The Latest Craniofacial Reconstructive Techniques Using Anchored Implants after Surgical Treatment of Nasal and Paranasal Sinuses Tumors
Abstract
:1. Introduction
2. Methods and Materials
3. Results
3.1. Prostheses Mounted on Anchored Implants
3.2. Indications
3.3. Contraindications
3.4. Implantation Procedure
- -
- bone parameters—thickness and shape,
- -
- implant stability,
- -
- minimizing the impact of unwanted forces on the implants,
- -
- planning locations for replacement implants in case of implant failure at the originally selected sites, and
- -
- maintaining the angles at which the implants are inserted so that other prosthetic components can subsequently be fixed to them.
- -
- longer time required for soft tissue healing;
- -
- simultaneous removal of oncological lesions and insertion of anchored implants;
- -
- planned radiotherapy treatment.
3.5. Silicone Prosthesis vs. 3D-Printed Prosthesis
- -
- taking an impression of the area for the prosthesis,
- -
- fitting of the external structure developed from archival photographs of the patient’s face,
- -
- modelling the prosthesis in wax to obtain its final shape, and
- -
- acceptance of the prosthesis, learning to use the system.
3.6. Prognostic Factors Influencing Implantation Success
3.6.1. Tissue Irradiation Dose
3.6.2. Number of Implants and Their Interposition, Implantation Technique
3.6.3. Implant Site
3.6.4. Patient’s Age
3.6.5. Aftercare
3.7. Own Experiences
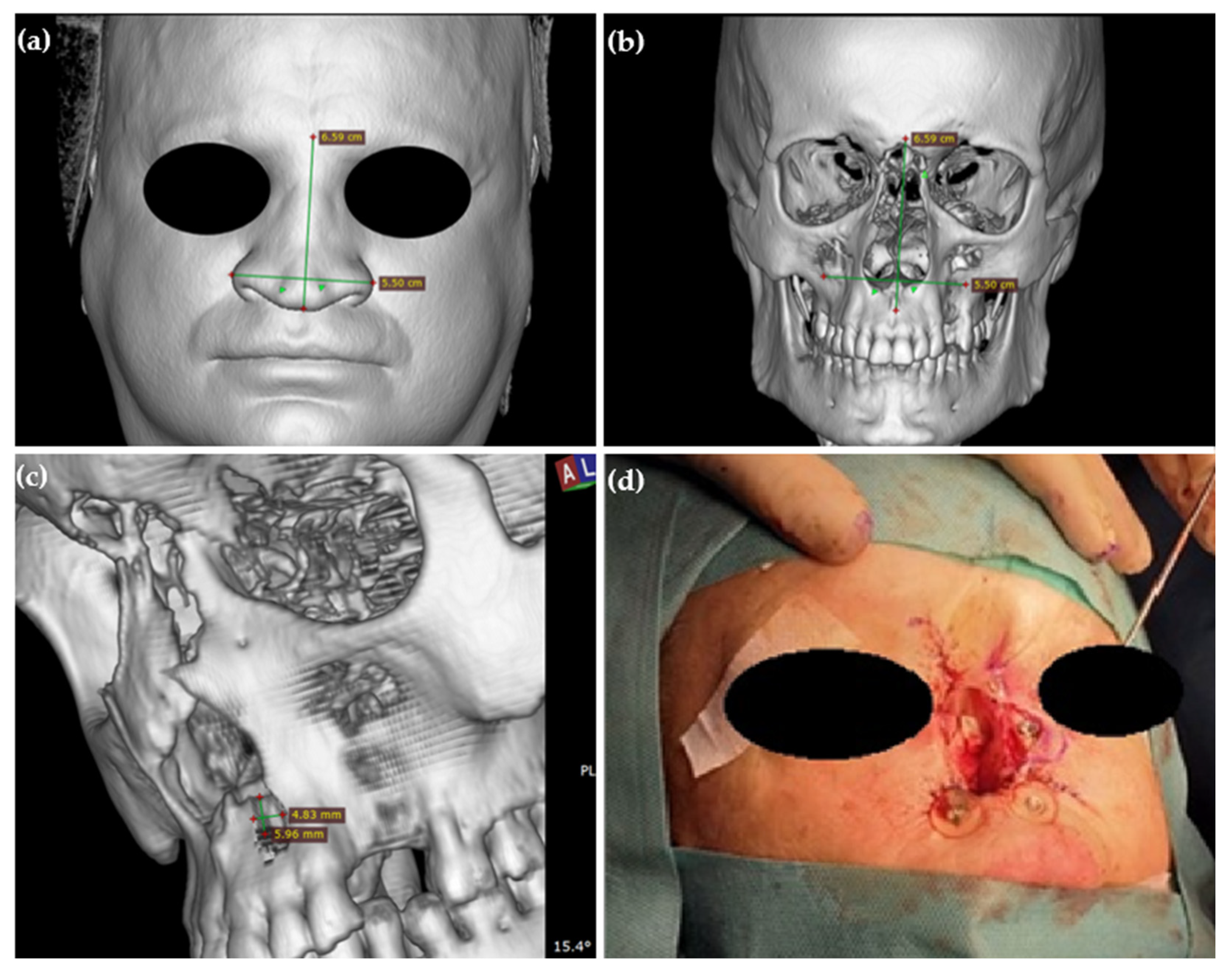
3.7.1. Case Report
3.7.2. Surgical Technique
3.7.3. Follow-Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kilic, S.; Shukla, P.A.; Marchiano, E.J.; Patel, R.H.; Baredes, S.; Liu, J.K.; Eloy, J.A. Malignant Primary Neoplasms of the Nasal Cavity and Paranasal Sinus. Curr. Otorhinolaryngol. Rep. 2016, 4, 249–258. [Google Scholar] [CrossRef]
- Agarwal, S.; van Zante, A.; Granados, M.L. Combined Neuroendocrine and Squamous Cell Carcinoma of the Sinonasal Tract: A Morphologic and Immunohistochemical Analysis and Review of Literature. Head Neck Pathol. 2022, 16, 1019–1033. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Taboni, S.; Carobbio, A.L.C.; Emanuelli, E.; Maroldi, R.; Bossi, P.; Nicolai, P. Sinonasal Squamous Cell Carcinoma, a Narrative Reappraisal of the Current Evidence. Cancers 2021, 13, 2835. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.B.; Jensen, K.; Friborg, J.; Smulders, B.; Andersen, E.; Samsøe, E.; Johansen, J.; Hansen, C.R.; Andersen, M.; Nielsen, M.S.; et al. Target coverage and local recurrences after radiotherapy for sinonasal cancer in Denmark 2008–2015. A DAHANCA study. Acta Oncol. 2022, 61, 120–126. [Google Scholar] [CrossRef]
- Taylor, M.A.; Saba, N.F. Cancer of the Paranasal Sinuses. Hematol. Oncol. Clin. N. Am. 2021, 35, 949–962. [Google Scholar] [CrossRef]
- Abdelmeguid, A.S.; Teeramatwanich, W.; Roberts, D.B.; Amit, M.; Ferraroto, R.; Glisson, B.S.; Kupferman, M.E.; Su, S.Y.; Phan, J.; Garden, A.S.; et al. Neoadjuvant chemotherapy for locoregionally advanced squamous cell carcinoma of the paranasal sinuses. Cancer 2021, 127, 1788–1795. [Google Scholar] [CrossRef]
- Wrobel, C.; Keppeler, D.; Meyer, A.C. Optimized fitting of a midface implant to anchor a magnetic nasal prosthesis using 3D printing. HNO 2022, 70, 200–205. [Google Scholar] [CrossRef]
- Vitomir, K.S.; Filip, I.; Vojkan, L.; Igor, Đ.; Lukasz, P. Survival rate of disk and screw-type implants used for the retention of extraoral prostheses. J. Prosthet. Dent. 2022, 127, 499–507. [Google Scholar] [CrossRef]
- Rogers, S.N.; Adatia, A.; Hackett, S.; Boscarino, A.; Patel, A.; Lowe, D.; Butterworth, C.J. Changing trends in the microvascular reconstruction and oral rehabilitation following maxillary cancer. Eur. Arch. Oto-Rhino-Laryngol. 2022, 279, 4113–4126. [Google Scholar] [CrossRef]
- Gunawardena, D.A.; Howes, D.; Fleming, S.; Clark, J.; Leinkram, D.; Palme, C.; Dusseldorp, J.R. Reconstruction of a maxillectomy and rhinectomy defect utilising a novel subperiosteal prosthesis and magnet-retained nasal prosthesis: A case report. Oral Oncol. 2022, 135, 106222. [Google Scholar] [CrossRef]
- Alberga, J.; Eggels, I.; Visser, A.; van Minnen, B.; Korfage, A.; Vissink, A.; Raghoebar, G. Outcome of implants placed to retain craniofacial prostheses—A retrospective cohort study with a follow-up of up to 30 years. Clin. Implant Dent. Relat. Res. 2022, 24, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Tso, T.V.; Chao, D.; Tanner, J.; Freymiller, E.G.; Jayanetti, J. Prosthetic reconstruction of a patient with an irradiated total rhinectomy with navigated surgical placement of a single zygomatic implant: A clinical report. J. Prosthet. Dent. 2021, 125, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Gaur, V.; Perumal, S.M.; Rahmaan, F.; Pałka, Ł. A practical approach to orofacial rehabilitation in a patient after inferior maxillectomy and rhinectomy with mono framework construction supported on a zygomatic implant placed in the glabella: A case report. Maxillofac. Plast. Reconstr. Surg. 2021, 43, 25. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.B.; Ahmed, Z.U.; Huryn, J.M.; Ganly, I. Prosthetic rehabilitation of the geriatric oncologic rhinectomy patient utilizing a craniofacial implant-retained nasal prosthesis. Clin. Case Rep. 2020, 8, 278–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowak, S.M.; Silk, H.; Ilankovan, V. New approach for the anchorage of nasal implants without osseointegration using the Epiplating system for a magnetic prosthesis. Br. J. Oral Maxillofac. Surg. 2020, 58, 488–490. [Google Scholar] [CrossRef] [PubMed]
- Dings, J.P.J.; Vijverberg, M.A.; Hol, M.K.S.; Ulrich, D.J.O.; de Haan, A.F.J.; Verhage-Damen, G.W.; de Clonie Maclennan-Naphausen, M.T.P.; Kruyt, I.J.; Ghaeminia, H.; Bruekers-Schipper, G.B.; et al. Autologous versus prosthetic nasal and auricular reconstruction—Patient, professional and layperson perceptions. Int. J. Oral Maxillofac. Surg. 2020, 49, 1271–1278. [Google Scholar] [CrossRef]
- Moore, P.; Grinsell, D.; Lyons, B.; Hewson, I. Outcomes of dental and craniofacial osseointegrated implantation in head and neck cancer patients. Head Neck 2019, 41, 3290–3298. [Google Scholar] [CrossRef]
- Unkovskiy, A.; Spintzyk, S.; Brom, J.; Huettig, F.; Keutel, C. Direct 3D printing of silicone facial prostheses: A preliminary experience in digital workflow. J. Prosthet. Dent. 2018, 120, 303–308. [Google Scholar] [CrossRef]
- Subramaniam, S.S.; Breik, O.; Cadd, B.; Peart, G.; Wiesenfeld, D.; Heggie, A.; Gibbons, S.D.; Nastri, A. Long-term outcomes of craniofacial implants for the restoration of facial defects. Int. J. Oral Maxillofac. Surg. 2018, 47, 773–782. [Google Scholar] [CrossRef] [Green Version]
- Papaspyrou, G.; Yildiz, C.; Bozzato, V.; Bohr, C.; Schneider, M.; Hecker, D.; Schick, B.; Al Kadah, B. Prosthetic supply of facial defects: Long-term experience and retrospective analysis on 99 patients. Eur. Arch. Oto-Rhino-Laryngol. 2018, 275, 607–613. [Google Scholar] [CrossRef]
- Khorasani, M.; Janbaz, P.; Rayati, F. Maxillofacial reconstruction with Medpor porous polyethylene implant: A case series study. J. Korean Assoc. Oral Maxillofac. Surg. 2018, 44, 128–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bansal, K.; Rawat, P.; Pruthi, G.; Jain, V. Prosthodontic rehabilitation of combined oronasal defect in patients with non-Hodgkin’s lymphoma using two different attachments: Two case reports. J. Indian Prosthodont. Soc. 2018, 18, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Burton, M.J.; Niparko, J.K.; Johansson, C.B.; Tjellstrom, A. Titanium-anchored prostheses in otology. Otolaryngol. Clin. N. Am. 1996, 29, 301–310. [Google Scholar] [CrossRef]
- Visser, A.; Raghoebar, G.M.; van Oort, R.P.; Vissink, A. Fate of implant-retained craniofacial prostheses: Life span and aftercare. Int. J. Oral Maxillofac. Implant. 2008, 23, 89–98. [Google Scholar]
- Schoen, P.J.; Raghoebar, G.M.; van Oort, R.P.; Reintsema, H.; van der Laan, B.F.; Burlage, F.R.; Roodenburg, J.L.; Vissink, A. Treatment outcome of bone-anchored craniofacial prostheses after tumor surgery. Cancer 2001, 92, 3045–3050. [Google Scholar] [CrossRef]
- Dings, J.P.J.; Merkx, M.A.W.; de Clonie Maclennan-Naphausen, M.T.P.; van de Pol, P.; Maal, T.J.J.; Meijer, G.J. Maxillofacial prosthetic rehabilitation: A survey on the quality of life. J. Prosthet. Dent. 2018, 120, 780–786. [Google Scholar] [CrossRef]
- Available online: https://southernimplants.com/ (accessed on 1 March 2023).
- D’Agostino, A.; Lombardo, G.; Favero, V.; Signoriello, A.; Bressan, A.; Lonardi, F.; Nocini, R.; Trevisiol, L. Complications related to zygomatic implants placement: A retrospective evaluation with 5 years follow-up. J. Cranio-Maxillofac. Surg. 2021, 49, 620–627. [Google Scholar] [CrossRef]
- Vistafix, C. Treatment and Surgery Guide. A Bone Anchored Facial Prosthetic Solution. Bone Anchored Solutions. Available online: https://mss-p-007-delivery.sitecorecontenthub.cloud/api/public/content/VFX001-Vistafix-Surgery-Guide.pdf (accessed on 22 November 2022).
- Huang, Y.H.; Seelaus, R.; Zhao, L.; Patel, P.K.; Cohen, M. Virtual surgical planning and 3D printing in prosthetic orbital reconstruction with percutaneous implants: A technical case report. Int. Med. Case Rep. J. 2016, 9, 341–345. [Google Scholar] [CrossRef] [Green Version]
- Martinez Plaza, A.; Perez de Perceval Tara, M.; Marin Fernandez, A.B.; Bullejos Martinez, E.; Roman Ramos, M.; Fernandez Valades, R.; Espana Lopez, A. Bilateral auricular reconstruction with osseointegrated implant-retained prostheses. Optimization of aesthetic outcomes using virtual planning. J. Stomatol. Oral Maxillofac. Surg. 2019, 120, 579–583. [Google Scholar] [CrossRef]
- Wei, L.A.; Brown, J.J.; Hosek, D.K.; Burkat, C.N. Osseointegrated implants for orbito-facial prostheses: Preoperative planning tips and intraoperative pearls. Orbit 2016, 35, 55–61. [Google Scholar] [CrossRef]
- Guo, T.; Gulati, K.; Arora, H.; Han, P.; Fournier, B.; Ivanovski, S. Orchestrating soft tissue integration at the transmucosal region of titanium implants. Acta Biomater. 2021, 124, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Abdullah Abdul, M.; Dasmawati, M.; Din Tengku Noor Daimah, T.; Yahya, S.; Akil Hazizan, M.; Rajion Zainul, A. Fabrication of nasal prosthesis utilising an affordable 3D printer. Int. J. Adv. Manuf. Technol. 2019, 100, 1907–1912. [Google Scholar] [CrossRef]
- Biswas, L.; Chen, J.; De Angelis, J.; Singh, A.; Owen-Woods, C.; Ding, Z.; Pujol, J.M.; Kumar, N.; Zeng, F.; Ramasamy, S.K.; et al. Lymphatic vessels in bone support regeneration after injury. Cell 2023, 186, 382–397.e324. [Google Scholar] [CrossRef] [PubMed]
- ElKhashab, M.A.; Radi, I.A.W.; Elkhadem, A.H. Implant prognosis in irradiated versus non-irradiated nasal, orbital and auricular sites. Int. J. Oral Maxillofac. Surg. 2020, 49, 636–648. [Google Scholar] [CrossRef]
- Granstrom, G. Craniofacial osseointegration. Oral Dis. 2007, 13, 261–269. [Google Scholar] [CrossRef]
- Nishimura, R.D.; Roumanas, E.; Moy, P.K.; Sugai, T. Nasal defects and osseointegrated implants: UCLA experience. J. Prosthet. Dent. 1996, 76, 597–602. [Google Scholar] [CrossRef]
- Chrcanovic, B.R.; Nilsson, J.; Thor, A. Survival and complications of implants to support craniofacial prosthesis: A systematic review. J. Craniomaxillofac. Surg. 2016, 44, 1536–1552. [Google Scholar] [CrossRef]
- Brandao, T.B.; Vechiato Filho, A.J.; de Souza Batista, V.E.; Prado Ribeiro, A.C.; Filho, H.N.; Chilvarquer, I.; Nunn, M.E.; Santos-Silva, A.R.; Barao, V.A.R.; Wee, A.G. Assessment of treatment outcomes for facial prostheses in patients with craniofacial defects: A pilot retrospective study. J. Prosthet. Dent. 2017, 118, 235–241. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Sivan, U.; Tan, S.L.; Lippo, L.; De Angelis, J.; Labella, R.; Singh, A.; Chatzis, A.; Cheuk, S.; Medhghalchi, M.; et al. High-resolution 3D imaging uncovers organ-specific vascular control of tissue aging. Sci. Adv. 2021, 7, eabd7819. [Google Scholar] [CrossRef]
- Korfage, A.; Raghoebar, G.M.; Noorda, W.D.; Plaat, B.E.; Vissink, A.; Visser, A. Recommendations for implant-retained nasal prostheses after ablative tumor surgery: Minimal surgical aftercare, high implant survival, and satisfied patients. Head Neck 2016, 38, E619–E624. [Google Scholar] [CrossRef]
- Kruyt, I.J.; Nelissen, R.C.; Johansson, M.L.; Mylanus, E.A.M.; Hol, M.K.S. The IPS-scale: A new soft tissue assessment scale for percutaneous and transcutaneous implants for bone conduction devices. Clin. Otolaryngol. 2017, 42, 1410–1413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cenda, P.; Sokola, M.; Nazim-Zygadlo, E.; Modrzejewski, M.; Dobosz, P.; Kozok, A. Typical surgical reconstruction for nose and paranasal sinuses oncology. Otolaryngol. Pol. 2008, 62, 412–414. [Google Scholar] [CrossRef]
- Ma, Y.; Lloyd, M.S. Systematic review of Medpor versus autologous ear reconstruction. J. Craniofacial Surg. 2022, 33, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Landry, M.; Hankins, M.; Berkovic, J.; Nathan, C.A. Delayed Infection of Porous Polyethylene Implants After Oncologic Maxillectomy and Reconstruction: 2 Case Reports and Review of Literature. Ear Nose Throat J. 2021, 100, 1023S–1026S. [Google Scholar] [CrossRef]
- Federspil, P.A. Implant-retained craniofacial prostheses for facial defects. GMS Curr. Top. Otorhinolaryngol. Head Neck Surg. 2009, 8, Doc03. [Google Scholar] [CrossRef] [PubMed]
- den Besten, C.A.; Stalfors, J.; Wigren, S.; Blechert, J.I.; Flynn, M.; Eeg-Olofsson, M.; Aggarwal, R.; Green, K.; Nelissen, R.C.; Mylanus, E.A.; et al. Stability, Survival, and Tolerability of an Auditory Osseointegrated Implant for Bone Conduction Hearing: Long-Term Follow-Up of a Randomized Controlled Trial. Otol. Neurotol. 2016, 37, 1077–1083. [Google Scholar] [CrossRef] [Green Version]
- Cochlear. Baha 5 System. A Summary of Clinical Evidence. Table Summarising the BI300 Implant Survival Rates Reported in the Corresponding Publications. Bone Anchored Solutions. Available online: https://mss-p-007-delivery.sitecorecontenthub.cloud/api/public/content/87928126b25f4fbf9dee47c1873812aa?v=8e967170 (accessed on 1 March 2023).
- Szybiak, B.; Pieńkowski, P.; Pazdrowski, J.; Majchrzak, E.; Wegner, A.; Łuczewski, Ł.; Szewczyk, M.; Sowka, M.; Golusinski, P.; Golusinski, W. [Nr 104] Rekonstrukcja twarzy—Nosa zewnętrznego z wykorzystaniem systemu VistaFix—Opis przypadku. Zesz. Nauk. WCO Lett. Oncol. Sci. 2013, 10, 57. [Google Scholar] [CrossRef]
- Evrard, L.; Glineur, R. Use of extraoral implants for nasal rehabilitation with epithesis after carcinologic surgery. A case report. Rev. Med. Brux. 2013, 34, 423–427. [Google Scholar]



| Number of Patients with Craniofacial Implants | Number of Patients with Nasal Prostheses | Type of the Implant | ||
|---|---|---|---|---|
| 1. | Wrobel C. et al. (2022) [7] | 1 | 1 | Medicon Epiplating system |
| 2. | Konstantinović S. et al. (2022) [8] | 26 | 11 | Disc implants |
| 3. | Rogers S. et al. (2022) [9] | 12 | 6 | Zygomatic implants |
| 4. | Gunawardena D. et al. (2022) [10] | 1 | 1 | Implant abutments KLS Martin self-screwing screws |
| 5. | Alberga J. et al. (2022) [11] | 201 | 45 | The Nobel Biocare and Entific Medical Systems |
| 6. | Tso T. et al. (2021) [12] | 1 | 1 | Zygomatic implants |
| 7. | Gaur V. et al. (2021) [13] | 1 | 1 | Zygomatic implants |
| 8. | Rosen E.B. et al. (2020) [14] | 1 | 1 | Vistafix System |
| 9. | Nowak S. M. et al. (2020) [15] | 1 | 1 | Medicon Epiplating system |
| 10. | Dings J. P. J et al. (2020) [16] | 41 | 24 | No data |
| 11. | Moore P. et al. (2019) [17] | 54 | 13 | Vistafix System, Institut Straumann AG implants |
| 12. | Unkovskiy A. et al. (2018) [18] | 1 | 1 | Vistafix System |
| 13. | Subramaniam S. S. et al. (2018) [19] | 110 | 15 | Vistafix System, Institut Straumann AG implants, The Nobel Biocare and Entific Medical Systems |
| 14. | Papaspyrou G. et al. (2018) [20] | 99 | 19 | Medicon implant System, Vistafix System, Leibinger System, Straumann System |
| 16. | Khorasani M. et al. (2018) [21] | 16 | 11 | Medpor porous polyethylene implants |
| 17. | Dings J. et al. (2018) | 38 | 14 | No data |
| 18. | Bansal K. et al. (2018) [22] | 2 | 2 | Magfit DX |
| No. | Implant Site | Skin Thickness before Abrading | Skin Thickness after Abrading | Implant VX1300 Length | Abutment VXA300 Length |
|---|---|---|---|---|---|
| 1. | Maxillary bone, nose floor, right side | 7 mm | 5 mm | 4 mm | 7.5 mm |
| 2. | Maxillary bone, nose floor, left side | 6.5 mm | 4.5 mm | 4 mm | 7.5 mm |
| 3. | Frontal process of maxilla, left side | 3 mm | 2 mm | 3 mm | 4.5 mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dżaman, K.; Ziemska-Gorczyca, M.; Anurin, I.; Błaszczyk, M. The Latest Craniofacial Reconstructive Techniques Using Anchored Implants after Surgical Treatment of Nasal and Paranasal Sinuses Tumors. Healthcare 2023, 11, 1663. https://doi.org/10.3390/healthcare11121663
Dżaman K, Ziemska-Gorczyca M, Anurin I, Błaszczyk M. The Latest Craniofacial Reconstructive Techniques Using Anchored Implants after Surgical Treatment of Nasal and Paranasal Sinuses Tumors. Healthcare. 2023; 11(12):1663. https://doi.org/10.3390/healthcare11121663
Chicago/Turabian StyleDżaman, Karolina, Marlena Ziemska-Gorczyca, Igor Anurin, and Magdalena Błaszczyk. 2023. "The Latest Craniofacial Reconstructive Techniques Using Anchored Implants after Surgical Treatment of Nasal and Paranasal Sinuses Tumors" Healthcare 11, no. 12: 1663. https://doi.org/10.3390/healthcare11121663





