Evaluating Classification Systems of Diabetic Foot Ulcer Severity: A 12-Year Retrospective Study on Factors Impacting Survival
Abstract
:1. Introduction
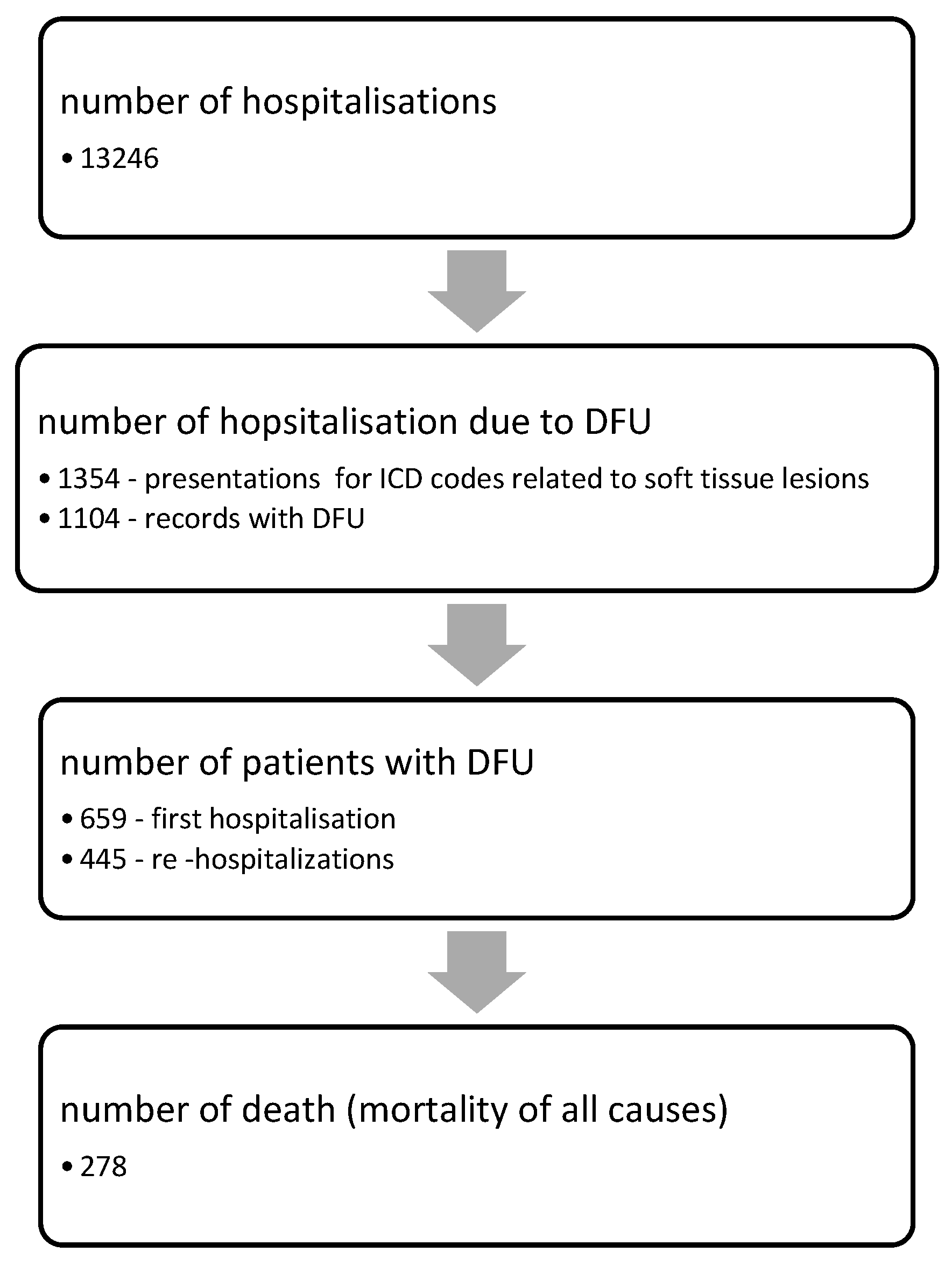
2. Materials and Methods
2.1. Data Collection
- -
- Patients over 18 years of age, diagnosed with any type of diabetes (type 1, type 2, or other specific types);
- -
- Patients admitted to the Diabetes, Nutrition, and Metabolic Diseases Clinic during the aforementioned time period;
- -
- Patients with an International Statistical Classification of Diseases, 10th Revision (ICD-10) [17] code corresponding to diabetic foot pathology in the discharge diagnostic, or soft tissue lesions.
- Social and demographic factors: area of residence (rural or urban), sex, and age;
- History of diabetes: disease duration, type of treatment (metformin, non-insulin antidiabetic agents, insulin, and association of insulin with other antidiabetic agents);
- Presence of specific diabetic chronic complications (retinopathy, nephropathy, neuropathy, peripheral artery disease), high blood pressure (HBP), and cardiovascular disease evaluated during the first hospitalization;
- Biological markers: glycated hemoglobin (HbA1c), inflammation markers (white cell blood count, fibrinogen, CRP, ferritin), lipid profile, renal function, total proteins, albumin, ASAT, ALAT, and uric acid;
- Clinical characteristics of wounds: location (toes, under metatarsals, dorsum of foot, heel), depth (using a sterile blunt nasal probe), extension (evaluating the surface of the wound), and perfusion;
- Infection: the presence of symptoms and signs of inflammation and/or pus, abnormal specific biochemical test results, and the presence of osteolysis on foot X-ray.
- In this study, five clinical classification systems for diabetic foot were used:
- a.
- The University of Texas Staging System for Diabetic Foot Ulcers uses four grades (0—pre-ulcerative or post-ulcerative wound entirely epithelialized; 1—superficial lesion, not involving the tendon, capsule, or bone; 2—wound penetrating tendon or capsule; and 3—wound penetrating bone or joint), which are influenced by the existence of infection (Stage B), ischemia (Stage C), or both (Stage D) [18]. Scores higher than BII are considered predictors of foot amputation [19];
- b.
- The Wagner–Meggitt classification of foot ulcers evaluates the clinical characteristics of foot ulcers (extent and depth) [20];
- c.
- The Saint Elian Wound Score System (SEWSS) [21] includes 10 variables organized into three categories: anatomical factors, aggravating factors, and factors related to ulceration. The first category evaluates anatomical factors, the site, and topographical aspects. In the second category of aggravating factors, four variables are included: ischemia, infection, edema, and neuropathy. In the case of infection, the indications of the IDSA (Infectious Diseases Society of America) [12] are used; therefore, a mild infection is one that affects only the superficial layers of the skin and is characterized by erythema between 0.5 mm and 2 cm, induration, pain, local heat, and purulent secretions [21]. Moderate infection is identified by erythema > 2 cm, and infection of the muscle, tendons, bone, or joint. Osteomyelitis is diagnosed via radiography or biopsy. Severe infection is characterized by a systemic inflammatory response or severe metabolic disorders that require hospitalization or are difficult to manage [21]. The third category of ulceration-related factors includes three variables: depth, area, and healing phase. The result of this scoring system is the sum of all criteria met by ulceration, ranging from six to thirty points. The severity of the ulceration is established by the obtained value, a distinct prognosis being proposed for each of the three degrees: I—mild, probable successful cure; II—moderate, with a partial threat to the foot, where the prognosis depends on the “state-of-the-art” therapies used and the patient’s biological response; and III—severe and life-threatening, where the prognosis does not depend on “state-of-the-art” therapies as a result of the patient’s poor biological response [22];
- d.
- The SINBAD Classification System and Score is an acronym that comes from six elements graded according to their severity: ulcer Site, Ischemia, Neuropathy, Bacterial infection, Area, and Depth [23]. The total score ranges between 0 and 6, which is divided into three categories related to the risk of lower limb amputation: low grade, 0–2; moderate grade, 3–4; and high grade, 5–6 [24,25];
- e.
- The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System (WIfI) is a grading system that evaluates the severity of a wound (ranging from 0 for no ulcer to 3 for an extensive, deep ulcer or gangrene involving the forefoot and/or midfoot), ischemia (ranging from 0 to 3, evaluated through the ankle–brachial index, ankle systolic pressure, or transcutaneous oximetry), and foot infection (from absent to severe: limb- and/or life-threatening) [26]. Based on the associations of the scores obtained for each grade, the WIfI stage (1–5) is then determined [27];
- The necessity of amputation of the lower limb for DFU during the first hospitalization was registered. Patients who needed a limb amputation were transferred to a surgical clinic in the same hospital.
2.2. Statistical Analyses
2.3. Ethical Considerations
3. Results
4. Discussion
4.1. Survival Rate
4.2. Cardiovascular Disease as a Predictor of Mortality in DFU Patients
4.3. Renal Disease as a Predictor of Mortality in Patients with DFUs
4.4. Ulcer Severity as a Predictor of Mortality in Patients with DFUs
4.5. Strength and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ibrahim, A. IDF Clinical Practice Recommendation on the Diabetic Foot: A Guide for Healthcare Professionals. Diabetes Res. Clin. Pract. 2017, 127, 285–287. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 11. Microvascular Complications and Foot Care: Standards of Medical Care in Diabetes—2019. Diabetes Care 2018, 42, S124–S138. [Google Scholar] [CrossRef] [Green Version]
- Sriyani, K.A.; Wasalathanthri, S.; Hettiarachchi, P.; Prathapan, S. Predictors of Diabetic Foot and Leg Ulcers in a Developing Country with a Rapid Increase in the Prevalence of Diabetes Mellitus. PLoS ONE 2013, 8, e80856. [Google Scholar] [CrossRef]
- Jain, A.K.C. A Simple New Classification for Diabetic Foot Ulcers. Med. Sci. 2015, 4, 2109–2120. [Google Scholar] [CrossRef]
- Khalid, I.; Imran, M.; Imran, M.; Akhtar, M.A.; Khan, S.; Amanullah, K.; Khalid, T.J. From Epidemic to Pandemic: Comparing Hospital Staff Emotional Experience Between MERS and COVID-19. Clin. Med. Res. 2021, 19, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Jenab, Y. Diabetic Foot Ulcer Is a Significant Predictor of Silent Myocardial Ischemia in Women with Type 2 Diabetes. J. Diabetes Metab. 2011, 161, 2. [Google Scholar] [CrossRef] [Green Version]
- Everett, E.; Mathioudakis, N. Update on Management of Diabetic Foot Ulcers. Ann. N. Y. Acad. Sci. 2018, 1411, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Bondor, C.I.; Veresiu, I.A.; Florea, B.; Vinik, E.J.; Vinik, A.I.; Gavan, N.A. Epidemiology of Diabetic Foot Ulcers and Amputations in Romania: Results of a Cross-Sectional Quality of Life Questionnaire Based Survey. J. Diabetes Res. 2016, 2016, e5439521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, D.G.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers and Their Recurrence. N. Engl. J. Med. 2017, 376, 2367–2375. [Google Scholar] [CrossRef]
- Mishra, S.C.; Chhatbar, K.C.; Kashikar, A.; Mehndiratta, A. Diabetic Foot. BMJ 2017, 359, j5064. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Sun, S.; Gao, Y.; Ran, X. Global Mortality of Diabetic Foot Ulcer: A Systematic Review and Meta-Analysis of Observational Studies. Diabetes Obes. Metab. 2023, 25, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, B.A.; Berendt, A.R.; Cornia, P.B.; Pile, J.C.; Peters, E.J.G.; Armstrong, D.G.; Deery, H.G.; Embil, J.M.; Joseph, W.S.; Karchmer, A.W.; et al. Executive Summary: 2012 Infectious Diseases Society of America Clinical Practice Guideline for the Diagnosis and Treatment of Diabetic Foot Infectionsa. Clin. Infect. Dis. 2012, 54, 1679–1684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monteiro-Soares, M.; Hamilton, E.J.; Russell, D.A.; Srisawasdi, G.; Boyko, E.J.; Mills, J.L.; Jeffcoate, W.; Game, F. Guidelines on the Classification of Foot Ulcers in People with Diabetes (IWGDF 2023 Update). Diabetes Metab. Res. Rev. 2023, e3648, Online ahead of print. [Google Scholar] [CrossRef]
- Monteiro-Soares, M.; Hamilton, E.J.; Russell, D.A.; Srisawasdi, G.; Boyko, E.J.; Mills, J.L.; Jeffcoate, W.; Game, F. Classification of Foot Ulcers in People with Diabetes: A Systematic Review. Diabetes Metab. Res. Rev. 2023, e3645, Online ahead of print. [Google Scholar] [CrossRef]
- Monteiro-Soares, M.; Boyko, E.J.; Jeffcoate, W.; Mills, J.L.; Russell, D.; Morbach, S.; Game, F. Diabetic foot ulcer classifications: A critical review. Diabetes Metab Res Rev. 2020, 36, e3272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaka, A.S.; Landsteiner, A.; Ensrud, K.E.; Logan, B.; Sowerby, C.; Ullman, K.; Yoon, P.; Wilt, T.J.; Sultan, S. Risk prediction models for diabetic foot ulcer development or amputation: A review of reviews. J Foot Ankle Res. 2023, 16, 13. [Google Scholar] [CrossRef] [PubMed]
- ICD-10 Version: 2019. Available online: https://icd.who.int/browse10/2019/en (accessed on 3 June 2023).
- Armstrong, D.G. The University of Texas Diabetic Foot Classification System. Ostomy. Wound Manag. 1996, 42, 60–61. [Google Scholar]
- Armstrong, D.G.; Lavery, L.A.; Harkless, L.B. Validation of a Diabetic Wound Classification System: The Contribution of Depth, Infection, and Ischemia to Risk of Amputation. Diabetes Care 1998, 21, 855–859. [Google Scholar] [CrossRef]
- Mehraj, M. A Review of Wagner Classification and Current Concepts in Management of Diabetic Foot. Int. J. Orthop. Sci. 2018, 4, 933–935. [Google Scholar] [CrossRef] [Green Version]
- Martínez-De Jesús, F.R. A Checklist System to Score Healing Progress of Diabetic Foot Ulcers. Int. J. Low. Extrem. Wounds 2010, 9, 74–83. [Google Scholar] [CrossRef]
- Martinez-De Jesús, F.R.; Hernandez-Luevano, E.; Rodriguez-Ramírez, N.; Cendejas-Alatorre, R.; Muñoa Prado, J.A.; Carrera Maigua, F.; Zambrano-Loaiza, E. Validation of the Ischaemia Severity Scale (ISS) Based on Non-Invasive Vascular Assessments (SEWSS) for Predicting Outcomes of Diabetic Foot Attack. J. Clin. Med. 2022, 11, 7195. [Google Scholar] [CrossRef] [PubMed]
- Ince, P.; Abbas, Z.G.; Lutale, J.K.; Basit, A.; Ali, S.M.; Chohan, F.; Morbach, S.; Möllenberg, J.; Game, F.L.; Jeffcoate, W.J. Use of the SINBAD Classification System and Score in Comparing Outcome of Foot Ulcer Management on Three Continents. Diabetes Care 2008, 31, 964–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, B.-J.; Choi, H.J.; Kang, J.S.; Tak, M.S.; Park, E.S. Comparison of Five Systems of Classification of Diabetic Foot Ulcers and Predictive Factors for Amputation. Int. Wound J. 2017, 14, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Kwak, S.H.; Kim, J.H.; Choi, H.J. Prediction of Diabetic Foot Amputation Using Newly Revised DIRECT Coding System: Comparison of Accuracy with That of Five Existing Classification Systems. Int. Wound J. 2023, 20, 359–371. [Google Scholar] [CrossRef]
- Mills, J.L.; Conte, M.S.; Armstrong, D.G.; Pomposelli, F.B.; Schanzer, A.; Sidawy, A.N.; Andros, G. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: Risk Stratification Based on Wound, Ischemia, and Foot Infection (WIfI). J. Vasc. Surg. 2014, 59, 220–234.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, W.P.; Loretz, L.; Hanesian, C.; Flahive, J.; Bostrom, J.; Lunig, N.; Schanzer, A.; Messina, L. Society for Vascular Surgery Wound, Ischemia, Foot Infection (WIfI) Score Correlates with the Intensity of Multimodal Limb Treatment and Patient-Centered Outcomes in Patients with Threatened Limbs Managed in a Limb Preservation Center. J. Vasc. Surg. 2017, 66, 488–498.e2. [Google Scholar] [CrossRef]
- SPSS Software|IBM. Available online: https://www.ibm.com/spss (accessed on 12 June 2023).
- Parizad, N.; Hajimohammadi, K.; Goli, R. Surgical Debridement, Maggot Therapy, Negative Pressure Wound Therapy, and Silver Foam Dressing Revive Hope for Patients with Diabetic Foot Ulcer: A Case Report. Int. J. Surg. Case Rep. 2021, 82, 105931. [Google Scholar] [CrossRef]
- Dietrich, I.; Braga, G.A.; de Melo, F.G.; da Costa Silva Silva, A.C.C. The Diabetic Foot as a Proxy for Cardiovascular Events and Mortality Review. Curr. Atheroscler. Rep. 2017, 19, 44. [Google Scholar] [CrossRef]
- Shlipak, M.G.; Sarnak, M.J.; Katz, R.; Fried, L.F.; Seliger, S.L.; Newman, A.B.; Siscovick, D.S.; Stehman-Breen, C. Cystatin C and the Risk of Death and Cardiovascular Events among Elderly Persons. N. Engl. J. Med. 2005, 352, 2049–2060. [Google Scholar] [CrossRef]
- Ogawa, Y.; Goto, T.; Tamasawa, N.; Matsui, J.; Tando, Y.; Sugimoto, K.; Tomotsune, K.; Kimura, M.; Yasujima, M.; Suda, T. Serum Cystatin C in Diabetic Patients. Not Only an Indicator for Renal Dysfunction in Patients with Overt Nephropathy but Also a Predictor for Cardiovascular Events in Patients without Nephropathy. Diabetes Res. Clin. Pract. 2008, 79, 357–361. [Google Scholar] [CrossRef]
- Jeyaraman, K.; Berhane, T.; Hamilton, M.; Chandra, A.P.; Falhammar, H. Mortality in Patients with Diabetic Foot Ulcer: A Retrospective Study of 513 Cases from a Single Centre in the Northern Territory of Australia. BMC Endocr. Disord. 2019, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Morbach, S.; Furchert, H.; Gröblinghoff, U.; Hoffmeier, H.; Kersten, K.; Klauke, G.-T.; Klemp, U.; Roden, T.; Icks, A.; Haastert, B.; et al. Long-Term Prognosis of Diabetic Foot Patients and Their Limbs: Amputation and Death over the Course of a Decade. Diabetes Care 2012, 35, 2021–2027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aragón-Sánchez, J.; Víquez-Molina, G.; López-Valverde, M.E.; Aragón-Hernández, J.; Rojas-Bonilla, J.M.; Murillo-Vargas, C. Long-Term Mortality of a Cohort of Patients Undergoing Surgical Treatment for Diabetic Foot Infections. An 8-Year Follow-up Study. Int. J. Low. Extrem. Wounds 2023, 22, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Mohammedi, K.; Woodward, M.; Hirakawa, Y.; Zoungas, S.; Colagiuri, S.; Hamet, P.; Harrap, S.; Poulter, N.; Matthews, D.R.; Marre, M.; et al. Presentations of Major Peripheral Arterial Disease and Risk of Major Outcomes in Patients with Type 2 Diabetes: Results from the ADVANCE-ON Study. Cardiovasc. Diabetol. 2016, 15, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosi, L.M.; Jones, A.S.; Topliss, D.J.; Bach, L.A. Demographics and Outcomes of Inpatients with Diabetic Foot Ulcers Treated Conservatively and Surgically in a Metropolitan Hospital Network. Diabetes Res. Clin. Pract. 2021, 175, 108821. [Google Scholar] [CrossRef] [PubMed]
- Bundó, M.; Vlacho, B.; Llussà, J.; Bobé, I.; Aivar, M.; Ciria, C.; Martínez-Sánchez, A.; Real, J.; Mata-Cases, M.; Cos, X.; et al. Prediction of Outcomes in Subjects with Type 2 Diabetes and Diabetic Foot Ulcers in Catalonian Primary Care Centers: A Multicenter Observational Study. J. Foot Ankle Res. 2023, 16, 8. [Google Scholar] [CrossRef]
- Brownrigg, J.R.W.; Davey, J.; Holt, P.J.; Davis, W.A.; Thompson, M.M.; Ray, K.K.; Hinchliffe, R.J. The Association of Ulceration of the Foot with Cardiovascular and All-Cause Mortality in Patients with Diabetes: A Meta-Analysis. Diabetologia 2012, 55, 2906–2912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C.S. Oxidative Stress and Metabolic Disorders: Pathogenesis and Therapeutic Strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef]
- Ndip, A.; Williams, A.; Jude, E.B.; Serracino-Inglott, F.; Richardson, S.; Smyth, J.V.; Boulton, A.J.M.; Alexander, M.Y. The RANKL/RANK/OPG Signaling Pathway Mediates Medial Arterial Calcification in Diabetic Charcot Neuroarthropathy. Diabetes 2011, 60, 2187–2196. [Google Scholar] [CrossRef] [Green Version]
- Higgins, C.L.; Isbilir, S.; Basto, P.; Chen, I.Y.; Vaduganathan, M.; Vaduganathan, P.; Reardon, M.J.; Lawrie, G.; Peterson, L.; Morrisett, J.D. Distribution of Alkaline Phosphatase, Osteopontin, RANK Ligand and Osteoprotegerin in Calcified Human Carotid Atheroma. Protein J. 2015, 34, 315–328. [Google Scholar] [CrossRef]
- Sattler, A.M.; Schoppet, M.; Schaefer, J.R.; Hofbauer, L.C. Novel Aspects on RANK Ligand and Osteoprotegerin in Osteoporosis and Vascular Disease. Calcif. Tissue Int. 2004, 74, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Lauri, C.; Tamminga, M.; Glaudemans, A.W.J.M.; Juárez Orozco, L.E.; Erba, P.A.; Jutte, P.C.; Lipsky, B.A.; IJzerman, M.J.; Signore, A.; Slart, R.H.J.A. Detection of Osteomyelitis in the Diabetic Foot by Imaging Techniques: A Systematic Review and Meta-Analysis Comparing MRI, White Blood Cell Scintigraphy, and FDG-PET. Diabetes Care 2017, 40, 1111–1120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jhamb, S.; Vangaveti, V.N.; Malabu, U.H. Genetic and Molecular Basis of Diabetic Foot Ulcers: Clinical Review. J. Tissue Viability 2016, 25, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, L.; Du, R.; Yan, J.; Liu, N.; Yuan, W.; Jiang, Y.; Xu, S.; Ye, F.; Yuan, G.; et al. CML/RAGE Signal Induces Calcification Cascade in Diabetes. Diabetol. Metab. Syndr. 2016, 8, 83. [Google Scholar] [CrossRef] [Green Version]
- Kay, A.M.; Simpson, C.L.; Stewart, J.A. The Role of AGE/RAGE Signaling in Diabetes-Mediated Vascular Calcification. J. Diabetes Res. 2016, 2016, 6809703. [Google Scholar] [CrossRef] [Green Version]
- Roustit, M.; Loader, J.; Deusenbery, C.; Baltzis, D.; Veves, A. Endothelial Dysfunction as a Link Between Cardiovascular Risk Factors and Peripheral Neuropathy in Diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 3401–3408. [Google Scholar] [CrossRef]
- Kuehl, M.; Stevens, M.J. Cardiovascular Autonomic Neuropathies as Complications of Diabetes Mellitus. Nat. Rev. Endocrinol. 2012, 8, 405–416. [Google Scholar] [CrossRef]
- Baltzis, D.; Roustit, M.; Grammatikopoulou, M.G.; Katsaboukas, D.; Athanasiou, V.; Iakovou, I.; Veves, A.; Manes, C.; Trakatelli, M.-C. Diabetic Peripheral Neuropathy as a Predictor of Asymptomatic Myocardial Ischemia in Type 2 Diabetes Mellitus: A Cross-Sectional Study. Adv. Ther. 2016, 33, 1840–1847. [Google Scholar] [CrossRef] [Green Version]
- Demirtas, D.; Kucukosmanoglu, M. In patients with diabetic foot, improved left ventricular functions are detected by strain echocardiography after the diabetic foot treatment: A cross-sectional study. Medicine 2019, 98, e17217. [Google Scholar] [CrossRef]
- Vuorlaakso, M.; Kiiski, J.; Salonen, T.; Karppelin, M.; Helminen, M.; Kaartinen, I. Major Amputation Profoundly Increases Mortality in Patients with Diabetic Foot Infection. Front. Surg. 2021, 8, 655902. [Google Scholar] [CrossRef]
- Dòria, M.; Rosado, V.; Pacheco, L.R.; Hernández, M.; Betriu, À.; Valls, J.; Franch-Nadal, J.; Fernández, E.; Mauricio, D. Prevalence of Diabetic Foot Disease in Patients with Diabetes Mellitus under Renal Replacement Therapy in Lleida, Spain. BioMed Res. Int. 2016, 2016, 7217586. [Google Scholar] [CrossRef] [Green Version]
- McFarlane, S.I.; Sowers, J.R. Cardiovascular Endocrinology 1: Aldosterone Function in Diabetes Mellitus: Effects on Cardiovascular and Renal Disease. J. Clin. Endocrinol. Metab. 2003, 88, 516–523. [Google Scholar] [CrossRef]
- Rubio, J.A.; Jiménez, S.; Lázaro-Martínez, J.L. Mortality in Patients with Diabetic Foot Ulcers: Causes, Risk Factors, and Their Association with Evolution and Severity of Ulcer. J. Clin. Med. 2020, 9, 3009. [Google Scholar] [CrossRef] [PubMed]
- Sen, P.; Demirdal, T. Evaluation of Mortality Risk Factors in Diabetic Foot Infections. Int. Wound J. 2020, 17, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Schofield, H.; Haycocks, S.; Robinson, A.; Edmonds, M.; Anderson, S.G.; Heald, A.H. Mortality in 98 Type 1 Diabetes Mellitus and Type 2 Diabetes Mellitus: Foot Ulcer Location Is an Independent Risk Determinant. Diabet. Med. J. Br. Diabet. Assoc. 2021, 38, e14568. [Google Scholar] [CrossRef]


| Characteristics | Mean | SD (Std.err.) |
|---|---|---|
| Age (years) | 61.34 | 11.062 (0.431) |
| Years since diagnosis | 11.24 | 8.79 (0.334) |
| Hospitalization (days) | 19.68 | 13.38(0.522) |
| Hb (g/dL) | 12.45 | 1.88 (0.07) |
| Fibrinogen (mg/dL) | 478.61 | 132.89 (5.42) |
| CRP (mg/dL) | 6.72 | 8.48 (0.46) |
| Ferritin (µg/mL) | 382.20 | 436.33 (40.86) |
| HbA1c (%) | 9.55 | 2.19 (0.09) |
| Albumin (g/dL) | 34.85 | 8.08 (0.68) |
| Glomerular filtration rate (GFR mL/min/1.73 m2) | 70.35 | 26.63 (1.05) |
| WBC | 10,848.39 | 4897.42 (192.53) |
| N | % | |
| Type of diabetes | ||
| T1DM | 80 | 12.4 |
| T2DM | 568 | 86.19 |
| Other types of DM | 11 | 1.67 |
| Sex (male) | 435 | 66 |
| Area of residence (urban) | 339 | 51.4 |
| Obesity | 208 | 31.6 |
| Diabetic ketoacidosis | 98 | 14.9 |
| DM treatment | ||
| Non-insulin antidiabetic drugs | 500 | 75.9 |
| Insulin alone or in combination with non-insulin therapy | 191 | 29 |
| Diabetic retinopathy | 431 | 65.4 |
| Diabetic nephropathy | 210 | 31.9 |
| Chronic kidney disease | 231 | 35.1 |
| Diabetic neuropathy | 619 | 88.5 |
| Peripheral artery disease | 419 | 63.6 |
| Hypertension | 483 | 73.3 |
| Cardiovascular diseases | 394 | 59.8 |
| Charcot’s foot | 21 | 3.2 |
| Classification System | N | % |
|---|---|---|
| Texas classification (stage) | ||
| A0 | 6 | 0.9 |
| AI | 74 | 11.2 |
| AII | 10 | 1.5 |
| AIII | 1 | 0.2 |
| B0 | 1 | 0.2 |
| BI | 140 | 21.2 |
| BII | 127 | 19.3 |
| BIII | 101 | 15.3 |
| C0 | 2 | 0.3 |
| CI | 8 | 1.2 |
| CII | 5 | 0.8 |
| CIII | 23 | 3.5 |
| D0 | 3 | 0.5 |
| DI | 17 | 2.6 |
| DII | 40 | 6.1 |
| DIII | 101 | 15.3 |
| Wagner–Meggitt classification | ||
| Grade 0 | 10 | 1.5 |
| Grade 1 | 213 | 32.4 |
| Grade 2 | 192 | 29.2 |
| Grade 3 | 103 | 15.7 |
| Grade 4 | 117 | 17.8 |
| Grade 5 | 23 | 3.5 |
| SEWSS | ||
| Mild | 35 | 5.3 |
| Moderate | 565 | 85.7 |
| Severe | 59 | 9.0 |
| SINBAD Classification System and Score | ||
| Low grade | 287 | 43.6 |
| Moderate grade | 269 | 40.8 |
| High grade | 54 | 8.2 |
| WIfI Classification System | ||
| Stage 1 | 64 | 9.7 |
| Stage 2 | 171 | 25.9 |
| Stage 3 | 76 | 11.5 |
| Stage 4 | 192 | 29.1 |
| Stage 5 | 155 | 23.5 |
| Lesions (depth) | ||
| Superficial | 231 | 35.1 |
| Deep ulcers | 223 | 33.8 |
| Ulcers penetrating all layers | 205 | 31.1 |
| Interval Start Time (Years) | Number Entering Interval | Number Exposed to Risk | Cumulative Proportion Surviving at End of Interval | Std. Error of Cumulative Proportion Surviving at End of Interval |
|---|---|---|---|---|
| 5 | 462 | 462.000 | 0.66 | 0.02 |
| 10 | 393 | 393.000 | 0.59 | 0.02 |
| 12 | 385 | 194.500 | 0.57 | 0.02 |
| p. | HR | 95.0% CI for HR | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Age | 0.000 | 1.046 | 1.034 | 1.058 |
| Gender | 0.073 | 1.248 | 0.979 | 1.591 |
| Area of residence | 0.540 | 0.929 | 0.734 | 1.176 |
| Diabetes duration | 0.368 | 1.006 | 0.993 | 1.019 |
| Length of hospitalization | 0.014 | 1.010 | 1.002 | 1.018 |
| Type 1 DM | 0.701 | 1.262 | 0.385 | 4.137 |
| Type 2 DM | 0.429 | 1.584 | 0.507 | 4.947 |
| Diabetic retinopathy (DR) | 0.475 | |||
| DR (nonproliferative) | 0.513 | 0.914 | 0.700 | 1.195 |
| DR (proliferative) | 0.334 | 1.207 | 0.824 | 1.766 |
| DR (maculopathy) | 0.688 | 1.102 | 0.686 | 1.771 |
| Diabetic nephropathy | 0.012 | 1.360 | 1.069 | 1.731 |
| Chronic kidney disease | 0.004 | |||
| Stage 1 | 0.035 | 1.407 | 1.024 | 1.935 |
| Stage2 | 0.001 | 1.698 | 1.232 | 2.341 |
| Stage3 | 0.777 | 1.102 | 0.563 | 2.157 |
| Stage 4 | 0.026 | 1.953 | 1.085 | 3.515 |
| Peripheral artery disease | 0.204 | |||
| Stage 1 | 0.585 | 0.728 | 0.232 | 2.279 |
| Stage 2 | 0.153 | 1.392 | 0.884 | 2.192 |
| Stage 3 | 0.132 | 1.871 | 0.828 | 4.230 |
| Stage 4 | 0.121 | 1.234 | 0.946 | 1.609 |
| Cardiovascular diseases | 0.000 | 1.753 | 1.385 | 2.219 |
| Polyneuropathy | 0.729 | 0.917 | 0.561 | 1.498 |
| Charcot’s foot | 0.281 | 0.641 | 0.285 | 1.439 |
| Hypertension | 0.014 | 1.433 | 1.076 | 1.907 |
| Dyslipidemia | 0.320 | 0.879 | 0.682 | 1.133 |
| Obesity | 0.303 | 0.873 | 0.674 | 1.131 |
| Diabetic ketoacidosis | 0.962 | 1.008 | 0.721 | 1.409 |
| Insulin treatment ± other antidiabetics | 0.223 | 1.181 | 0.904 | 1.542 |
| Non-insulin treatment | 0.562 | 1.099 | 0.799 | 1.512 |
| Texas Staging System > IIB | 0.026 | 1.308 | 1.033 | 1.656 |
| SINBAD Classification System and Score—high grade | 0.001 | 1.520 | 1.182 | 1.954 |
| WIfI stage 4–5 | 0.004 | 1.455 | 1.124 | 1.883 |
| SEWSS median score | 0.026 | 1.039 | 1.005 | 1.075 |
| Wagner–Meggitt classification | 0.052 | 1.269 | 0.998 | 1.612 |
| Lesion depth | 0.015 | |||
| Deep ulcers | 0.007 | 1.680 | 1.151 | 2.450 |
| Ulcers intersecting all layers | 0.023 | 1.567 | 1.065 | 2.306 |
| Cellulitis | 0.375 | |||
| Cellulitis only in foot | 0.430 | 0.907 | 0.711 | 1.157 |
| Cellulitis above the foot | 0.203 | 0.672 | 0.365 | 1.239 |
| Fever | 0.539 | 0.870 | 0.557 | 1.357 |
| Osteolysis | 0.418 | 1.110 | 0.862 | 1.430 |
| Wound culture | 0.091 | |||
| Negative culture | 0.369 | 0.821 | 0.533 | 1.263 |
| Aerobes, Gram-positive | 0.044 | 0.734 | 0.543 | 0.991 |
| Aerobes, Gram-negative | 0.459 | 1.135 | 0.811 | 1.588 |
| Fungi | 0.619 | 1.428 | 0.351 | 5.804 |
| Hb | 0.015 | 0.925 | 0.868 | 0.985 |
| Hb < 10 g/dL | 0.010 | 1.586 | 1.118 | 2.252 |
| WBC | 0.843 | 1.000 | 1.000 | 1.000 |
| Fibrinogen (mg/dL) | 0.325 | 1.000 | 0.999 | 1.000 |
| CRP (mg/dL) | 0.490 | 1.007 | 0.986 | 1.029 |
| Glycemia (mg/dL) | 0.202 | 0.999 | 0.998 | 1.000 |
| Creatinine (mg/dL) | 0.096 | 1.008 | 0.999 | 1.017 |
| Albumin (g/dL) | 0.009 | 1.029 | 1.007 | 1.051 |
| Sideremia (μg/dL) | 0.174 | 0.994 | 0.986 | 1.003 |
| Ferritin (ng/dL) | 0.002 | 1.001 | 1.000 | 1.001 |
| LDL cholesterol (mg/dL) | 0.841 | 1.000 | 0.996 | 1.004 |
| GFR (mL/min/1.73 m2) | 0.000 | 0.991 | 0.987 | 0.996 |
| GFR < 60 mL/min/1.73 m2 | 0.000 | 1.609 | 1.261 | 2.052 |
| Hemocultures | 0.586 | |||
| Negative | 0.335 | 1.184 | 0.840 | 1.670 |
| Positive | 0.756 | 0.887 | 0.418 | 1.883 |
| HbA1c (%) | 0.904 | 1.053 | 0.457 | 2.426 |
| p. | HR | 95.0% CI | ||
|---|---|---|---|---|
| Inferior | Superior | |||
| Relationship between Texas Staging System and mortality risk (model 1) | ||||
| Texas (category) | 0.031 | 1.963 | 1.065 | 3.617 |
| Cardiovascular disease | 0.001 | 2.898 | 1.540 | 5.456 |
| Hypertension | 0.570 | 0.804 | 0.379 | 1.706 |
| Hb < 10 g/dL | 0.010 | 2.853 | 1.281 | 6.355 |
| GFR < 60 mL/min/1.73 m2 | 0.020 | 2.171 | 1.129 | 4.175 |
| Albumin (g/dL) | 0.069 | 1.034 | 0.997 | 1.072 |
| Age (years) | 0.004 | 1.042 | 1.013 | 1.073 |
| Sex (male) | 0.408 | 1.292 | 0.704 | 2.370 |
| Diabetes duration (years) | 0.228 | 0.978 | 0.943 | 1.014 |
| Relationship between Wagner–Meggitt classification and mortality risk (model 2) | ||||
| Wagner–Meggitt (category) | 0.042 | 1.889 | 1.024 | 3.483 |
| Cardiovascular disease | 0.001 | 2.829 | 1.489 | 5.376 |
| Hypertension | 0.589 | 0.812 | 0.382 | 1.727 |
| Hb < 10 g/dL | 0.029 | 2.508 | 1.098 | 5.733 |
| GFR < 60 mL/min/1.73 m2 | 0.006 | 2.470 | 1.297 | 4.705 |
| Albumin (g/dL) | 0.091 | 1.032 | 0.995 | 1.070 |
| Age (years) | 0.006 | 1.041 | 1.011 | 1.071 |
| Sex (male) | 0.341 | 1.347 | 0.730 | 2.485 |
| Diabetes duration (years) | 0.247 | 0.979 | 0.943 | 1.015 |
| Relationship between SEWSS and mortality risk (model 3) | ||||
| SEWSS (median score) | 0.067 | 1.823 | 0.959 | 3.466 |
| Cardiovascular disease | 0.003 | 2.717 | 1.407 | 5.247 |
| Hypertension | 0.678 | 0.852 | 0.399 | 1.816 |
| Hb < 10 g/dL | 0.016 | 2.722 | 1.208 | 6.135 |
| GFR < 60 mL/min/1.73 m2 | 0.006 | 2.454 | 1.297 | 4.645 |
| Albumin (g/dL) | 0.068 | 1.035 | 0.997 | 1.074 |
| Age (years) | 0.002 | 1.048 | 1.018 | 1.079 |
| Sex (male) | 0.264 | 1.408 | 0.772 | 2.569 |
| Diabetes duration (years) | 0.119 | 0.969 | 0.932 | 1.008 |
| Relationship between SINBAD and mortality risk (model 4) | ||||
| SINBAD (score 5–6) | 0.007 | 2.333 | 1.258 | 4.326 |
| Cardiovascular disease | 0.002 | 2.967 | 1.472 | 5.975 |
| Hypertension | 0.161 | 0.580 | 0.271 | 1.243 |
| Hb < 10 g/dL | 0.001 | 5.939 | 2.605 | 13.54 |
| GFR < 60 mL/min/1.73 m2 | 0.070 | 1.916 | 0.949 | 3.869 |
| Albumin (g/dL) | 0.065 | 1.049 | 0.997 | 1.103 |
| Age (years) | 0.102 | 1.026 | 0.995 | 1.058 |
| Sex (male) | 0.319 | 1.374 | 0.735 | 2.568 |
| Diabetes duration (years) | 0.030 | 0.953 | 0.913 | 0.995 |
| Relationship between WIfI and mortality risk (model 5) | ||||
| WIfI (stage 4–5) | 0.077 | 1.755 | 0.940 | 3.277 |
| Cardiovascular disease | 0.001 | 2.956 | 1.569 | 5.568 |
| Hypertension | 0.370 | 0.708 | 0.333 | 1.506 |
| Hb < 10 g/dL | 0.002 | 3.479 | 1.589 | 7.613 |
| GFR < 60 mL/min/1.73 m2 | 0.079 | 1.037 | 0.937 | 3.274 |
| Albumin (g/dL) | 0.058 | 1.035 | 0.999 | 1.076 |
| Age (years) | 0.024 | 1.033 | 1.004 | 1.063 |
| Sex (male) | 0.195 | 1.494 | 0.814 | 2.741 |
| Diabetes duration (years) | 0.120 | 0.970 | 0.933 | 1.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niță, O.; Arhire, L.I.; Mihalache, L.; Popa, A.D.; Niță, G.; Gherasim, A.; Graur, M. Evaluating Classification Systems of Diabetic Foot Ulcer Severity: A 12-Year Retrospective Study on Factors Impacting Survival. Healthcare 2023, 11, 2077. https://doi.org/10.3390/healthcare11142077
Niță O, Arhire LI, Mihalache L, Popa AD, Niță G, Gherasim A, Graur M. Evaluating Classification Systems of Diabetic Foot Ulcer Severity: A 12-Year Retrospective Study on Factors Impacting Survival. Healthcare. 2023; 11(14):2077. https://doi.org/10.3390/healthcare11142077
Chicago/Turabian StyleNiță, Otilia, Lidia Iuliana Arhire, Laura Mihalache, Alina Delia Popa, George Niță, Andreea Gherasim, and Mariana Graur. 2023. "Evaluating Classification Systems of Diabetic Foot Ulcer Severity: A 12-Year Retrospective Study on Factors Impacting Survival" Healthcare 11, no. 14: 2077. https://doi.org/10.3390/healthcare11142077
APA StyleNiță, O., Arhire, L. I., Mihalache, L., Popa, A. D., Niță, G., Gherasim, A., & Graur, M. (2023). Evaluating Classification Systems of Diabetic Foot Ulcer Severity: A 12-Year Retrospective Study on Factors Impacting Survival. Healthcare, 11(14), 2077. https://doi.org/10.3390/healthcare11142077






