Outcomes of Percutaneous Coronary Intervention in Elderly Patients with Rheumatoid Arthritis: A Nationwide Population-Based Cohort Study
Abstract
1. Introduction
2. Materials and Methods
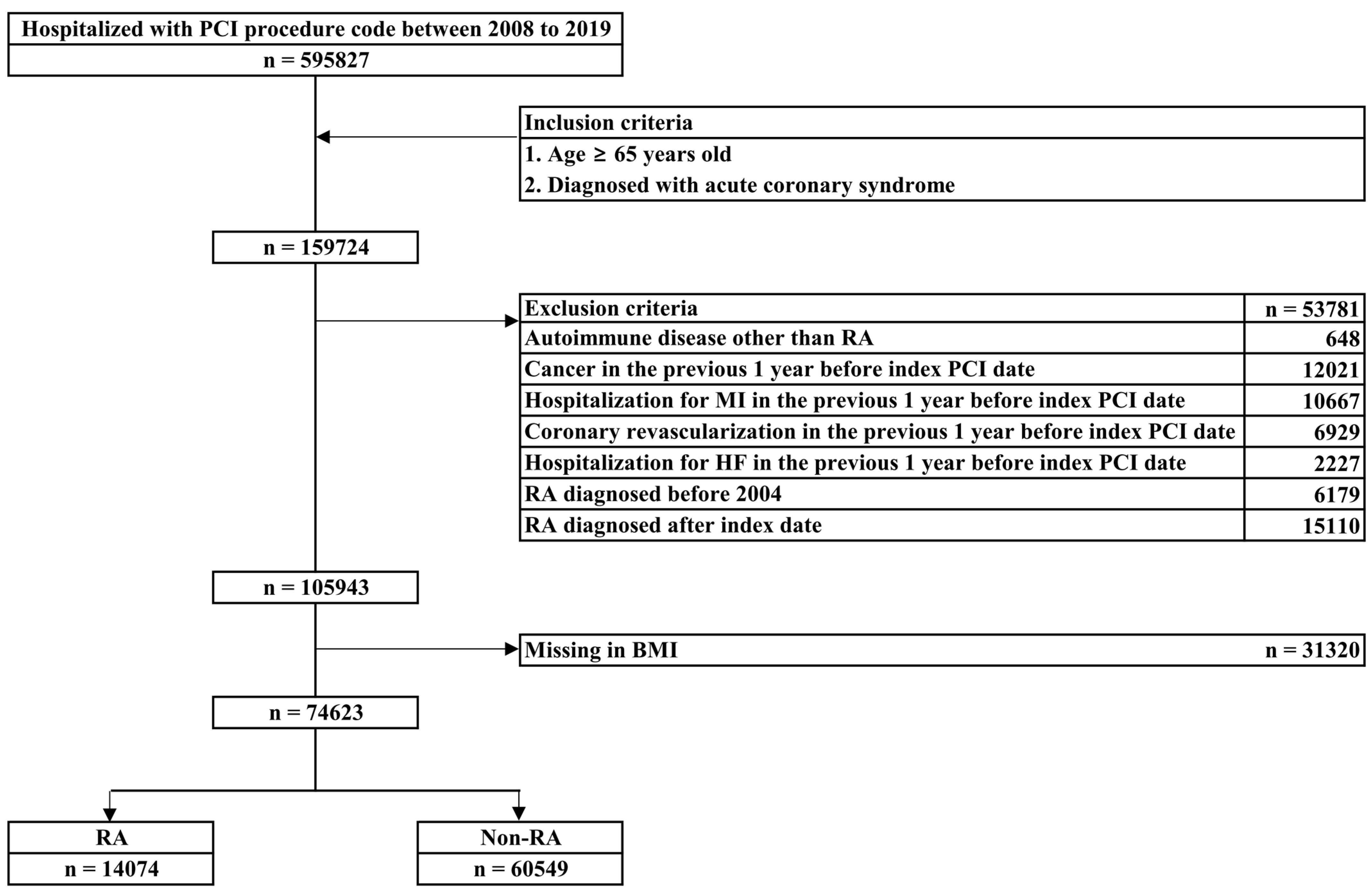
2.1. Data Sources and Study Population
2.2. Comorbidities
2.3. Study End-Points
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Participants
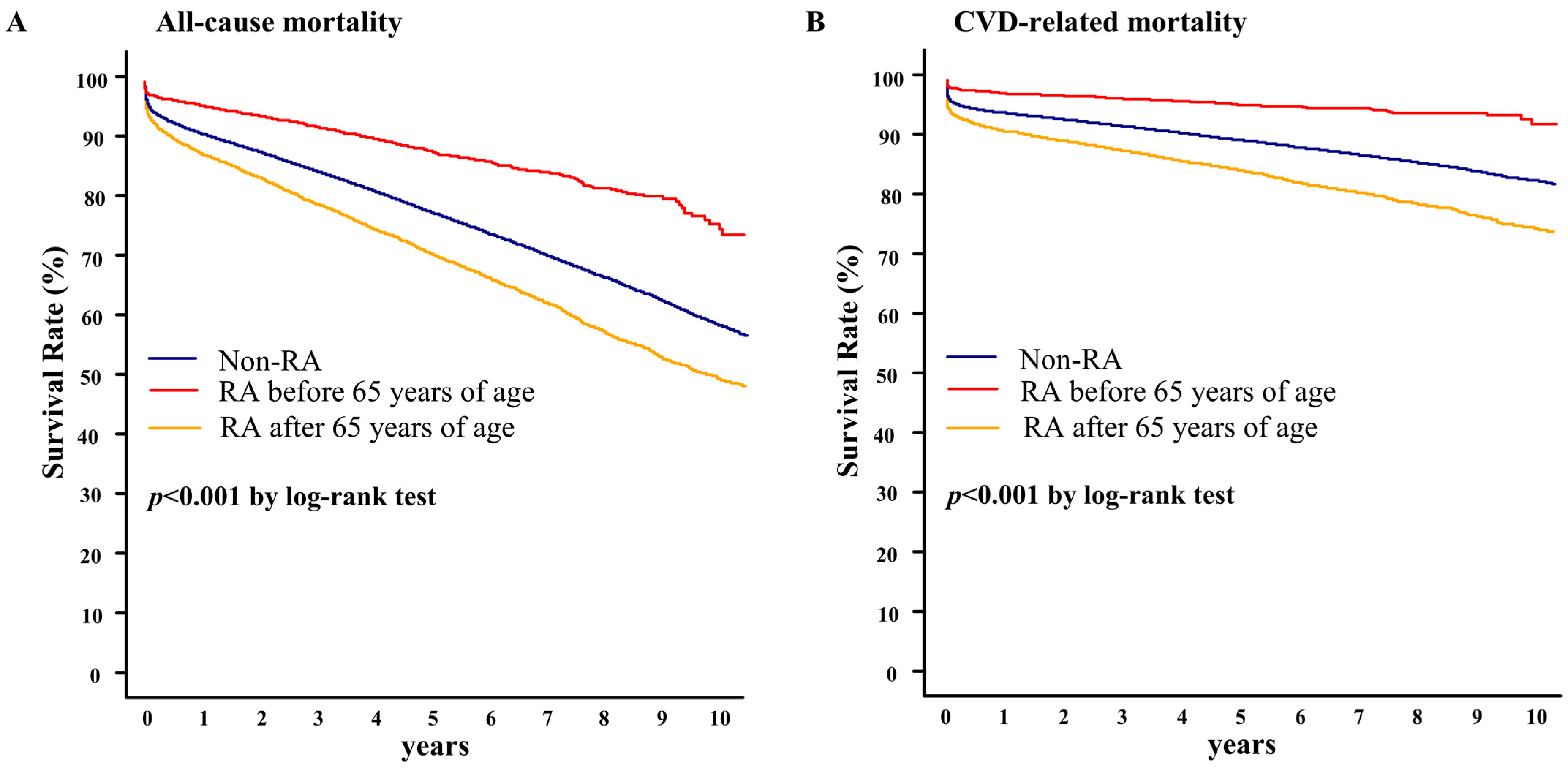
3.2. Association of RA and Clinical Outcomes in Elderly Patients Treated with PCI
3.3. Clinical Factors Associated with the Survival Outcomes in Elderly Patients Treated with PCI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Avina-Zubieta, J.A.; Thomas, J.; Sadatsafavi, M.; Lehman, A.J.; Lacaille, D. Risk of incident cardiovascular events in patients with rheumatoid arthritis: A meta-analysis of observational studies. Ann. Rheum. Dis. 2012, 71, 1524–1529. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.J.L.; van Halm, V.P.; Voskuyl, A.E.; Smulders, Y.M.; Boers, M.; Lems, W.F.; Visser, M.; Stehouwer, C.D.A.; Dekker, J.M.; Nijpels, G.; et al. Does rheumatoid arthritis equal diabetes mellitus as an independent risk foctor for cardiovascular disease? A prospective study. Arthritis Rheum. 2009, 61, 1571–1579. [Google Scholar] [CrossRef]
- Safiri, S.; Kolahi, A.A.; Hoy, D.; Smith, E.; Bettampadi, D.; Mansournia, M.A.; Almasi-Hashiani, A.; Ashrafi-Asgarabad, A.; Moradi-Lakeh, M.; Qorbani, M.; et al. Global, reginal and national burden of rheumatoid arthritis 1990-2017: A systemic analysis of the global burden of disease study 2017. Ann. Rheum. Dis. 2019, 78, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Fraenkel, L.; Rabidou, N.; Dhar, R. Are rheumatologists’ treatment decisions influenced by patients’ age? Rheumatology 2006, 45, 1555–1557. [Google Scholar] [CrossRef]
- Serhal, L.; Lwin, M.N.; Holroyd, C.; Edwards, C.J. Rheumatoid arthritis in the elderly: Characteristics and treatment considerations. Autoimmun. Rev. 2020, 19, 102528. [Google Scholar] [CrossRef] [PubMed]
- Ruban, T.N.; Jacob, B.J.; Pope, J.E.; Keystone, E.C.; Bombardier, C.; Kuriya, B. The influence of age at disease onset on disease activity and disability: Results from the Ontario best practices research initiative. Clin. Rheumatol. 2016, 35, 759–763. [Google Scholar] [CrossRef]
- Doornum, S.V.; Brand, C.; Sundararajan, V.; Ajani, A.E.; Wicks, I.P. Rheumatoid arthritis patients receive less frequent acute reperfusion and secondary prevention therapy after myocardial infarction compared with the general population. Arthritis Res. Ther. 2010, 12, R183. [Google Scholar] [CrossRef]
- Brieger, D.; Eagle, K.A.; Goodman, S.G.; Steg, P.G.; Budaj, A.; White, K.; Montalescot, G.; GRACE Investigators. Acute coronary syndromes without chest pains, an underdiagnosed and undertreated high-risk group: Insights from the global registry of acute coronary events. Chest 2004, 126, 461–469. [Google Scholar] [CrossRef]
- Martinez, S.; Mohamed, M.; Potts, J.; Abhishek, A.; Roddy, E.; Savage, M.; Bharadwaj, A.; Kwok, C.S.; Bagur, R.; Mamas, M.A. Percutaneous coronary intervention outcomes in patients with rheumatoid arthritis, systemic lupus erythematosus and systemic sclerosis. Rheumatology 2020, 59, 2512–2522. [Google Scholar] [CrossRef]
- Lai, C.-H.; Lai, W.-W.; Chiou, M.-J.; Lin, W.-C.; Yang, Y.-J.; Li, C.-Y.; Tsai, L.-M. Outcomes of percutaneous coronary intervention in patients with rheumatoid arthritis and systemic lupus erythematosus: An 11-year nationwide cohort study. Ann. Rheum. Dis. 2016, 75, 1350–1356. [Google Scholar] [CrossRef]
- Mccoy, S.S.; Crowson, C.S.; Maradit-Kremers, H.; Therneau, T.M.; Roger, V.L.; Matteson, E.L.; Gabriel, S.E. Longterm outcomes and treatment after myocardial infarction in patients with rheumatoid arthritis. J. Rheumatol. 2013, 40, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Varghese, J.J.; Koneru, S.; Scaife, S.L.; Zahnd, W.E.; Francis, M.L. Mortality after coronary artery revascularization of patients with rheumatoid arthritis. J. Thorac. Cardiovasc. Surg. 2010, 140, 91–96. [Google Scholar] [CrossRef] [PubMed]
- National Institute on Alcohol Abuse and Alcoholism. Alcohol Facts and Statistics; Glossary. Available online: https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/alcohol-facts-and-statistics (accessed on 1 June 2021).
- Chung, C.P.; Oeser, A.; Raggi, P.; Gebretsadik, T.; Shintani, A.K.; Sokka, T.; Pincus, T.; Avalos, I.; Stein, C.M. Increased coronary-artery atherosclerosis in rheumatoid arthritis: Relationship to disease duration and cardiovascular risk factors. Arthritis Rheum. 2005, 52, 3045–3053. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, L.; Delzell, E.; Muntner, P.; Hillegass, W.B.; Safford, M.M.; Millan, I.Y.N.; Crowson, C.S.; Curtis, J.R. The association between inflammatory markers, serum lipids and the risk of cardiovascular events in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2014, 73, 1301–1308. [Google Scholar] [CrossRef]
- Avina-Zubieta, J.A.; Abrahamowicz, M.; De Vera, M.A.; Choi, H.K.; Sayre, E.C.; Rahman, M.M.; Sylvestre, M.; Wynant, W.; Esdaile, J.M.; Lacaille, D. Immediate and past cumulative effects of oral glucocorticoids on the risk of acute myocardial infarction in rheumatoid arthritis: A population-based study. Rheumatology 2013, 52, 68–75. [Google Scholar] [CrossRef]
- Padol, I.T.; Hunt, R.H. Association of myocardial infarctions with COX-2 inhibition may be related to immunomodulation towards a Th1 response resulting in atheromatous plaque instability: An evidence-based interpretation. Rheumatology 2010, 49, 837–843. [Google Scholar] [CrossRef]
- England, B.R.; Thiele, G.M.; Anderson, D.R.; Mikuls, T.R. Increased cardiovascular risk in rheumatoid arthritis: Mechanisms and implications. BMJ 2018, 361, k1036. [Google Scholar] [CrossRef]
- Kang, E.H.; Liao, K.P.; Kim, S.C. Cardiovascular safety of biologics and JAK inhibitors in patients with rheumatoid arthritis. Curr. Rheumatol. Rep. 2018, 20, 42. [Google Scholar] [CrossRef]
- Radovits, B.J.; Fransen, J.; Eijsbouts, A.; van Riel, P.L.; Laan, F.J. Missed opportunities in the treatment of elderly patients with rheumatoid arthritis. Rheumatology 2009, 48, 906–910. [Google Scholar] [CrossRef]
- Doornum, S.V.; Brand, C.; King, B.; Sundararajan, V. Increased case fatality rates following a first acute cardiovascular event in patients with rheumatoid arthritis. Arthritis Rheum. 2006, 54, 2061–2068. [Google Scholar] [CrossRef]
- Mantel, A.; Holmqvist, M.; Jernberg, T.; Wallberg-Jonsson, S.; Askling, J. Rheumatoid arthritis is associated with with a more severe presentation of acute coronary syndrome and worse short-term outcome. Eur. Heart J. 2015, 36, 3413–3422. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.M.; Kwok, S.-K.; Ju, J.H.; Lee, S.-W.; Song, J.J.; Yoon, C.-. H, Park, Y.-B.; Park, S.-H. Risk factors associated with inadequate control of disease activity in elderly patients with rheumatoid arthritis: Results from a nationwide Korean college of rheumatology biologics (KOBIO) registry. PLoS ONE 2018, 13, e0205651. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Gonzolez, R.; Leon, L.; Loza, E.; Redondo, M.; Garcia de Yébenes, M.J.; Carmona, L. Adherence to biologic therapies and associated factors in rheumatoid arthritis, spondyoarthritis and psoriatic arthritis: A systemic literature review. Clin. Exp. Rheumatol. 2015, 33, 559–569. [Google Scholar]
- Innala, L.; Berglin, E.; Moller, B.; Ljung, L.; Smedby, T.; Sodergren, A.; Magnusson, S.; Rantapaa-Dahlqvist, S.; Wallberg-Jonsson, S. Age at onset determines severity and choice of treatment in early rheumatoid arthritis: A prospective study. Arthritis Res. Ther. 2014, 16, R94. [Google Scholar] [CrossRef] [PubMed]
- Numasawa, Y.; Inohara, T.; Ishii, H.; Yamaji, K.; Kohsaka, S.; Sawano, M.; Kodaira, M.; Uemura, S.; Kadota, K.; Amano, T.; et al. Comparison of outcomes after percutaneous coronary intervention in elderly patients, including 10628 nonagenarians: Insights from a Japanese nation registry (J-PCI Registry). J. Am. Heart Assoc. 2019, 8, e011183. [Google Scholar] [CrossRef]
- Batchelor, W.B.; Anstrom, K.J.; Muhlbaier, L.H.; Grosswald, R.; Weintraub, W.S.; O’Neil, W.W.; Peterson, E.D. Contemporary outcome trends in the elderly undergoing percutaneous coronary interventions: Results in 7,472 octogenarians. National Cardiovascular Network Collaboration. J. Am. Coll Cardiol. 2000, 36, 723–730. [Google Scholar] [CrossRef]
- Johnman, C.; Oldroyd, K.G.; Mackay, D.F.; Slack, R.; Pell, A.C.; Flapan, A.D.; Jennings, K.P.; Eteiba, H.; Irving, J.; Pell, J.P. Percutaneous coronary intervention in the elderly: Changes in case-mix and periprocedural outcomes in 31758 patients treated between 2000 and 2007. Circ. Cardiovasc. Interv. 2010, 3, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.M.H.; Maier, A.B.; Stinissen, P.; Masson, P.; Lories, R.J.; Keyser, F.D. The influence of ageing on the development and management of rheumatoid arthritis. Nat. Rev. Rheumaol. 2013, 9, 604–613. [Google Scholar] [CrossRef]
- Dawson, L.P.; Dinh, D.; O’Brien, J.; Duffy, S.J.; Guymer, E.; Brennan, A.; Clark, D.; Oqueli, E.; Hiew, C.; Freeman, M.; et al. Outcomes of percutaneous coronary intervention in patients with rheumatoid arthritis. Am. J. Cardiol. 2021, 140, 39–46. [Google Scholar] [CrossRef]
- Wong, B.; Lee, K.; El-Jack, S. Very elderly patients with acute coronary syndromes treated with percutaneous coronary intervention. Heart Lung Circ. 2021, 30, 1337–1342. [Google Scholar] [CrossRef]
- Changal, K.H.; Mir, T.; Khan, S.; Nazir, S.; Elzanatey, A.; Meenakshisundaram, C.; Mubbasher, S.; Sheikh, M.A. Drug-eluting stents versus bare-metal stents in large coronary artery revascularization: Systemic review and meta-analysis. Cardiovasc. Revasc. Med. 2021, 23, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, R.; Bonaa, K.H.; Efthimiou, O.; Varenne, O.; Baldo, A.; Urban, P.; Kaiser, C.; Remkes, W.; Raber, L.; de Balder, A.; et al. Drug-eluting or bare-metal stents for percutaneous coronary intervention; a systemic review and individual patient data meta-analysis of randomized clinical trials. Lancet 2019, 393, 2503–2510. [Google Scholar] [CrossRef] [PubMed]
- Afilalo, J.; Alexander, K.P.; Mack, M.J.; Maurer, M.S.; Green, P.; Allen, L.A.; Popma, J.J.; Ferrucci, L.; Forman, D.E. Fraility assessment in the cardiovascular care of older adults. J. Am. Coll Cardiol. 2014, 63, 747–762. [Google Scholar] [CrossRef] [PubMed]

| Variables | Total | RA | Without RA | p-Value |
|---|---|---|---|---|
| (n = 74,623) | (n = 14,074) | (n = 60,549) | ||
| Age at index date (years), mean ± SD | 73.7 ± 6.0 | 74.6 ± 6.1 | 73.5 ± 6.0 | <0.001 |
| Age at index date, n (%) | <0.001 | |||
| 65 to 69 years | 21,644 (29.0) | 3389 (24.1) | 18,255 (30.2) | |
| 70 to 79 years | 39,764 (53.3) | 7630 (54.2) | 32,134 (53.1) | |
| ≥80 years | 13,215 (17.7) | 3055 (21.7) | 10,160 (16.8) | |
| Sex (women), n (%) | 30,084 (40.3) | 7791 (55.4) | 22,293 (36.8) | <0.001 |
| BMI (kg/㎡), mean ± SD | 24.2 ± 3.1 | 24.4 ± 3.2 | 24.1 ± 3.0 | <0.001 |
| Current smoking, n (%) | 14,881 (19.9) | 2015 (14.3) | 12,866 (21.3) | <0.001 |
| Alcohol abuse, n (%) | 249 (0.3) | 59 (0.4) | 190 (0.3) | 0.061 |
| Age at the time of diagnosis of RA, n (%) | <0.001 | |||
| <65 years | - | 3987 (28.3) | - | |
| ≥65 years | - | 10,087 (71.7) | - | |
| Duration of RA (year), n (%) | - | 5.9 ± 3.5 | - | <0.001 |
| <1 | - | 1079 (7.7) | - | |
| 1 to 5 | - | 5023 (35.7) | - | |
| >5 | - | 7972 (56.6) | - | |
| Comorbidities, n (%) | ||||
| Hypertension | 64,269 (86.1) | 12,449 (88.5) | 51,820 (85.6) | <0.001 |
| Dyslipidemia | 16,565 (22.2) | 3350 (23.8) | 13,215 (21.8) | <0.001 |
| Diabetes mellitus | 40,307 (54.0) | 8208 (58.3) | 32,099 (53.0) | <0.001 |
| Atrial fibrillation | 5820 (7.8) | 1156 (8.2) | 4664 (7.7) | 0.044 |
| Venous thromboembolism | 1841 (2.5) | 425 (3.0) | 1416 (2.3) | <0.001 |
| Peripheral vascular disease | 11,696 (15.7) | 2744 (19.5) | 8952 (14.8) | <0.001 |
| stroke/TIA | 10,887 (14.6) | 2247 (16.0) | 8640 (14.3) | <0.001 |
| heart failure | 14,851 (19.9) | 3108 (22.1) | 11,743 (19.4) | <0.001 |
| COPD | 5943 (8.0) | 1221 (8.7) | 4722 (7.8) | 0.001 |
| Moderate-to-severe CKD | 823 (1.1) | 165 (1.2) | 658 (1.1) | 0.406 |
| Use of drugs, n (%) | ||||
| ACEI/ARB | 11,883 (15.9) | 2318 (16.5) | 9565 (15.8) | 0.051 |
| Beta-blockers | 57,072 (76.5) | 10,659 (75.7) | 46,413 (76.7) | 0.021 |
| Anticoagulants | 3509 (4.7) | 744 (5.3) | 2765 (4.6) | <0.001 |
| Antiplatelets | 74,545 (99.9) | 14,057 (99.9) | 60,488 (99.9) | 0.604 |
| Statins | 66,971 (89.8) | 12,879 (91.5) | 54,092 (89.3) | <0.001 |
| Other lipid-lowering agents | 4226 (5.7) | 865 (6.2) | 3361 (5.6) | 0.006 |
| Variable | Total | With RA | Without RA | p-Value |
|---|---|---|---|---|
| (n = 74,623) | (n = 14,074) | (n = 60,549) | ||
| Diagnosis at PCI index date, n (%) | <0.001 | |||
| STEMI | 28,662 (38.4) | 4925 (35.0) | 23,737 (39.2) | <0.001 |
| NSTEMI | 10,009 (13.4) | 1996 (14.2) | 8013 (13.2) | 0.003 |
| Unstable angina | 35,952 (48.2) | 7153 (50.8) | 28,799 (47.6) | <0.001 |
| Type of implanted stents *, n (%) | 0.010 | |||
| Drug-eluting stents | 72,341 (96.9) | 13,659 (97.1) | 58,682 (96.9) | 0.418 |
| Bare metal stents | 1282 (1.7) | 216 (1.5) | 1066 (1.8) | 0.069 |
| Both drug-eluting and bare metal stents | 265 (0.4) | 38 (0.3) | 227 (0.4) | 0.071 |
| Number of implanted stents *, n (%) | 0.056 | |||
| single-vessel PCI | 65,536 (87.8) | 12,378 (88.0) | 53,158 (87.8) | 0.620 |
| multi-vessel PCI | 8352 (11.2) | 1535 (10.9) | 6817 (11.3) | 0.239 |
| Variable | Univariable Models | Multivariable Models | ||
|---|---|---|---|---|
| Crude OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value | |
| All-cause mortality | ||||
| Age (at index date) | 1.11 (1.108–1.115) | <0.001 | 1.11 (1.106–1.113) | <0.001 |
| Male | 1.06 (1.02–1.09) | 0.001 | 1.17 (1.12–1.21) | <0.001 |
| Current smoking | 1.30 (1.25–1.35) | <0.001 | 1.46 (1.40–1.53) | <0.001 |
| Obesity | 0.91 (0.91–0.92) | <0.001 | 0.94 (0.94–0.95) | <0.001 |
| Stroke/TIA | 1.85 (1.77–1.93) | <0.001 | 1.72 (1.64–1.80) | <0.001 |
| Heart failure | 1.65 (1.59–1.72) | <0.001 | 1.28 (1.23–1.34) | <0.001 |
| Hypertension | 1.21 (1.16–1.27) | <0.001 | 1.13 (1.07–1.19) | <0.001 |
| Dyslipidemia | 0.93 (0.89–0.97) | <0.001 | 0.99 (0.95–1.03) | 0.637 |
| Diabetes mellitus | 1.42 (1.37–1.47) | <0.001 | 1.46 (1.41–1.51) | <0.001 |
| COPD | 2.60 (2.46–2.74) | <0.001 | 2.08 (1.96–2.20) | <0.001 |
| Moderate-to-severe CKD | 4.20 (3.65–4.84) | <0.001 | 4.53 (3.90–5.28) | <0.001 |
| Diagnosis at PCI index date | ||||
| Unstable angina (reference) | 1.00 | 1.00 | ||
| STEMI | 2.14 (2.07–2.22) | <0.001 | 1.91 (1.84–1.98) | <0.001 |
| NSTEMI | 1.44 (1.37–1.51) | <0.001 | 1.13 (1.07–1.19) | <0.001 |
| CVD-related mortality | ||||
| Age (at index date) | 1.10 (1.095–1.102) | <0.001 | 1.09 (1.08–1.09) | <0.001 |
| Male | 0.85 (0.82–0.89) | <0.001 | 0.93 (0.88–0.97) | 0.003 |
| Current smoking | 1.14 (1.08–1.20) | <0.001 | 1.29 (1.22–1.37) | <0.001 |
| Obesity | 0.93 (0.92–0.93) | <0.001 | 0.96 (0.95–0.97) | <0.001 |
| Stroke/TIA | 1.86 (1.76–1.96) | <0.001 | 1.75 (1.65–1.85) | <0.001 |
| Heart failure | 1.95 (1.85–2.04) | <0.001 | 1.55 (1.47–1.63) | <0.001 |
| Hypertension | 1.16 (1.09–1.24) | <0.001 | 1.09 (1.02–1.17) | 0.011 |
| Dyslipidemia | 1.00 (0.95–1.06) | 0.870 | 1.03 (0.97–1.09) | 0.297 |
| Diabetes mellitus | 1.30 (1.25–1.36) | <0.001 | 1.28 (1.22–1.34) | <0.001 |
| COPD | 1.89 (1.77–2.02) | <0.001 | 1.49 (1.39–1.60) | <0.001 |
| Moderate-to-severe CKD | 1.61 (1.34–1.91) | <0.001 | 1.49 (1.23–1.79) | <0.001 |
| Diagnosis at PCI index date | ||||
| Unstable angina (reference) | 1.00 | 1.00 | ||
| STEMI | 2.84 (2.71–2.99) | <0.001 | 2.52 (2.40–2.66) | <0.001 |
| NSTEMI | 1.86 (1.74–2.00) | <0.001 | 1.51 (1.40–1.62) | <0.001 |
| Variable | Univariable Models | Multivariable Models | ||
|---|---|---|---|---|
| Crude OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value | |
| All-cause mortality | ||||
| Age at diagnosis of RA | ||||
| ···Young-onset RA (reference) | 1.00 | 1.00 | ||
| ···Elderly-onset RA | 3.35 (3.03–3.70) | <0.001 | 1.80 (1.59–2.03) | <0.001 |
| Age (at index date) | 1.10 (1.10–1.11) | <0.001 | 1.07 (1.06–1.08) | <0.001 |
| Male | 1.20 (1.11–1.29) | <0.001 | 1.19 (1.09–1.30) | <0.001 |
| Current smoking | 1.30 (1.25–1.35) | <0.001 | 1.46 (1.40–1.53) | <0.001 |
| Obesity | 0.91 (0.89–0.92) | <0.001 | 0.94 (0.92–0.95) | <0.001 |
| Stroke/TIA | 1.77 (1.61–1.95) | <0.001 | 1.65 (1.49–1.83) | <0.001 |
| Heart failure | 1.51 (1.39–1.64) | <0.001 | 1.19 (1.08–1.31) | <0.001 |
| Hypertension | 1.19 (1.06–1.34) | 0.004 | 1.10 (0.97–1.26) | 0.145 |
| Dyslipidemia | 1.01 (0.92–1.10) | 0.873 | 1.05 (0.96–1.16) | 0.288 |
| Diabetes mellitus | 1.31 (1.21–1.41) | <0.001 | 1.37 (1.26–1.49) | <0.001 |
| COPD | 2.37 (2.11–2.67) | <0.001 | 1.85 (1.63–2.11) | <0.001 |
| Moderate-to-severe CKD | 4.08 (2.99–5.62) | <0.001 | 4.84 (3.44–6.85) | <0.001 |
| Diagnosis at PCI index date | ||||
| Unstable angina (reference) | 1.00 | 1.00 | ||
| STEMI | 2.62 (2.41–2.84) | <0.001 | 2.28 (2.09–2.49) | <0.001 |
| NSTEMI | 1.63 (1.45–1.82) | <0.001 | 1.31 (1.16–1.48) | <0.001 |
| CVD-related mortality | ||||
| Age at diagnosis of RA | ||||
| ···Young-onset RA (reference) | 1.00 | 1.00 | ||
| ···Elderly-onset RA | 2.67 (2.33–3.08) | <0.001 | 1.36 (1.15–1.62) | <0.001 |
| Age (at index date) | 1.09 (1.08–1.10) | <0.001 | 1.06 (1.05–1.07) | <0.001 |
| Male | 0.90 (0.82–1.00) | 0.046 | 0.89 (0.79–0.99) | 0.040 |
| Current smoking | 1.18 (1.03–1.35) | 0.016 | 1.33 (1.14–1.55) | <0.001 |
| Obesity | 0.92 (0.91–0.94) | <0.001 | 0.95 (0.94–0.97) | <0.001 |
| Stroke/TIA | 1.77 (1.57–2.00) | <0.001 | 1.69 (1.49–1.92) | <0.001 |
| Heart failure | 1.86 (1.66–2.06) | <0.001 | 1.49 (1.33–1.67) | <0.001 |
| Hypertension | 1.07 (0.91–1.26) | 0.409 | 0.99 (0.83–1.17) | 0.860 |
| Dyslipidemia | 1.15 (1.03–1.29) | 0.013 | 1.15 (1.02–1.29) | 0.025 |
| Diabetes mellitus | 1.23 (1.11–1.36) | <0.001 | 1.25 (1.12–1.39) | <0.001 |
| COPD | 1.85 (1.59–2.15) | <0.001 | 1.47 (1.25–1.72) | <0.001 |
| Moderate-to-severe CKD | 1.36 (0.88–2.02) | 0.149 | 1.36 (0.86–2.08) | 0.173 |
| Diagnosis at PCI index date | ||||
| Unstable angina (reference) | 1.00 | 1.00 | ||
| STEMI | 3.51 (3.13–3.94) | <0.001 | 3.05 (2.71–3.43) | <0.001 |
| NSTEMI | 2.17 (1.82–2.53) | <0.001 | 1.77 (1.51–2.08) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.Y.; Moon, H.; Kim, S.-S.; Kim, H.-S. Outcomes of Percutaneous Coronary Intervention in Elderly Patients with Rheumatoid Arthritis: A Nationwide Population-Based Cohort Study. Healthcare 2023, 11, 1381. https://doi.org/10.3390/healthcare11101381
Kim BY, Moon H, Kim S-S, Kim H-S. Outcomes of Percutaneous Coronary Intervention in Elderly Patients with Rheumatoid Arthritis: A Nationwide Population-Based Cohort Study. Healthcare. 2023; 11(10):1381. https://doi.org/10.3390/healthcare11101381
Chicago/Turabian StyleKim, Bo Young, HyeSung Moon, Sung-Soo Kim, and Hyun-Sook Kim. 2023. "Outcomes of Percutaneous Coronary Intervention in Elderly Patients with Rheumatoid Arthritis: A Nationwide Population-Based Cohort Study" Healthcare 11, no. 10: 1381. https://doi.org/10.3390/healthcare11101381
APA StyleKim, B. Y., Moon, H., Kim, S.-S., & Kim, H.-S. (2023). Outcomes of Percutaneous Coronary Intervention in Elderly Patients with Rheumatoid Arthritis: A Nationwide Population-Based Cohort Study. Healthcare, 11(10), 1381. https://doi.org/10.3390/healthcare11101381







