | 13 | 7148 | 549, 84 |
| Non-randomized clinical trial | 13 | 5154 | 396, 46 |
| In-vitro study | 10 | 4156 | 415, 60 |
| Clinical report | 5 | 1585 | 317, 00 |
| In vivo study | 9 | 4831 | 536, 77 |
| Guideline | 7 | 3111 | 444, 4 |
| Systematic review | 5 | 1750 | 350, 00 |
| Case report | 2 | 696 | 348, 00 |
| Cross-sectional | 1 | 320 | 320, 00 |
| Number of authors | |||
| 1–2 | 13 | 4852 | 373, 23 |
| 3–4 | 24 | 11,365 | 473, 54 |
| 5–6 | 20 | 7609 | 380, 45 |
| >6 | 44 | 19,084 | 433, 73 |
| Rank | Keyword | Frequency Total (Percent) | Total Link Strength | Frequency in 1981–1991 | Frequency in 1992–2002 | Frequency in 2003–2013 | Frequency in 2014–2016 |
|---|---|---|---|---|---|---|---|
| 1 | midazolam | 5 (3.45%) | 33 | 0 | 4 | 1 | 0 |
| 2 | cytochrome p450 | 5 (3.45%) | 23 | 0 | 4 | 1 | 0 |
| 3 | pharmacokinetics | 4 (2.76%) | 20 | 0 | 1 | 3 | 0 |
| 4 | drug metabolism | 4 (2.76%) | 18 | 0 | 3 | 1 | 0 |
| 5 | cyp3a4 | 4 (2.76%) | 13 | 0 | 4 | 0 | 0 |
| 6 | sedation | 3 (2.07%) | 19 | 0 | 2 | 1 | 0 |
| 7 | critical care | 3 (2.07%) | 15 | 0 | 1 | 1 | 0 |
| 8 | mechanical ventilation | 3 (2.07%) | 15 | 0 | 1 | 2 | 0 |
| 9 | anesthesia | 3 (2.07%) | 8 | 0 | 1 | 2 | 0 |
| 10 | propofol | 2 (1.38%) | 14 | 0 | 2 | 0 | 0 |
| 11 | elevated plus-maze | 2 (1.38%) | 13 | 0 | 2 | 0 | 0 |
| 12 | delirium | 2 (1.38%) | 11 | 0 | 0 | 2 | 0 |
| 13 | apoptosis | 2 (1.38%) | 10 | 0 | 0 | 2 | 0 |
| 14 | mortality | 2 (1.38%) | 10 | 0 | 0 | 2 | 0 |
| 15 | epilepsy | 2 (1.38%) | 8 | 0 | 1 | 1 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corôa, M.C.P.; Mendes, P.F.S.; Baia-da-Silva, D.C.; Souza-Monteiro, D.; Ferreira, M.K.M.; Braga, G.L.C.; Damasceno, T.V.; Perdigão, J.M.; Lima, R.R. What Is Known about Midazolam? A Bibliometric Approach of the Literature. Healthcare 2023, 11, 96. https://doi.org/10.3390/healthcare11010096
Corôa MCP, Mendes PFS, Baia-da-Silva DC, Souza-Monteiro D, Ferreira MKM, Braga GLC, Damasceno TV, Perdigão JM, Lima RR. What Is Known about Midazolam? A Bibliometric Approach of the Literature. Healthcare. 2023; 11(1):96. https://doi.org/10.3390/healthcare11010096
Chicago/Turabian StyleCorôa, Maria Claudia Pinheiro, Paulo Fernando Santos Mendes, Daiane Claydes Baia-da-Silva, Deiweson Souza-Monteiro, Maria Karolina Martins Ferreira, Glenda Luciana Costa Braga, Taissa Viana Damasceno, José Messias Perdigão, and Rafael Rodrigues Lima. 2023. "What Is Known about Midazolam? A Bibliometric Approach of the Literature" Healthcare 11, no. 1: 96. https://doi.org/10.3390/healthcare11010096
APA StyleCorôa, M. C. P., Mendes, P. F. S., Baia-da-Silva, D. C., Souza-Monteiro, D., Ferreira, M. K. M., Braga, G. L. C., Damasceno, T. V., Perdigão, J. M., & Lima, R. R. (2023). What Is Known about Midazolam? A Bibliometric Approach of the Literature. Healthcare, 11(1), 96. https://doi.org/10.3390/healthcare11010096







