Study Protocol for the Evaluation of Multidisciplinary Medication Reconciliation Service in Adult Patients Undergoing Thoracic and Cardiovascular Surgery (The MERITS Study): A Single-Center Controlled before-and-after Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Setting
2.3. Inclusion and Exclusion Criteria
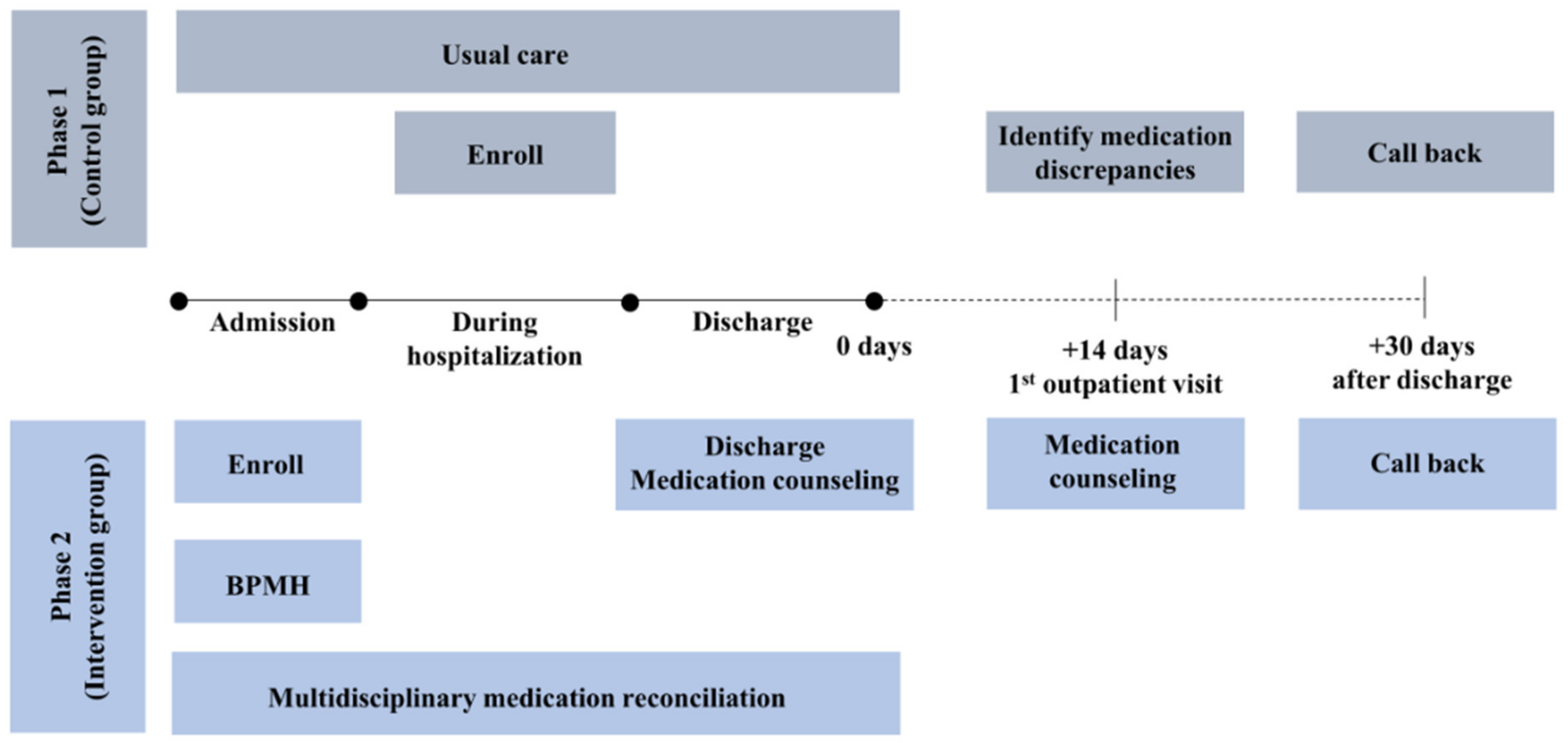
2.4. Intervention
2.4.1. Phase 1: Control group
2.4.2. Phase 2: Intervention group
2.5. Outcomes Measurement
2.5.1. Primary Outcome
2.5.2. Secondary Outcome
2.6. Sample Size
2.7. Data Collection and Management
2.8. Statistical Analysis
2.9. Patient and Public Involvement
2.10. Trial Status
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tam, V.C.; Knowles, S.R.; Cornish, P.L.; Fine, N.; Marchesano, R.; Etchells, E.E. Frequency, type and clinical importance of medication history errors at admission to hospital: A systematic review. Cmaj 2005, 173, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Paulino, E.I.; Bouvy, M.L.; Gastelurrutia, M.A.; Guerreiro, M.; Buurma, H. Drug related problems identified by European community pharmacists in patients discharged from hospital. Pharm. World Sci. 2004, 26, 353–360. [Google Scholar] [PubMed]
- Santell, J.P. Reconciliation failures lead to medication errors. Jt. Comm. J. Qual. Patient Saf. 2006, 32, 225–229. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Medication Safety in Transition of Care: Technical Report; World Health Organization: Geneve, Switzerland, 2019; Available online: https://apps.who.int/iris/handle/10665/325453 (accessed on 9 June 2023).
- Abdellatif, A.; Bagian, J.P.; Barajas, E.R.; Cohen, M.; Cousins, D.; Denham, C.R.; Essinger, K.; Gegelashvili, G.; Glenister, H.; Hoffman, C.; et al. Assuring Medication Accuracy at Transitions in Care: Patient Safety Solutions, Volume 1, Solution 6, May 2007. Jt. Comm. J. Qual. Patient Saf. 2007, 33, 450–453. [Google Scholar]
- Mekonnen, A.B.; McLachlan, A.J.; Jo-anne, E.B. Effectiveness of pharmacist-led medication reconciliation programmes on clinical outcomes at hospital transitions: A systematic review and meta-analysis. BMJ Open 2016, 6, e010003. [Google Scholar] [CrossRef] [PubMed]
- Chiewchantanakit, D.; Meakchai, A.; Pituchaturont, N.; Dilokthornsakul, P.; Dhippayom, T. The effectiveness of medication reconciliation to prevent medication error: A systematic review and meta-analysis. Res. Social. Adm. Pharm. 2020, 16, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, A.A.; Jackson, S.H. Age-related changes in pharmacokinetics and pharmacodynamics: Basic principles and practical applications. Br. J. Clin. Pharmacol. 2004, 57, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Davies, E.A.; O’Mahony, M.S. Adverse drug reactions in special populations—The elderly. Br. J. Clin. Pharmacol. 2015, 80, 796–807. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Jung, H.W.; Lee, J.H.; Lim, J.; Do Moon, S.; Yoon, S.W.; Moon, H.; Lee, S.R.; Kim, H.; Lee, S.R.; et al. Clinical Frailty Scale, K-FRAIL questionnaire, and clinical outcomes in an acute hospitalist unit in Korea. Korean J. Intern. Med. 2021, 36, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Delgado Sánchez, O.; Anoz Jiménez, L.; Serrano Fabiá, A.; Nicolás Pico, J. Conciliation in medication. Med. Clin. 2007, 129, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Beckett, R.D.; Crank, C.W.; Wehmeyer, A. Effectiveness and feasibility of pharmacist-led admission medication reconciliation for geriatric patients. J. Pharm. Pract. 2012, 25, 136–141. [Google Scholar] [CrossRef] [PubMed]
- PCNE Classification for Drug-Related Problems V9.1, p. 1. Available online: https://www.pcne.org/upload/files/417_PCNE_classification_V9-1_final.pdf (accessed on 1 May 2023).
- Cornu, P.; Steurbaut, S.; Leysen, T.; De Baere, E.; Ligneel, C.; Mets, T.; Dupont, A.G. Effect of medication reconciliation at hospital admission on medication discrepancies during hospitalization and at discharge for geriatric patients. Ann. Pharmacother. 2012, 46, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Eggink, R.N.; Lenderink, A.W.; Widdershoven, J.W.M.G.; van den Bemt, P.M.L.A. The effect of a clinical pharmacist discharge service on medication discrepancies in patients with heart failure. Pharm. World Sci. 2010, 32, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Abu Farha, R.; Yousef, A.; Gharaibeh, L.; Alkhalaileh, W.; Mukattash, T.; Alefishat, E. Medication discrepancies among hospitalized patients with hypertension: Assessment of prevalence and risk factors. BMC Health Serv. Res. 2021, 21, 1338. [Google Scholar] [CrossRef] [PubMed]
- Caleres, G.; Modig, S.; Midlöv, P.; Chalmers, J.; Bondesson, Å. Medication Discrepancies in Discharge Summaries and Associated Risk Factors for Elderly Patients with Many Drugs. Drugs-Real World Outcomes 2020, 7, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Leguelinel-Blache, G.; Arnaud, F.; Bouvet, S.; Dubois, F.; Castelli, C.; Roux-Marson, C.; Ray, V.; Sotto, A.; Kinowski, J.M. Impact of admission medication reconciliation performed by clinical pharmacists on medication safety. Eur. J. Intern. Med. 2014, 25, 808–814. [Google Scholar] [CrossRef]
- Kwan, Y.; Fernandes, O.A.; Nagge, J.J.; Wong, G.G.; Huh, J.-H.; Hurn, D.A.; Pond, G.R.; Bajcar, J.M. Pharmacist Medication Assessments in a Surgical Preadmission Clinic. AMA Arch. Intern. Med. 2007, 167, 1034–1040. [Google Scholar] [CrossRef]
| Time Point | Study Period | ||||
|---|---|---|---|---|---|
| Enrolment and Allocation | Post-Allocation | ||||
| Admission | Hospitalization | Discharge | First Outpatient Visit (about 2 Weeks after the Discharge) | 30 Days after the Discharge | |
| Enrolment | |||||
| Eligibility screen | X | ||||
| Informed consent | X | ||||
| Allocation | X | ||||
| Intervention | |||||
| Phase 1: Control group (December 2021~) |  | ||||
| Phase 2: Intervention group (After phase 1 ends~) |  | ||||
| Assessments | |||||
| Baseline characteristic | X | X | |||
| Clinical Frailty Score | X | ||||
| Medication discrepancies | X | X | X | ||
| Number of pharmacist interventions | X | ||||
| Medication appropriate index score | X | ||||
| Patient’s satisfaction | X | ||||
| Drug-related problems | X | X | |||
| Readmission | X | X | |||
| Emergency department visit | X | X | |||
| All-cause death | X | X | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Kim, A.J.; Chae, H.-W.; Heo, K.-N.; Kim, Y.; Kim, S.H.; Cho, Y.S.; Lee, H.J.; Lee, J.-Y. Study Protocol for the Evaluation of Multidisciplinary Medication Reconciliation Service in Adult Patients Undergoing Thoracic and Cardiovascular Surgery (The MERITS Study): A Single-Center Controlled before-and-after Study. Healthcare 2023, 11, 1778. https://doi.org/10.3390/healthcare11121778
Park S, Kim AJ, Chae H-W, Heo K-N, Kim Y, Kim SH, Cho YS, Lee HJ, Lee J-Y. Study Protocol for the Evaluation of Multidisciplinary Medication Reconciliation Service in Adult Patients Undergoing Thoracic and Cardiovascular Surgery (The MERITS Study): A Single-Center Controlled before-and-after Study. Healthcare. 2023; 11(12):1778. https://doi.org/10.3390/healthcare11121778
Chicago/Turabian StylePark, Soyoung, A Jeong Kim, Hyun-Woo Chae, Kyu-Nam Heo, Yookyung Kim, Sung Hwan Kim, Yoon Sook Cho, Hyun Joo Lee, and Ju-Yeun Lee. 2023. "Study Protocol for the Evaluation of Multidisciplinary Medication Reconciliation Service in Adult Patients Undergoing Thoracic and Cardiovascular Surgery (The MERITS Study): A Single-Center Controlled before-and-after Study" Healthcare 11, no. 12: 1778. https://doi.org/10.3390/healthcare11121778
APA StylePark, S., Kim, A. J., Chae, H.-W., Heo, K.-N., Kim, Y., Kim, S. H., Cho, Y. S., Lee, H. J., & Lee, J.-Y. (2023). Study Protocol for the Evaluation of Multidisciplinary Medication Reconciliation Service in Adult Patients Undergoing Thoracic and Cardiovascular Surgery (The MERITS Study): A Single-Center Controlled before-and-after Study. Healthcare, 11(12), 1778. https://doi.org/10.3390/healthcare11121778






