Clinic and Home-Based Exercise with Blood Flow Restriction Resolves Thigh Muscle Atrophy after Anterior Cruciate Ligament Reconstruction with the Bone-Patellar Tendon-Bone Autograft: A Case Report
Abstract
:1. Introduction
2. Materials and Methods
2.1. Case Presentation
2.2. Differential Diagnosis
2.3. Treatment Methods
2.3.1. Preoperative Rehabilitation
2.3.2. Surgical Technique and Postoperative Precautions
2.3.3. Immediate Postoperative Rehabilitation
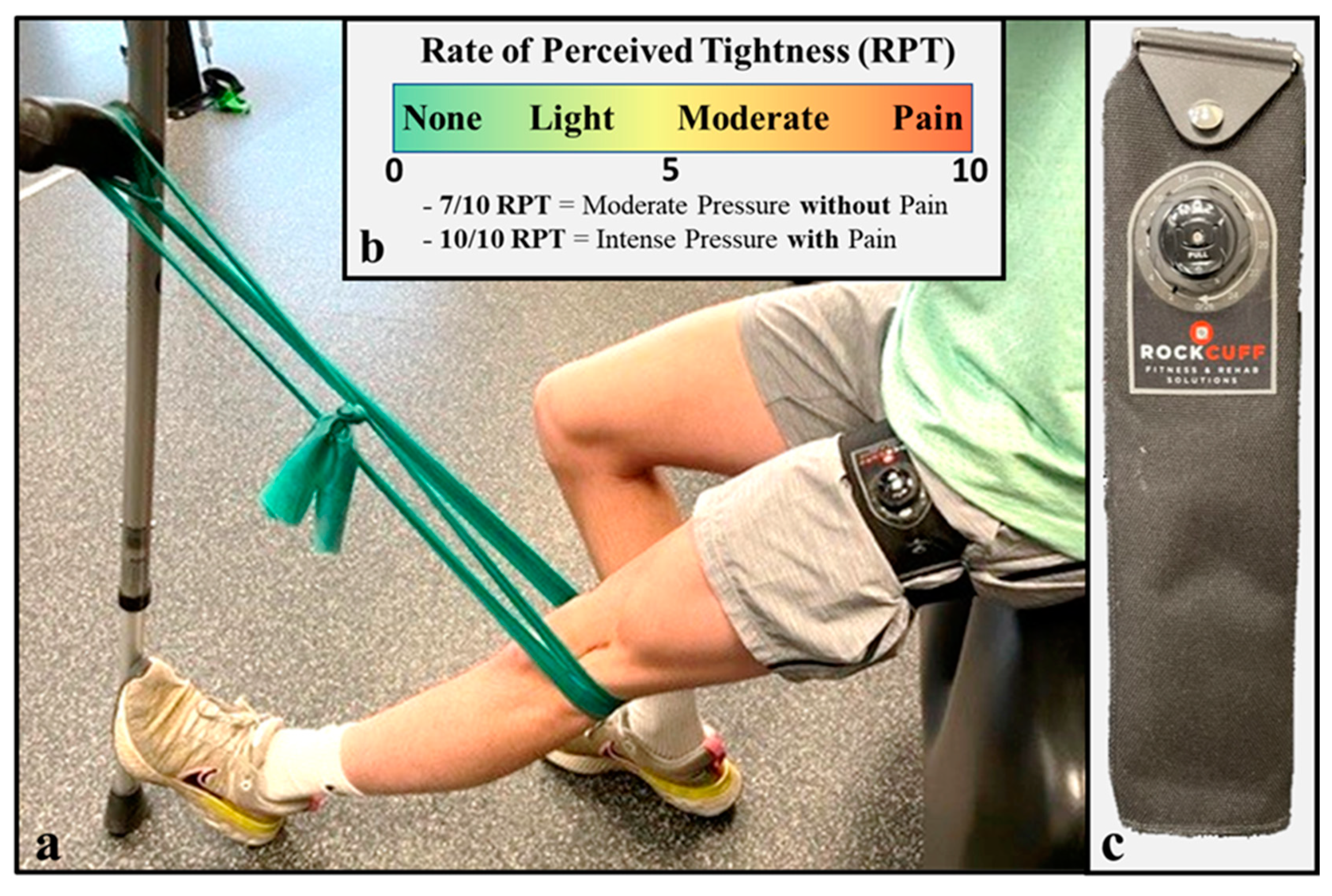
2.3.4. Clinic-Based Exercise with Blood Flow Restriction
2.3.5. Home-Based Exercise with Practical Blood Flow Restriction
2.3.6. Yielding/Holding Isometric Exercise Program
2.3.7. Data Collection
3. Results
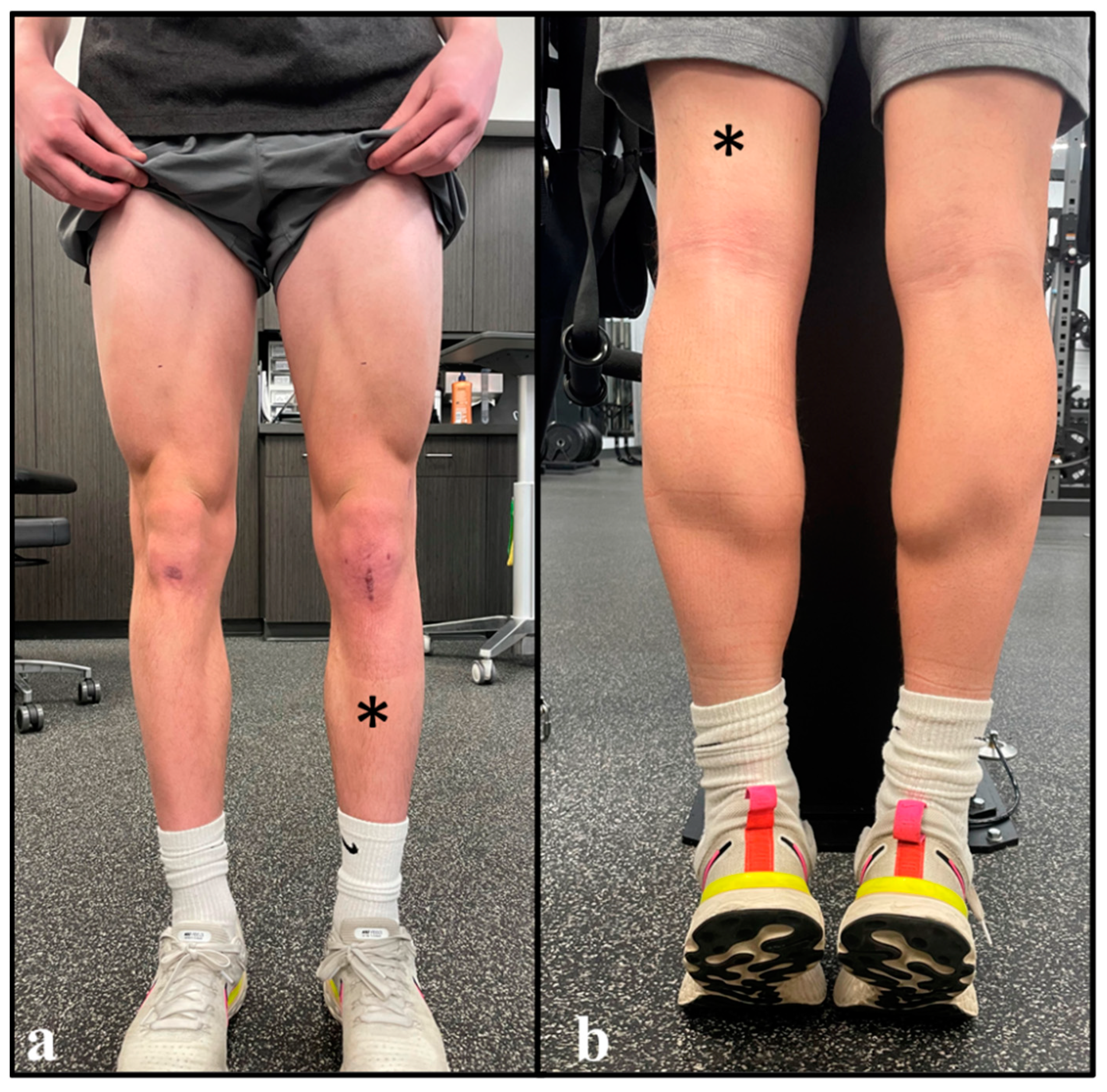
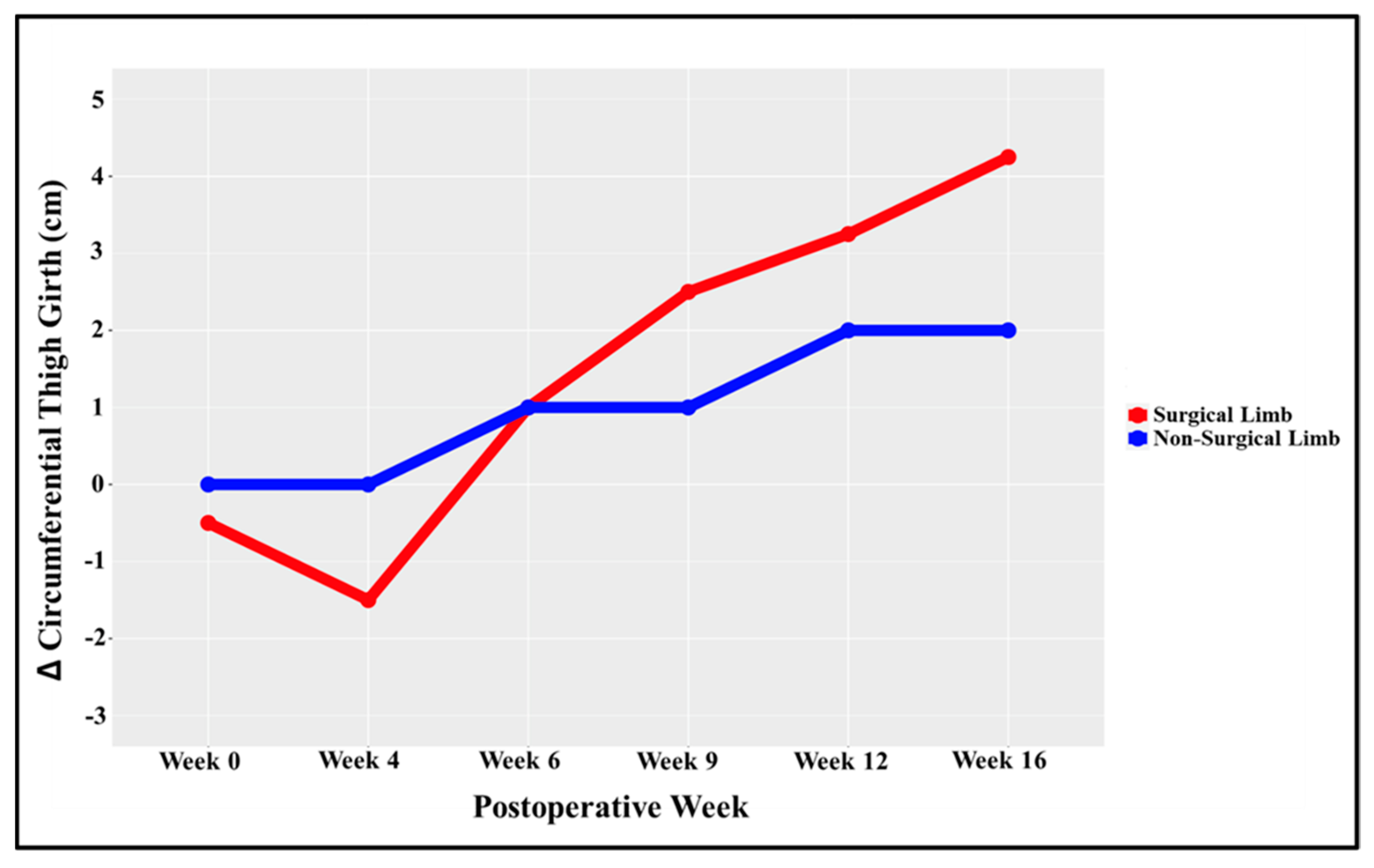
3.1. Outcomes
3.2. Follow-Up
4. Discussion
4.1. Safety
4.2. Prescribing Blood Flow Restriction
4.3. Limb Dominance
4.4. Strength Outcome
4.5. Case Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andersson, A.C. Knee Laxity and Function After Conservative Treatment of Anterior Cruciate Ligament Injuries. Int. J. Sport. Med. 1993, 14, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Eastlack, M.E.; Axe, M.J.; Snyder-Mackler, L. Laxity, instability, and functional outcome after ACL injury: Copers versus noncopers. Med. Sci. Sport. Exerc. 1999, 31, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Sommerfeldt, M.; Raheem, A.; Whittaker, J.; Hui, C.; Otto, D. Recurrent Instability Episodes and Meniscal or Cartilage Damage After Anterior Cruciate Ligament Injury: A Systematic Review. Orthop. J. Sport. Med. 2018, 6, 2325967118786507. [Google Scholar] [CrossRef] [Green Version]
- Kawashima, I.; Tsukahara, T.; Sakai, T.; Kawai, R.; Ishizuka, S.; Hiraiwa, H.; Imagama, S. Delayed anterior cruciate ligament reconstruction increases the incidence of medial meniscal bucket handle tears and medial compartment chondral injuries in patients aged 40 years and older. Arch. Orthop. Trauma Surg. 2021, 141, 971–975. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.-C.; Yoon, Y.-C.; Wang, J.-H.; Bae, J.-H. Anatomical versus Non-Anatomical Single Bundle Anterior Cruciate Ligament Reconstruction: A Cadaveric Study of Comparison of Knee Stability. Clin. Orthop. Surg. 2012, 4, 249–255. [Google Scholar] [CrossRef] [Green Version]
- Eliya, Y.; Nawar, K.; Rothrauff, B.B.; Lesniak, B.P.; Musahl, V.; de Sa, D. Anatomical anterior cruciate ligament reconstruction (ACLR) results in fewer rates of atraumatic graft rupture, and higher rates of rotatory knee stability: A meta-analysis. J. ISAKOS 2020, 5, 359–370. [Google Scholar] [CrossRef]
- Diermeier, T.; Rothrauff, B.B.; Engebretsen, L.; Lynch, A.D.; Ayeni, O.R.; Paterno, M.V.; Xerogeanes, J.W.; Fu, F.H.; Karlsson, J.; Musahl, V.; et al. Treatment After Anterior Cruciate Ligament Injury: Panther Symposium ACL Treatment Consensus Group. Orthop. J. Sport. Med. 2020, 8, 2325967120931097. [Google Scholar] [CrossRef]
- Carmichael, J.R.; Cross, M.J. Why bone-patella tendon-bone grafts should still be considered the gold standard for anterior cruciate ligament reconstruction. Br. J. Sport. Med. 2009, 43, 323–325. [Google Scholar] [CrossRef]
- Gifstad, T.; Foss, O.A.; Engebretsen, L.; Lind, M.; Forssblad, M.; Albrektsen, G.; Drogset, J.O. Lower risk of revision with patellar tendon autografts compared with hamstring autografts: A registry study based on 45,998 primary ACL reconstructions in Scandinavia. Am. J. Sport. Med. 2014, 42, 2319–2328. [Google Scholar] [CrossRef]
- MOON Knee Group; Sullivan, J.P.; Huston, L.J.; Zajichek, A.; Reinke, E.K.; Andrish, J.T.; Brophy, R.H.; Dunn, W.R.; Flanigan, D.C.; Kaeding, C.C.; et al. Incidence and Predictors of Subsequent Surgery After Anterior Cruciate Ligament Reconstruction: A 6-Year Follow-up Study. Am. J. Sport. Med. 2020, 48, 2418–2428. [Google Scholar] [CrossRef]
- Akpinar, B.; Thorhauer, E.; Irrgang, J.J.; Tashman, S.; Fu, F.H.; Anderst, W.J. Alteration of Knee Kinematics After Anatomic Anterior Cruciate Ligament Reconstruction Is Dependent on Associated Meniscal Injury. Am. J. Sport. Med. 2018, 46, 1158–1165. [Google Scholar] [CrossRef]
- Tang, X.; Marshall, B.; Wang, J.H.; Zhu, J.; Li, J.; Smolinski, P.; Fu, F.H. Lateral Meniscal Posterior Root Repair With Anterior Cruciate Ligament Reconstruction Better Restores Knee Stability. Am. J. Sport. Med. 2019, 47, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, B.; Robb, C.; Thomas, M.; Thompson, P.; Spalding, T. Factors That Predict Failure in Anatomic Single-Bundle Anterior Cruciate Ligament Reconstruction. Am. J. Sport. Med. 2017, 45, 1529–1536. [Google Scholar] [CrossRef]
- Sarraj, M.; Coughlin, R.P.; Solow, M.; Ekhtiari, S.; Simunovic, N.; Krych, A.J.; MacDonald, P.; Ayeni, O.R. Anterior cruciate ligament reconstruction with concomitant meniscal surgery: A systematic review and meta-analysis of outcomes. Knee Surg. Sport. Traumatol. Arthrosc. 2019, 27, 3441–3452. [Google Scholar] [CrossRef]
- Wall, B.T.; Dirks, M.L.; Snijders, T.; Senden, J.M.G.; Dolmans, J.; van Loon, L.J.C. Substantial skeletal muscle loss occurs during only 5 days of disuse. Acta Physiol. 2014, 210, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Iversen, E.; Røstad, V.; Larmo, A. Intermittent blood flow restriction does not reduce atrophy following anterior cruciate ligament reconstruction. J. Sport Health Sci. 2016, 5, 115–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birchmeier, T.; Lisee, C.; Kane, K.; Brazier, B.; Triplett, A.; Kuenze, C. Quadriceps Muscle Size Following ACL Injury and Reconstruction: A Systematic Review. J. Orthop. Res. 2020, 38, 598–608. [Google Scholar] [CrossRef]
- Garcia, S.; Curran, M.T.; Palmieri-Smith, R.M. Longitudinal Assessment of Quadriceps Muscle Morphology Before and After Anterior Cruciate Ligament Reconstruction and Its Associations With Patient-Reported Outcomes. Sport. Health A Multidiscip. Approach 2020, 12, 271–278. [Google Scholar] [CrossRef]
- Rice, D.A.; McNair, P.J. Quadriceps Arthrogenic Muscle Inhibition: Neural Mechanisms and Treatment Perspectives. Semin. Arthritis Rheum. 2010, 40, 250–266. [Google Scholar] [CrossRef]
- Pietrosimone, B.; Lepley, A.S.; Kuenze, C.; Harkey, M.S.; Hart, J.M.; Blackburn, J.T.; Norte, G. Arthrogenic Muscle Inhibition Following Anterior Cruciate Ligament Injury. J. Sport Rehabil. 2022, 31, 694–706. [Google Scholar] [CrossRef]
- Žargi, T.G.; Drobnič, M.; Vauhnik, R.; Koder, J.; Kacin, A. Factors predicting quadriceps femoris muscle atrophy during the first 12 weeks following anterior cruciate ligament reconstruction. Knee 2017, 24, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Berg, H.E.; Dudley, G.A.; Hather, B.; Tesch, P.A. Work capacity and metabolic and morphologic characteristics of the human quadriceps muscle in response to unloading. Clin. Physiol. Funct. Imaging 1993, 13, 337–347. [Google Scholar] [CrossRef]
- Berg, H.E.; Larsson, L.; Tesch, P.A. Lower limb skeletal muscle function after 6 wk of bed rest. J. Appl. Physiol. 1997, 82, 182–188. [Google Scholar] [CrossRef] [PubMed]
- De Boer, M.D.; Selby, A.; Atherton, P.; Smith, K.; Seynnes, O.R.; Maganaris, C.N.; Maffulli, N.; Movin, T.; Narici, M.V.; Rennie, M.J. The temporal responses of protein synthesis, gene expression and cell signalling in human quadriceps muscle and patellar tendon to disuse. J. Physiol. 2007, 585, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Atherton, P.J.; Greenhaff, P.L.; Phillips, S.M.; Bodine, S.C.; Adams, C.M.; Lang, C.H.; Andrushko, J.W.; Lanovaz, J.L.; Björkman, K.M.; Kontulainen, S.A.; et al. Control of skeletal muscle atrophy in response to disuse: Clinical/preclinical contentions and fallacies of evidence. Am. J. Physiol. Metab. 2016, 311, E594–E604. [Google Scholar] [CrossRef] [Green Version]
- Lepley, L.K.; Davi, S.M.; Burland, J.P.; Lepley, A.S. Muscle Atrophy After ACL Injury: Implications for Clinical Practice. Sport. Health A Multidiscip. Approach 2020, 12, 579–586. [Google Scholar] [CrossRef]
- Arangio, G.A.; Chen, C.; Kalady, M.; Reed, J.F. Thigh Muscle Size and Strength After Anterior Cruciate Ligament Reconstruction and Rehabilitation. J. Orthop. Sport. Phys. Ther. 1997, 26, 238–243. [Google Scholar] [CrossRef]
- Takarada, Y.; Takazawa, H.; Ishii, N. Applications of vascular occlusion diminish disuse atrophy of knee extensor muscles. Med. Sci. Sport. Exerc. 2000, 32, 2035–2039. [Google Scholar] [CrossRef] [Green Version]
- Phillips, S.M.; Glover, E.I.; Rennie, M.J.; Kilroe, S.P.; Fulford, J.; Holwerda, A.M.; Jackman, S.R.; Lee, B.P.; Gijsen, A.P.; Wall, B.T.; et al. Alterations of protein turnover underlying disuse atrophy in human skeletal muscle. J. Appl. Physiol. 2009, 107, 645–654. [Google Scholar] [CrossRef] [Green Version]
- Wall, B.T.; Snijders, T.; Senden, J.M.G.; Ottenbros, C.L.P.; Gijsen, A.P.; Verdijk, L.B.; van Loon, L.J.C. Disuse Impairs the Muscle Protein Synthetic Response to Protein Ingestion in Healthy Men. J. Clin. Endocrinol. Metab. 2013, 98, 4872–4881. [Google Scholar] [CrossRef] [Green Version]
- Lepley, L.K.; Lepley, A.S.; Onate, J.A.; Grooms, D. Eccentric Exercise to Enhance Neuromuscular Control. Sport. Health A Multidiscip. Approach 2017, 9, 333–340. [Google Scholar] [CrossRef]
- Franchi, M.V.; Reeves, N.D.; Narici, M.V. Skeletal Muscle Remodeling in Response to Eccentric vs. Concentric Loading: Morphological, Molecular, and Metabolic Adaptations. Front. Physiol. 2017, 8, 447. [Google Scholar] [CrossRef] [Green Version]
- Gokeler, A.; Neuhaus, D.; Benjaminse, A.; Grooms, D.R.; Baumeister, J. Principles of Motor Learning to Support Neuroplasticity After ACL Injury: Implications for Optimizing Performance and Reducing Risk of Second ACL Injury. Sport. Med. 2019, 49, 853–865. [Google Scholar] [CrossRef] [Green Version]
- Harkey, M.S.; Gribble, P.A.; Pietrosimone, B.G. Disinhibitory Interventions and Voluntary Quadriceps Activation: A Systematic Review. J. Athl. Train. 2014, 49, 411–421. [Google Scholar] [CrossRef] [Green Version]
- Charles, D.; White, R.; Reyes, C.; Palmer, D. A systematic review of the effects of blood flow restriction training on quadriceps muscle atrophy and circumference post acl reconstruction. Int. J. Sport. Phys. Ther. 2020, 15, 882–891. [Google Scholar] [CrossRef]
- Hirsch, K.R.; Wolfe, R.R.; Ferrando, A.A. Pre- and Post-Surgical Nutrition for Preservation of Muscle Mass, Strength, and Functionality Following Orthopedic Surgery. Nutrients 2021, 13, 1675. [Google Scholar] [CrossRef]
- Howard, E.E.; Margolis, L.M.; Fussell, M.A.; Rios, C.G.; Meisterling, E.M.; Lena, C.J.; Pasiakos, S.M.; Rodriguez, N.R. Effect of High-Protein Diets on Integrated Myofibrillar Protein Synthesis before Anterior Cruciate Ligament Reconstruction: A Randomized Controlled Pilot Study. Nutrients 2022, 14, 563. [Google Scholar] [CrossRef] [PubMed]
- Kilgas, M.A.; Lytle, L.L.; Drum, S.N.; Elmer, S.J. Exercise with Blood Flow Restriction to Improve Quadriceps Function Long After ACL Reconstruction. Int. J. Sport. Med. 2019, 40, 650–656. [Google Scholar] [CrossRef] [Green Version]
- Hughes, L.; Paton, B.; Rosenblatt, B.; Gissane, C.; Patterson, S. Blood flow restriction training in clinical musculoskeletal rehabilitation: A systematic review and meta-analysis. Br. J. Sport. Med. 2017, 51, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Hughes, L.; Rosenblatt, B.; Paton, B.; Patterson, S.D. Blood Flow Restriction Training in Rehabilitation Following Anterior Cruciate Ligament Reconstructive Surgery: A Review. Tech. Orthop. 2018, 33, 106–113. [Google Scholar] [CrossRef]
- Ladlow, P.; Coppack, R.; Dharm-Datta, S.; Conway, D.; Sellon, E.; Patterson, S.D.; Bennett, A.N. Low-Load Resistance Training with Blood Flow Restriction Improves Clinical Outcomes in Musculoskeletal Rehabilitation: A Single-Blind Randomized Controlled Trial. Front. Physiol. 2018, 9, 1269. [Google Scholar] [CrossRef]
- Patterson, S.D.; Burr, J.F.; Warmington, S. Editorial: Blood Flow Restriction: Rehabilitation to Performance. Front. Physiol. 2021, 12, 566421. [Google Scholar] [CrossRef]
- Lu, Y.; Patel, B.H.; Kym, C.; Nwachukwu, B.U.; Beletksy, A.; Forsythe, B.; Chahla, J. Perioperative Blood Flow Restriction Rehabilitation in Patients Undergoing ACL Reconstruction: A Systematic Review. Orthop. J. Sport. Med. 2020, 8, 2325967120906822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koc, B.B.; Truyens, A.; Heymans, M.J.; Jansen, E.J.; Schotanus, M.G. Effect of Low-Load Blood Flow Restriction Training After Anterior Cruciate Ligament Reconstruction: A Systematic Review. Int. J. Sport. Phys. Ther. 2022, 17, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Caetano, D.; Oliveira, C.; Correia, C.; Barbosa, P.; Montes, A.; Carvalho, P. Rehabilitation outcomes and parameters of blood flow restriction training in ACL injury: A scoping review. Phys. Ther. Sport 2021, 49, 129–137. [Google Scholar] [CrossRef]
- Patterson, S.D.; Hughes, L.; Warmington, S.; Burr, J.; Scott, B.; Owens, J.; Abe, T.; Nielsen, J.; Libardi, C.A.; Laurentino, G.; et al. Blood Flow Restriction Exercise: Considerations of Methodology, Application, and Safety. Front. Physiol. 2019, 10, 533. [Google Scholar] [CrossRef]
- Ohta, H.; Kurosawa, H.; Ikeda, H.; Iwase, Y.; Satou, N.; Nakamura, S. Low-load resistance muscular training with moderate restriction of blood flow after anterior cruciate ligament reconstruction. Acta Orthop. 2003, 74, 62–68. [Google Scholar] [CrossRef] [Green Version]
- Czamara, A.; Krzemińska, K.; Widuchowski, W.; Dragan, S.L. The Muscle Strength of the Knee Joint after ACL Reconstruction Depends on the Number and Frequency of Supervised Physiotherapy Visits. Int. J. Environ. Res. Public Health 2021, 18, 10588. [Google Scholar] [CrossRef]
- Saper, M.; Butler, L.; Giampetruzzi, N.; Greenberg, E.; Link, M.; Prati, V.; Weaver, A. Physical Therapy Visit Utilization is Not Associated with Hop Test Performance after Acl Reconstruction in Pediatric and Adolescent Athletes: A Multicenter Study. Orthop. J. Sport. Med. 2022, 10, 2325967121s00513. [Google Scholar] [CrossRef]
- Bielitzki, R.; Behrendt, T.; Behrens, M.; Schega, L. Current Techniques Used for Practical Blood Flow Restriction Training: A Systematic Review. J. Strength Cond. Res. 2021, 35, 2936–2951. [Google Scholar] [CrossRef] [PubMed]
- Thiebaud, R.S.; Abe, T.; Loenneke, J.P.; Garcia, T.; Shirazi, Y.; McArthur, R. Acute Muscular Responses to Practical Low-Load Blood Flow Restriction Exercise Versus Traditional Low-Load Blood Flow Restriction and High-/Low-Load Exercise. J. Sport Rehabilitation 2020, 29, 984–992. [Google Scholar] [CrossRef]
- Schoenfeld, B.J.; Ogborn, D.; Krieger, J.W. Dose-response relationship between weekly resistance training volume and increases in muscle mass: A systematic review and meta-analysis. J. Sport. Sci. 2017, 35, 1073–1082. [Google Scholar] [CrossRef]
- Lasevicius, T.; Ugrinowitsch, C.; Schoenfeld, B.J.; Roschel, H.; Tavares, L.D.; De Souza, E.O.; Laurentino, G.; Tricoli, V. Effects of different intensities of resistance training with equated volume load on muscle strength and hypertrophy. Eur. J. Sport Sci. 2018, 18, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Bickley, L.S.; Szilagyi, P.G.; Hoffman, R.M. Bates’ Pocket Guide to Physical Examination and History Taking, 9th ed.; Soriano, R.P., Ed.; Wolters Kluwer: Alphen aan den Rijn, The Netherlands, 2020. [Google Scholar]
- Sturgill, L.P.; Snyder-Mackler, L.; Manal, T.J.; Axe, M.J. Interrater Reliability of a Clinical Scale to Assess Knee Joint Effusion. J. Orthop. Sport. Phys. Ther. 2009, 39, 845–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulligan, E.P.; McGuffie, D.Q.; Coyner, K.; Khazzam, M. The reliability and diagnostic accuracy of assessing the translation endpoint during the lachman test. Int. J. Sport. Phys. Ther. 2015, 10, 52–61. [Google Scholar]
- Smith, P.A.; Bollier, M. Anterior Cruciate Ligament and Medial Collateral Ligament Injuries. J. Knee Surg. 2014, 27, 359–368. [Google Scholar] [CrossRef]
- Stratford, P.; Binkley, J. A Review of the McMurray Test: Definition, Interpretation, and Clinical Usefulness. J. Orthop. Sport. Phys. Ther. 1995, 22, 116–120. [Google Scholar] [CrossRef]
- Malanga, G.A.; Andrus, S.; Nadler, S.F.; McLean, J. Physical examination of the knee: A review of the original test description and scientific validity of common orthopedic tests. Arch. Phys. Med. Rehabilitation 2003, 84, 592–603. [Google Scholar] [CrossRef] [Green Version]
- Swinford, S.T.; LaPrade, R.; Engebretsen, L.; Cohen, M.; Safran, M. Biomechanics and physical examination of the posteromedial and posterolateral knee: State of the art. J. ISAKOS Jt. Disord. Orthop. Sport. Med. 2020, 5, 378–388. [Google Scholar] [CrossRef]
- Della Villa, F.; Buckthorpe, M.; Grassi, A.; Nabiuzzi, A.; Tosarelli, F.; Zaffagnini, S.; Della Villa, S. Systematic video analysis of ACL injuries in professional male football (soccer): Injury mechanisms, situational patterns and biomechanics study on 134 consecutive cases. Br. J. Sport. Med. 2020, 54, 1423–1432. [Google Scholar] [CrossRef]
- Sherman, M.F.; Lieber, L.; Bonamo, J.R.; Podesta, L.; Reiter, I. The long-term followup of primary anterior cruciate ligament repair. Am. J. Sport. Med. 1991, 19, 243–255. [Google Scholar] [CrossRef]
- Ozeki, N.; Seil, R.; Krych, A.J.; Koga, H. Surgical treatment of complex meniscus tear and disease: State of the art. J. ISAKOS 2021, 6, 35–45. [Google Scholar] [CrossRef]
- Pereira, H.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L.; Espregueira-Mendes, J. The Meniscus: Basic Science. In Meniscal Transplantation; Springer: Berlin/Heidelberg, Germany, 2013; pp. 7–14. [Google Scholar] [CrossRef]
- Peters, P.G.; Herbenick, M.A.; Anloague, P.A.; Markert, R.J.; Rubino, L.J. Knee range of motion: Reliability and agreement of 3 measurement methods. Am. J. Orthop. 2011, 40, E249–E252. [Google Scholar]
- Malempati, C.; Jurjans, J.; Noehren, B.; Ireland, M.L.; Johnson, D.L. Current Rehabilitation Concepts for Anterior Cruciate Ligament Surgery in Athletes. Orthopedics 2015, 38, 689–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Melick, N.; Cingel, R.E.H.V.; Brooijmans, F.; Neeter, C.; Van Tienen, T.; Hullegie, W.; Der Sanden, M.W.G.N.-V. Evidence-based clinical practice update: Practice guidelines for anterior cruciate ligament rehabilitation based on a systematic review and multidisciplinary consensus. Br. J. Sport. Med. 2016, 50, 1506–1515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solie, B.; Kiely, M.; Doney, C.; Schwery, N.; Jones, J.; Bjerke, B. Return to Elite-Level Basketball after Surgical Reconstruction of the Anterior Cruciate Ligament and Medial Collateral Ligament of the Knee with Meniscal Repairs—It Takes Teamwork and Time to Restore Performance: A Case Report. J. Orthop. Sport. Phys. Ther. Cases 2022, 2, 226–233. [Google Scholar]
- Shelbourne, K.D.; Klotz, C. What I have learned about the ACL: Utilizing a progressive rehabilitation scheme to achieve total knee symmetry after anterior cruciate ligament reconstruction. J. Orthop. Sci. 2006, 11, 318–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baxter, F.R.; Bach, J.S.; Detrez, F.; Cantournet, S.; Corté, L.; Cherkaoui, M.; Ku, D.N. Augmentation of Bone Tunnel Healing in Anterior Cruciate Ligament Grafts: Application of Calcium Phosphates and Other Materials. J. Tissue Eng. 2010, 1, 712370. [Google Scholar] [CrossRef]
- Potvin, J.R.; Fuglevand, A.J. A motor unit-based model of muscle fatigue. PLOS Comput. Biol. 2017, 13, e1005581. [Google Scholar] [CrossRef] [Green Version]
- Silbernagel, K.G.; Thomeé, R.; Eriksson, B.I.; Karlsson, J. Continued Sport. Activity, Using a Pain-Monitoring Model, during Rehabilitation in Patients with Achilles Tendinopathy. Am. J. Sport. Med. 2007, 35, 897–906. [Google Scholar] [CrossRef]
- Feil, S.; Newell, J.; Minogue, C.; Paessler, H.H. The Effectiveness of Supplementing a Standard Rehabilitation Program with Superimposed Neuromuscular Electrical Stimulation After Anterior Cruciate Ligament Reconstruction. Am. J. Sport. Med. 2011, 39, 1238–1247. [Google Scholar] [CrossRef]
- Lee, H.K.; Kim, H.J.; Kim, S.B.; Kang, N. A Review and Meta-Analysis of Interactive Metronome Training: Positive Effects for Motor Functioning. Percept. Mot. Ski. 2022, 129, 1614–1634. [Google Scholar] [CrossRef]
- Nascimento, D.D.C.; Rolnick, N.; Neto, I.V.D.S.; Severin, R.; Beal, F.L.R. A Useful Blood Flow Restriction Training Risk Stratification for Exercise and Rehabilitation. Front. Physiol. 2022, 13, 318. [Google Scholar] [CrossRef] [PubMed]
- Majors, I.B.; Mears, S.C.; Oholendt, C.K.; Hargett, N.A.; Barnes, C.L.; Stambough, J.B. Does Blood Flow Restriction Therapy Improve Leg Strength in Patients with a Painful Total Knee Arthroplasty? J. Arthroplast. 2022, 37, 1064–1068. [Google Scholar] [CrossRef]
- Wilson, J.M.; Lowery, R.P.; Joy, J.M.; Loenneke, J.P.; Naimo, M.A. Practical Blood Flow Restriction Training Increases Acute Determinants of Hypertrophy Without Increasing Indices of Muscle Damage. J. Strength Cond. Res. 2013, 27, 3068–3075. [Google Scholar] [CrossRef]
- Bell, Z.W.; Dankel, S.J.; Mattocks, K.T.; Buckner, S.L.; Jessee, M.B.; Mouser, J.G.; Abe, T.; Loenneke, J.P. An investigation into setting the blood flow restriction pressure based on perception of tightness. Physiol. Meas. 2018, 39, 105006. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.; Campos, Y.; Leitão, L.; Arriel, R.; Novaes, J.; Vianna, J. Does Acute Blood Flow Restriction with Pneumatic and Non-Pneumatic Non-Elastic Cuffs Promote Similar Responses in Blood Lactate, Growth Hormone, and Peptide Hormone? J. Hum. Kinet. 2020, 74, 85–97. [Google Scholar] [CrossRef]
- Aniceto, R.R.; Leandro, L.D.S. Practical Blood Flow Restriction Training: New Methodological Directions for Practice and Research. Sport. Med. Open 2022, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Monteerarat, Y.; Limthongthang, R.; Laohaprasitiporn, P.; Vathana, T. Reliability of capillary refill time for evaluation of tissue perfusion in simulated vascular occluded limbs. Eur. J. Trauma Emerg. Surg. 2022, 48, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Cuffe, M.; Novak, J.; Saithna, A.; Strohmeyer, H.S.; Slaven, E. Current Trends in Blood Flow Restriction. Front. Physiol. 2022, 13, 882472. [Google Scholar] [CrossRef] [PubMed]
- Loenneke, J.; Fahs, C.; Rossow, L.; Abe, T.; Bemben, M. The anabolic benefits of venous blood flow restriction training may be induced by muscle cell swelling. Med. Hypotheses 2012, 78, 151–154. [Google Scholar] [CrossRef]
- Morton, R.W.; Colenso-Semple, L.; Phillips, S.M. Training for strength and hypertrophy: An evidence-based approach. Curr. Opin. Physiol. 2019, 10, 90–95. [Google Scholar] [CrossRef]
- Schaefer, L.V.; Bittmann, F.N. Are there two forms of isometric muscle action? Results of the experimental study support a distinction between a holding and a pushing isometric muscle function. BMC Sport. Sci. Med. Rehabil. 2017, 9, 11. [Google Scholar] [CrossRef] [Green Version]
- Lim, H.Y.; Wong, S.H. Effects of isometric, eccentric, or heavy slow resistance exercises on pain and function in individuals with patellar tendinopathy: A systematic review. Physiother. Res. Int. 2018, 23, e1721. [Google Scholar] [CrossRef]
- Burton, I. Interventions for prevention and in-season management of patellar tendinopathy in athletes: A scoping review. Phys. Ther. Sport 2022, 55, 80–89. [Google Scholar] [CrossRef]
- Sinclair, J.; Taylor, P.J.; Jones, B.; Butters, B.; Bentley, I.; Edmundson, C.J. A Multi-Experiment Investigation of the Effects Stance Width on the Biomechanics of the Barbell Squat. Sports 2022, 10, 136. [Google Scholar] [CrossRef]
- Paxton, J.Z.; Hagerty, P.; Andrick, J.J.; Baar, K. Optimizing an Intermittent Stretch Paradigm Using ERK1/2 Phosphorylation Results in Increased Collagen Synthesis in Engineered Ligaments. Tissue Eng. Part A 2012, 18, 277–284. [Google Scholar] [CrossRef] [Green Version]
- Antonio, J.; Kenyon, M.; Ellerbroek, A.; Carson, C.; Burgess, V.; Tyler-Palmer, D.; Mike, J.; Roberts, J.; Angeli, G.; Peacock, C. Comparison of Dual-Energy X-ray Absorptiometry (DXA) Versus a Multi-Frequency Bioelectrical Impedance (InBody 770) Device for Body Composition Assessment after a 4-Week Hypoenergetic Diet. J. Funct. Morphol. Kinesiol. 2019, 4, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwery, N.A.; Kiely, M.T.; Larson, C.M.; Wulf, C.A.; Heikes, C.S.; Hess, R.W.; Giveans, M.R.; Solie, B.S.; Doney, C.P. Quadriceps Strength following Anterior Cruciate Ligament Reconstruction: Normative Values based on Sex, Graft Type and Meniscal Status at 3, 6 & 9 Months. Int. J. Sport. Phys. Ther. 2022, 17, 434–444. [Google Scholar] [CrossRef]
- Loenneke, J.P.; Wilson, J.M.; Wilson, G.J.; Pujol, T.J.; Bemben, M.G. Potential safety issues with blood flow restriction training. Scand. J. Med. Sci. Sport. 2011, 21, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Minniti, M.C.; Statkevich, A.P.; Kelly, R.L.; Rigsby, V.P.; Exline, M.M.; Rhon, D.I.; Clewley, D. The Safety of Blood Flow Restriction Training as a Therapeutic Intervention for Patients With Musculoskeletal Disorders: A Systematic Review. Am. J. Sport. Med. 2020, 48, 1773–1785. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Paton, B. Is There a Minimum Effective Dose for Vascular Occlusion During Blood Flow Restriction Training? Front. Physiol. 2022, 13, 838115. [Google Scholar] [CrossRef] [PubMed]
- Tramer, J.S.; Khalil, L.S.; Jildeh, T.R.; Abbas, M.J.; McGee, A.; Lau, M.J.; Moutzouros, V.; Okoroha, K.R. Blood Flow Restriction Therapy for 2 Weeks Prior to Anterior Cruciate Ligament Reconstruction Did Not Impact Quadriceps Strength Compared to Standard Therapy. Arthrosc. J. Arthrosc. Relat. Surg. 2022, 39, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Strandberg, S.; Lindström, M.; Wretling, M.-L.; Aspelin, P.; Shalabi, A. Muscle morphometric effect of anterior cruciate ligament injury measured by computed tomography: Aspects on using non-injured leg as control. BMC Musculoskelet. Disord. 2013, 14, 150. [Google Scholar] [CrossRef] [Green Version]
- Adams, D.J.; Mazzocca, A.D.; Fulkerson, J.P. Residual Strength of the Quadriceps Versus Patellar Tendon After Harvesting a Central Free Tendon Graft. Arthrosc. J. Arthrosc. Relat. Surg. 2006, 22, 76–79. [Google Scholar] [CrossRef]
- Johnston, P.T.; McClelland, J.A.; Feller, J.A.; Webster, K.E. Knee muscle strength after quadriceps tendon autograft anterior cruciate ligament reconstruction: Systematic review and meta-analysis. Knee Surg. Sport. Traumatol. Arthrosc. 2020, 29, 2918–2933. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.H.; Capin, J.J.; Zarzycki, R.; Snyder-Mackler, L. Athletes With Bone-Patellar Tendon-Bone Autograft for Anterior Cruciate Ligament Reconstruction Were Slower to Meet Rehabilitation Milestones and Return-to-Sport Criteria Than Athletes With Hamstring Tendon Autograft or Soft Tissue Allograft: Secondary Analysis From the ACL-SPORT. Trial. J. Orthop. Sport. Phys. Ther. 2020, 50, 259–266. [Google Scholar] [CrossRef]
- Kew, M.E.; Bodkin, S.; Diduch, D.R.; Brockmeier, S.F.; Lesevic, M.B.; Hart, J.M.; Werner, B.C. Reinjury Rates in Adolescent Patients 2 Years Following ACL Reconstruction. J. Pediatr. Orthop. 2022, 42, 90–95. [Google Scholar] [CrossRef]
- Marigi, E.M.; Hale, R.F.; Bernard, C.D.; Bates, N.; Stuart, M.J.; Hewett, T.E.; Krych, A.J. Are 6-Month Functional and Isokinetic Testing Measures Risk Factors for Second Anterior Cruciate Ligament Injuries at Long-T Follow-Up? J. Knee Surg. 2022. [Google Scholar] [CrossRef]
- Paterno, M.V.; Thomas, S.; VanEtten, K.T.; Schmitt, L.C. Confidence, ability to meet return to sport criteria, and second ACL injury risk associations after ACL-reconstruction. J. Orthop. Res. 2021, 40, 182–190. [Google Scholar] [CrossRef]
- Piussi, R.; Simonson, R.; Högberg, J.; Thomeé, R.; Samuelsson, K.; Senorski, E.H. Psychological Patient-reported Outcomes Cannot Predict a Second Anterior Cruciate Ligament Injury in Patients who Return to Sport. after an Anterior Cruciate Ligament Reconstruction. Int. J. Sport. Phys. Ther. 2022, 17, 1340–1350. [Google Scholar] [CrossRef]
- Bodkin, S.G.; Hertel, J.; Diduch, D.R.; Saliba, S.A.; Novicoff, W.M.; Brockmeier, S.F.; Miller, M.D.; Gwathmey, F.W.; Werner, B.C.; Hart, J.M. Predicting Anterior Cruciate Ligament Reinjury from Return-to-Activity Assessments at 6 Months Postsurgery: A Prospective Cohort Study. J. Athl. Train. 2022, 57, 325–333. [Google Scholar] [CrossRef]
- Roach, M.H.; Aderman, M.J.; Gee, S.M.; Peck, K.Y.; Roach, S.P.; Goss, D.L.; Posner, M.A.; Haley, C.A.; Svoboda, S.J.; Cameron, K.L. Influence of Graft Type on Lower Extremity Functional Test Performance and Failure Rate After Anterior Cruciate Ligament Reconstruction. Sport. Health A Multidiscip. Approach 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Marmura, H.; Getgood, A.M.; Spindler, K.P.; Kattan, M.W.; Briskin, I.; Bryant, D.M. Validation of a Risk Calculator to Personalize Graft Choice and Reduce Rupture Rates for Anterior Cruciate Ligament Reconstruction. Am. J. Sport. Med. 2021, 49, 1777–1785. [Google Scholar] [CrossRef]
- Kaeding, C.C.; Spindler, K.P.; Huston, L.J.; Zajichek, A.; MOON Knee Group. ACL Reconstruction In High School and College-aged Athletes: Does Autograft Choice Affect Recurrent ACL Revision Rates? Orthop. J. Sport. Med. 2019, 7 (Suppl. S5), 2325967119S0028. [Google Scholar] [CrossRef] [Green Version]
- Kyritsis, P.; Bahr, R.; Landreau, P.; Miladi, R.; Witvrouw, E. Likelihood of ACL graft rupture: Not meeting six clinical discharge criteria before return to sport is associated with a four times greater risk of rupture. Br. J. Sport. Med. 2016, 50, 946–951. [Google Scholar] [CrossRef]
- Sugimoto, D.; Myer, G.D.; Bush, H.M.; Klugman, M.F.; McKeon, J.M.M.; Hewett, T.E. Compliance with Neuromuscular Training and Anterior Cruciate Ligament Injury Risk Reduction in Female Athletes: A Meta-Analysis. J. Athl. Train. 2012, 47, 714–723. [Google Scholar] [CrossRef] [Green Version]
- Antonio, J.; Kenyon, M.; Ellerbroek, A.; Carson, C.; Tyler-Palmer, D.; Burgess, V.; Angeli, G.; Silver, T.; Jiannine, L.; Peacock, C. Body composition assessment: A comparison of the bod pod, InBody 770, and DXA. J. Exerc. Nutr. 2019, 2, 1–7. [Google Scholar]
- Patterson, S.; Hughes, L.; Head, P.; Warmington, S.; Brandner, C. Blood flow restriction training: A novel approach to augment clinical rehabilitation: How to do it. Br. J. Sport. Med. 2017, 51, 1648–1649. [Google Scholar] [CrossRef]
- Bell, Z.W.; Dankel, S.J.; Spitz, R.W.; Chatakondi, R.N.; Abe, T.; Loenneke, J.P. The Perceived Tightness Scale Does Not Provide Reliable Estimates of Blood Flow Restriction Pressure. J. Sport Rehabil. 2020, 29, 516–518. [Google Scholar] [CrossRef]


| Clinic-Based Exercise Prescription (2×/week) | Home-Based Exercise Prescription (Variable Frequency) | |
|---|---|---|
| Week 0–1 |
| HEP Frequency: 5×/day (3–4×/day on clinic-based rehabilitation days) |
| ||
| Week 1–2 |
| HEP Frequency: 4×/day (3×/day on clinic-based rehabilitation days) |
| ||
| Week 2–3 |
| HEP Frequency: 3×/day (2×/day on clinic-based rehabilitation days) |
| ||
| Week 3–4 |
| HEP Frequency: 2×/day (1×/day on clinic-based rehabilitation days) |
| ||
| Week 4–5 |
| HEP Frequency: 2×/day (1×/day on clinic-based rehabilitation days) |
| ||
| Week 5–6 |
| HEP Frequency: 2×/day (1×/day on clinic-based rehabilitation days) |
| ||
| Week 6–7 |
| HEP Frequency: 2×/day (1×/day on clinic-based rehabilitation days) |
| ||
| Week 7–8 |
| HEP Frequency: 2×/day (1×/day on clinic-based rehabilitation days) |
| ||
| Week 8–9 |
| HEP Frequency: 2×/day (1×/day on clinic-based rehabilitation days) |
| ||
| Week 9–10 |
| HEP Frequency: 2×/day (1×/day on clinic-based rehabilitation days) |
| ||
| Week 10–11 |
| HEP Frequency: 2×/day (1×/day on clinic-based rehabilitation days) |
| ||
| Week 11–12 |
| HEP Frequency: 2×/day (1×/day on clinic-based rehabilitation days) |
| ||
| Week 12–13 |
| HEP Frequency: 2×/day (1×/day on clinic-based rehabilitation days) |
| ||
| Week 13–14 |
| HEP Frequency: 2×/day (1×/day on clinic-based rehabilitation days) |
| ||
| Week 14–15 |
| HEP Frequency: 2×/day (1×/day on clinic-based rehabilitation days) |
| ||
| Week 15–16 |
| HEP Frequency: 2×/day (1×/day on clinic-based rehabilitation days) |
|
| Objective Measurement | Week 0 | Week 4 | Week 6 | Week 9 | Week 12 | Week 16 | |
|---|---|---|---|---|---|---|---|
| Effusion (0 –3+ Sweep Test) | Surgical | 3+ | 3+ | 2+ | 1+ | 1+ | Trace |
| Thigh Girth (cm) | Surgical | 47.5 | 46.5 | 49 | 50.5 | 51.25 | 52.25 |
| Non-Surgical | 48 | 48 | 49 | 49 | 50 | 50 | |
| Difference | −0.5 | −1.5 | 0 | 1.5 | 1.25 | 2.25 | |
| Calf Girth (cm) | Surgical | 35 | 35 | 35 | 36 | 36.25 | 36.5 |
| Non-Surgical | 35.25 | 35.25 | 35.25 | 35.5 | 35.5 | 35.5 | |
| Difference | −0.25 | −0.25 | −0.25 | 0.75 | 0.75 | 1 | |
| Range of Motion (degrees) | Surgical | 5-0-30 | 5-0-125 | 5-0-130 | 5-0-130 | 8-0-145 | 8-0-150 |
| Non-Surgical | 8-0-150 | 8-0-150 | 8-0-150 | 8-0-150 | 8-0-150 | 8-0-150 | |
| Straight-Leg Raise (degree lag) | Surgical | 5 | 0 | 0 | 0 | 0 | 0 |
| Quadriceps Strength/BW (%) | Surgical | NA | NA | NA | NA | 70 | 87 |
| Non-Surgical | NA | NA | NA | NA | 96 | 135 | |
| LSI | NA | NA | NA | NA | 73 | 66 | |
| KT-1000 (mm) | Surgical | NA | NA | NA | 5,6,7 | 4,5,6 | 4,5,6 |
| Non-Surgical | NA | NA | NA | 5,6,7 | 5,6,7 | 5,6,7 | |
| Lean Mass Analysis (kg) | Surgical | NA | NA | NA | NA | NA | 10.37 |
| Non-Surgical | NA | NA | NA | NA | NA | 10.02 | |
| Difference | NA | NA | NA | NA | NA | 0.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solie, B.S.; Eggleston, G.G.; Schwery, N.A.; Doney, C.P.; Kiely, M.T.; Larson, C.M. Clinic and Home-Based Exercise with Blood Flow Restriction Resolves Thigh Muscle Atrophy after Anterior Cruciate Ligament Reconstruction with the Bone-Patellar Tendon-Bone Autograft: A Case Report. Healthcare 2023, 11, 1885. https://doi.org/10.3390/healthcare11131885
Solie BS, Eggleston GG, Schwery NA, Doney CP, Kiely MT, Larson CM. Clinic and Home-Based Exercise with Blood Flow Restriction Resolves Thigh Muscle Atrophy after Anterior Cruciate Ligament Reconstruction with the Bone-Patellar Tendon-Bone Autograft: A Case Report. Healthcare. 2023; 11(13):1885. https://doi.org/10.3390/healthcare11131885
Chicago/Turabian StyleSolie, Braidy S., Garrett G. Eggleston, Nicole A. Schwery, Christopher P. Doney, Michael T. Kiely, and Christopher M. Larson. 2023. "Clinic and Home-Based Exercise with Blood Flow Restriction Resolves Thigh Muscle Atrophy after Anterior Cruciate Ligament Reconstruction with the Bone-Patellar Tendon-Bone Autograft: A Case Report" Healthcare 11, no. 13: 1885. https://doi.org/10.3390/healthcare11131885







