Human Error Analysis and Modeling of Medication-Related Adverse Events in Taiwan Using the Human Factors Analysis and Classification System and Logistic Regression
Abstract
1. Introduction
1.1. Medication Errors
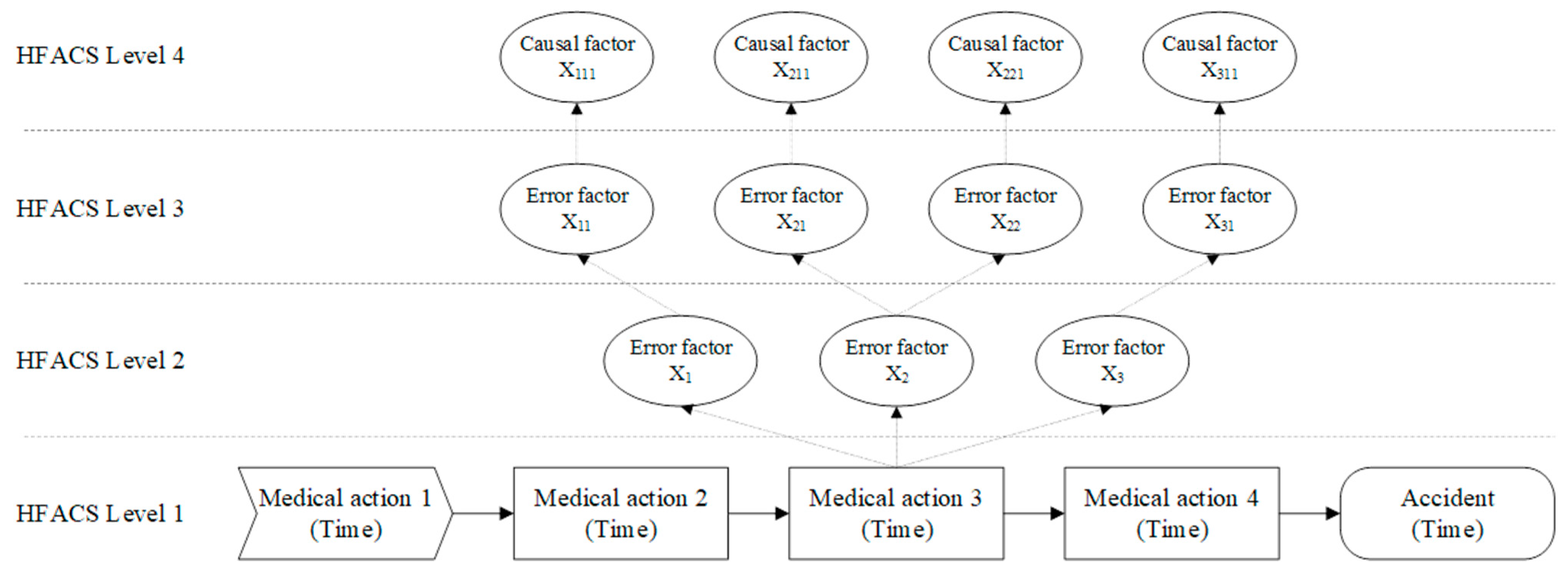
1.2. Human Factors Analysis and Classification System (HFACS)
1.3. Causality between Error Factors
1.4. The Purposes of this Study
2. Materials and Methods
2.1. Selection of Medication-Related Adverse Events
2.2. Medication Error Analysis
2.3. Logistic Regression
3. Results
3.1. Medication-Related Adverse Event Analysis
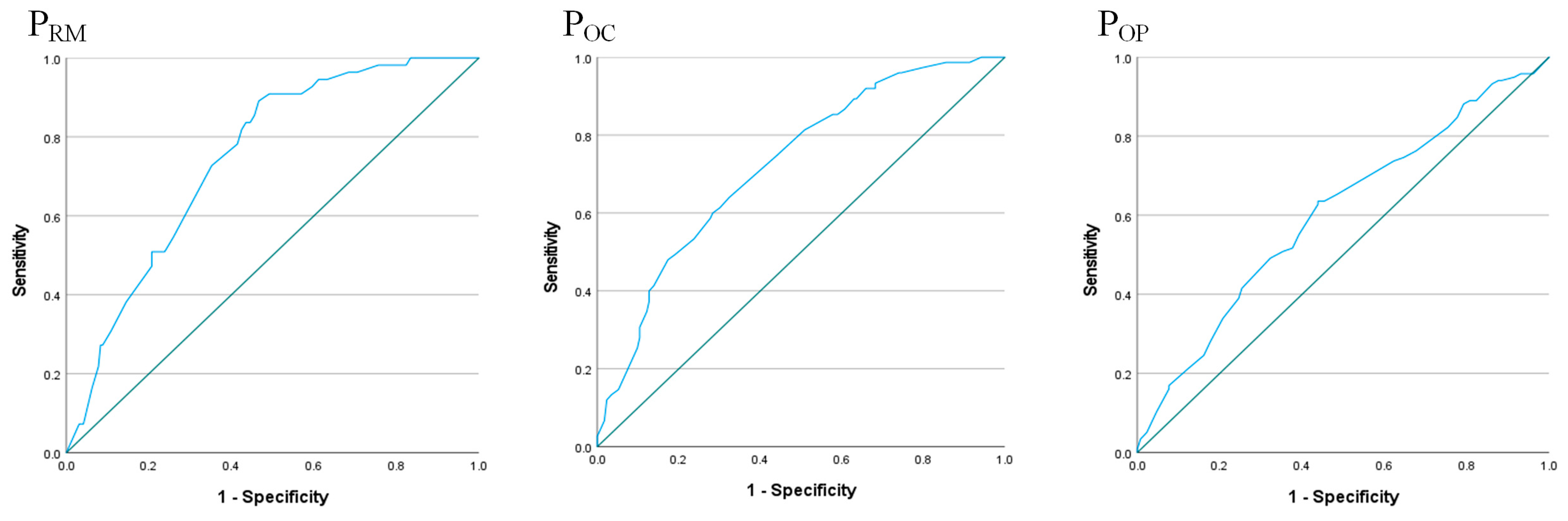
3.2. Logistic Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xie, X.; Guo, D. Human factors risk assessment and management: Process safety in engineering. Process. Saf. Environ. Prot. 2018, 113, 467–482. [Google Scholar]
- Hsieh, M.C.; Chiang, P.Y.; Wang, E.M.Y.; Kung, W.C.; Hu, Y.T.; Huang, M.S.; Hsieh, H.C. The investigation of human error analysis in adverse drug events in Taiwan—From the perspective of causality assessment. Hum. Factors Ergon. Man. 2019, 29, 340–349. [Google Scholar] [CrossRef]
- Kepner, S.; Jones, R. Patient Safety Trends in 2021: An Analysis of 288,882 Serious events and incidents from the nation’s largest event reporting database. J. Patient Saf. 2022, 4, 18–33. [Google Scholar] [CrossRef]
- Joint Commission of Taiwan. Taiwan Patient-Safety Reporting System; Annual Report 2020; Joint Commission of Taiwan: Taipei, Taiwan, 2021. [Google Scholar]
- Zarea, K.; Mohammadi, A.; Beiranvand, S.; Hassani, F.; Baraz, S. Iranian nurses’ medication errors: A survey of the types, the causes, and the related factors. Int. J. Afr. Nurs. Sci. 2018, 8, 112–116. [Google Scholar]
- Pham, J.C.; Story, J.L.; Hicks, R.W.; Shore, A.D.; Morlock, L.L.; Cheung, D.S.; Kelen, G.D.; Pronovost, P.J. National study on the frequency, types, causes, and consequences of voluntarily reported emergency department medication errors. J. Emerg. Med. 2011, 40, 485–492. [Google Scholar] [CrossRef]
- Aronson, J.K. Medication errors: Definitions and classification. Br. J. Clin. Pharmacol. 2009, 67, 599–604. [Google Scholar]
- Mieiro, D.B.; Oliveira, É.B.C.; Fonseca, R.E.P.D.; Mininel, V.A.; Zem-Mascarenhas, S.H.; Machado, R.C. Strategies to minimize medication errors in emergency units: An integrative review. Rev. Bras. Enferm. 2019, 72, 307–314. [Google Scholar] [CrossRef]
- Naseralallah, L.M.; Hussain, T.A.; Jaam, M.; Pawluk, S.A. Impact of pharmacist interventions on medication errors in hospitalized pediatric patients: A systematic review and meta-analysis. Int. J. Clin. Pharm. 2020, 42, 979–994. [Google Scholar] [CrossRef]
- Alagha, H.Z.; Badary, O.A.; Ibrahim, H.M.; Sabri, N.A. Reducing prescribing errors in the paediatric intensive care unit: An experience from Egypt. Acta Paediatr. 2011, 100, e169–e174. [Google Scholar] [CrossRef]
- Doherty, C.; Mc Donnell, C. Tenfold medication errors: 5 years’ experience at a university-affiliated pediatric hospital. Pediatrics 2012, 129, 916–924. [Google Scholar] [CrossRef]
- Suclupe, S.; Martinez-Zapata, M.J.; Mancebo, J.; Font-Vaquer, A.; Castillo-Masa, A.M.; Viñolas, I.; Morán, I.; Robleda, G. Medication errors in prescription and administration in critically ill patients. J. Adv. Nurs. 2020, 76, 1192–1200. [Google Scholar] [CrossRef] [PubMed]
- Freund, Y.; Goulet, H.; Bokobza, J.; Ghanem, A.; Carreira, S.; Madec, D.; Leroux, G.; Ray, P.; Boddaert, J.; Riou, B.; et al. Factors associated with adverse events resulting from medical errors in the emergency department: Two work better than one. J. Emerg. Med. 2013, 45, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Reason, J. Human Error; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Salvendy, G. (Ed.) . Handbook of Human Factors and Ergonomics; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Cigularov, K.P.; Chen, P.Y.; Rosecrance, J. The effects of error management climate and safety communication on safety: A multi-level study. Accid. Anal. Prev. 2010, 42, 1498–1506. [Google Scholar] [CrossRef]
- Harvey, B. The Oaks Colliery disaster of 1866: A case study in responsibility. Bus. Hist. 2016, 58, 501–531. [Google Scholar] [CrossRef]
- Li, W.C.; Harris, D. Pilot error and its relationship with higher organizational levels: HFACS analysis of 523 accidents. Aviat. Space Environ. Med. 2006, 77, 1056–1061. [Google Scholar] [PubMed]
- Li, W.C.; Harris, D.; Yu, C.S. Routes to failure: Analysis of 41 civil aviation accidents from the Republic of China using the human factors analysis and classification system. Accid. Anal. Prev. 2008, 40, 426–434. [Google Scholar] [CrossRef]
- Chiu, M.C.; Hsieh, M.C. Latent human error analysis and efficient improvement strategies by fuzzy TOPSIS in aviation maintenance tasks. Appl. Ergon. 2016, 54, 136–147. [Google Scholar] [CrossRef]
- Kılıc, B.; Gümüş, E. Application of HFACS to the nighttime aviation accidents and incidents. J. Aviat. 2020, 4, 10–16. [Google Scholar] [CrossRef]
- Hsieh, M.C.; Chiang, P.Y.; Lee, Y.C.; Wang, E.M.; Kung, W.C.; Hu, Y.T.; Huang, M.S.; Hsieh, H.C. An investigation of human errors in medication adverse event improvement priority using a hybrid approach. Healthcare 2021, 9, 442. [Google Scholar] [CrossRef]
- Li, C.; Tang, T.; Chatzimichailidou, M.M.; Jun, G.T.; Waterson, P. A hybrid human and organisational analysis method for railway accidents based on STAMP-HFACS and human information processing. Appl. Ergon. 2019, 79, 122–142. [Google Scholar] [CrossRef]
- Kaptan, M.; Sarıalioğlu, S.; Uğurlu, Ö.; Wang, J. The evolution of the HFACS method used in analysis of marine accidents: A review. Int. J. Ind. Ergon. 2021, 86, 103225. [Google Scholar] [CrossRef]
- Theophilus, S.C.; Esenowo, V.N.; Arewa, A.O.; Ifelebuegu, A.O.; Nnadi, E.O.; Mbanaso, F.U. Human factors analysis and classification system for the oil and gas industry (HFACS-OGI). Reliab. Eng. Syst. Saf. 2017, 167, 168–176. [Google Scholar] [CrossRef]
- Hsieh, M.C.; Wang, E.M.Y.; Lee, W.C.; Li, L.W.; Hsieh, C.Y.; Tsai, W.; Wang, C.P.; Huang, J.L.; Liu, T.C. Application of HFACS, fuzzy TOPSIS, and AHP for identifying important human error factors in emergency departments in Taiwan. Int. J. Ind. Ergon. 2018, 67, 171–179. [Google Scholar] [CrossRef]
- Hsieh, M.C.; Lo, Y.C.; Wang, E.Y.; Fang, Y.H.; Kung, W.C.; Huang, M.H.; Hu, Y.T. An investigation of adverse events in medication processes by HFACS and conditional probability. In Proceedings of the 23rd International Conference on Industrial Engineering and Engineering Management, Wuhan, China, 17–18 September 2016; Atlantis Press: Paris, France, 2017; pp. 51–55. [Google Scholar]
- Gao, J.; Bai, H.; Wang, D.; Wang, L.; Huo, C.; Hou, Y. Rapid security situation prediction of smart grid based on Markov Chain. In Proceedings of the 2019 IEEE 3rd Information Technology, Networking, Electronic and Automation Control Conference (ITNEC), Chengdu, China, 15–17 March 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 2386–2389. [Google Scholar]
- Mathur, P.; Khatri, S.K.; Sharma, M. Prediction of aviation accidents using logistic regression model. In Proceedings of the 2017 International Conference on Infocom Technologies and Unmanned Systems (Trends and Future Directions) (ICTUS), Dubai, United Arab Emirates, 18–20 December 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 725–728. [Google Scholar]
- Wu, X.; Hou, L.; Wen, Y.; Liu, W.; Wu, Z. Research on the relationship between causal factors and consequences of incidents occurred in tank farm using ordinal logistic regression. J. Loss Prev. Process Ind. 2019, 61, 287–297. [Google Scholar] [CrossRef]
- Wang, Y.H.; Ou, Y.; Deng, X.D.; Zhao, L.R.; Zhang, C.Y. The ship collision accidents based on logistic regression and big data. In Proceedings of the 2019 Chinese Control and Decision Conference (CCDC), Nanchang, China, 3–5 June 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 4438–4440. [Google Scholar]
- Konstandinidou, M.; Nivolianitou, Z.; Kefalogianni, E.; Caroni, C. In-depth analysis of the causal factors of incidents reported in the Greek petrochemical industry. Reliab. Eng. Syst. 2011, 96, 1448–1455. [Google Scholar] [CrossRef]
- Department of Energy. DOE Workbook: Conducting Accident Investigations; US Department of Energy: Washington DC, USA, 1999; Available online: http://158.132.155.107/posh97/private/AccidentPhenonmenon/investigation-workbook.pdf (accessed on 11 December 2017).
- Harrell, F.E. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis; Springer: New York, NY, USA, 2001; p. 608. [Google Scholar]
- Onder, S. Evaluation of occupational injuries with lost days among opencast coal mine workers through logistic regression models. Saf. Sci. 2013, 59, 86–92. [Google Scholar] [CrossRef]

| Error Factors of HFACS (Acronym) | Number of Errors | Occurrence Rate of Error Factors |
|---|---|---|
| Unsafe acts | ||
| Decision errors (d) | 119 | 47.98% |
| Skill-based errors (s) | 49 | 19.76% |
| Perceptual errors (p) | 23 | 9.27% |
| Violations (v) | 57 | 22.98% |
| Preconditions for unsafe acts | ||
| Technological environment (te) | 21 | 8.47% |
| Adverse mental states (ms) | 86 | 34.68% |
| Physical/mental limitations (l) | 92 | 37.10% |
| Communication, coordination, planning (cc) | 22 | 8.87% |
| Adverse physiological states (ps) | 27 | 10.89% |
| Unsafe supervision | ||
| Inadequate supervision (is) | 75 | 30.24% |
| Planned inappropriate operations (pi) | 58 | 23.39% |
| Failure to correct problem (fc) | 98 | 39.52% |
| Supervisory violations (sv) | 17 | 6.85% |
| Organizational influence | ||
| Resource management (rm) | 55 | 22.18% |
| Organizational climate (oc) | 75 | 30.24% |
| Organizational process (op) | 118 | 47.58% |
| The Error Factors of HFACS from Level 4 to Level 2 | The Occurrence Rate of Error Factors in Level 1 of HFACS | |||||
|---|---|---|---|---|---|---|
| Level 4 | Level 3 | Level 2 | Decision Errors | Skill-Based Errors | Perceptual Errors | Violations |
| Organizational climate | Planned inappropriate operations | Adverse mental states | 0.326 | 0.466 | 0.083 | 0.195 |
| Failure to correct problem | Adverse physiological states | 0.458 | 0.436 | 0.128 | 0.000 | |
| Resource management | Planned inappropriate operations | Adverse mental states | 0.398 | 0.089 | 0.273 | 0.196 |
| Failure to correct problem | Adverse physiological states | 0.537 | 0.08 | 0.381 | 0.000 | |
| Organizational process | Planned inappropriate operations | Adverse mental states | 0.375 | 0.288 | 0.192 | 0.151 |
| Failure to correct problem | Adverse physiological states | 0.513 | 0.263 | 0.279 | 0.000 | |
| The Error Factors of HFACS from Level 1 to Level 3 | The Occurrence Rate of Error Factors in Level 4 of HFACS | ||||
|---|---|---|---|---|---|
| Level 1 | Level 2 | Level 3 | Organizational Climate | Resource Management | Organizational Process |
| Skill-based errors | Adverse mental states | Planned inappropriate operations | 0.205 | 0.117 | 0.497 |
| Physical/mental limitations | Supervisory violations | 0.409 | 0.030 | 0.627 | |
| Perceptual errors | Adverse mental states | Planned inappropriate operations | 0.043 | 0.503 | 0.551 |
| Physical/mental limitations | Supervisory violations | 0.109 | 0.195 | 0.676 | |
| Decision errors | Adverse mental states | Planned inappropriate operations | 0.097 | 0.359 | 0.527 |
| Physical/mental limitations | Supervisory violations | 0.226 | 0.118 | 0.655 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, S.-H.; Hsieh, M.-C.; Huang, R.-F. Human Error Analysis and Modeling of Medication-Related Adverse Events in Taiwan Using the Human Factors Analysis and Classification System and Logistic Regression. Healthcare 2023, 11, 2063. https://doi.org/10.3390/healthcare11142063
Ko S-H, Hsieh M-C, Huang R-F. Human Error Analysis and Modeling of Medication-Related Adverse Events in Taiwan Using the Human Factors Analysis and Classification System and Logistic Regression. Healthcare. 2023; 11(14):2063. https://doi.org/10.3390/healthcare11142063
Chicago/Turabian StyleKo, Shu-Huan, Min-Chih Hsieh, and Run-Feng Huang. 2023. "Human Error Analysis and Modeling of Medication-Related Adverse Events in Taiwan Using the Human Factors Analysis and Classification System and Logistic Regression" Healthcare 11, no. 14: 2063. https://doi.org/10.3390/healthcare11142063
APA StyleKo, S.-H., Hsieh, M.-C., & Huang, R.-F. (2023). Human Error Analysis and Modeling of Medication-Related Adverse Events in Taiwan Using the Human Factors Analysis and Classification System and Logistic Regression. Healthcare, 11(14), 2063. https://doi.org/10.3390/healthcare11142063






