Epidemiological Overview of Urogenital Gonorrhea in Mexico (2003–2020)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Period Analyzed of Urogenital Gonorrhea (UG)
2.2. Operational Definition of a UG Case
2.3. Data Collection
2.4. Epidemiological Analysis of UG
2.5. Data Analysis
2.6. Seasonal Influence on UG Cases
3. Results
3.1. Behavior of New Cases and Incidence of UG by Sex
3.2. Susceptibility of UG per Age Groups and Sex
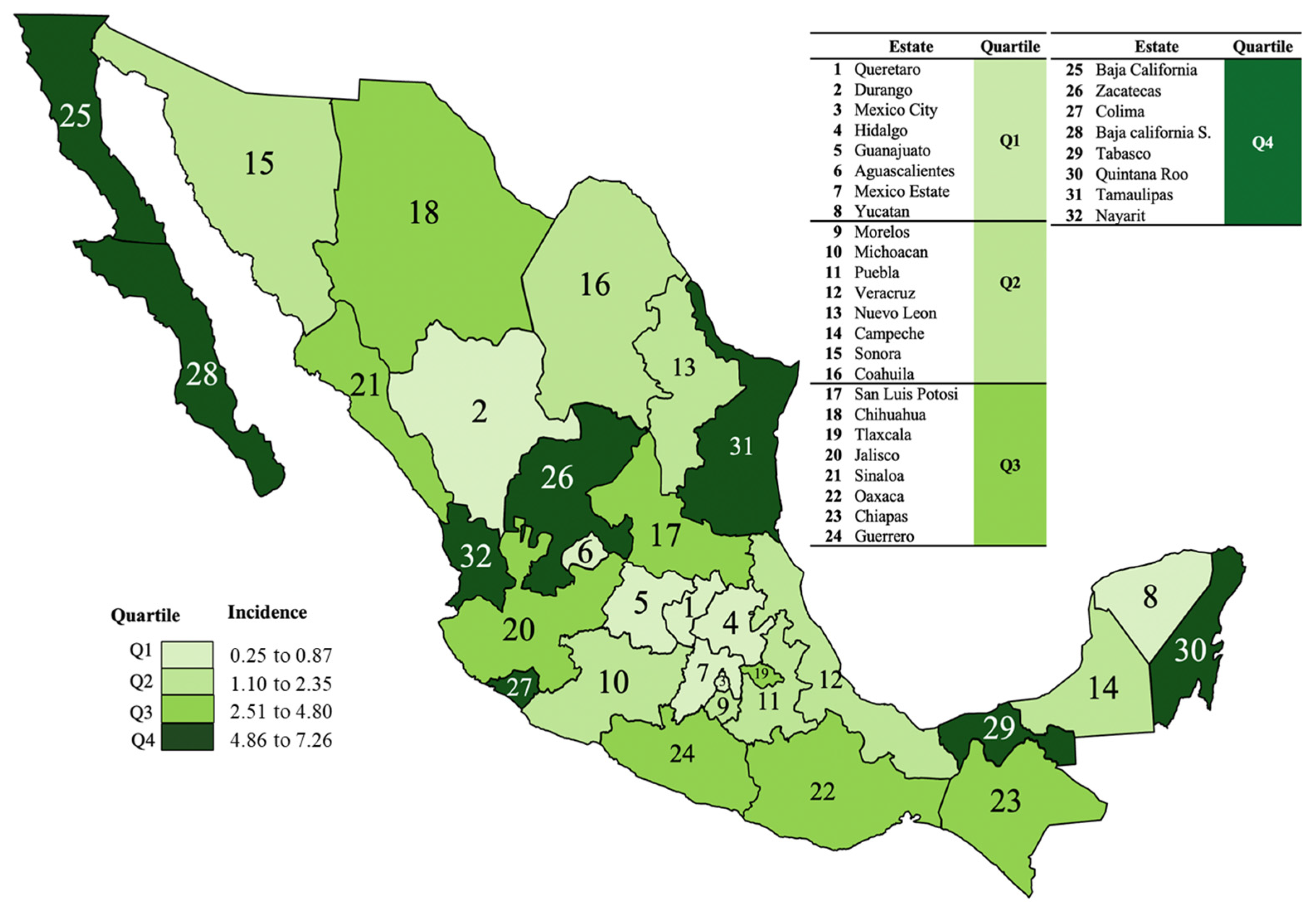
3.3. Geographical Distribution of Incidence of UG
3.4. Distribution of UG Cases by Season and Temperature
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wright, S.S.; Kreisel, K.M.; Hitt, J.C.; Pagaoa, M.A.; Weinstock, H.S.; Thorpe, P.G. Impact of the COVID-19 Pandemic on Centers for Disease Control and Prevention-Funded Sexually Transmitted Disease Programs. Sex. Transm. Dis. 2022, 49, e61–e63. [Google Scholar] [CrossRef] [PubMed]
- Rowley, J.; Vander Hoorn, S.; Korenromp, E.; Low, N.; Unemo, M.; Abu-Raddad, L.J. Chlamydia, gonorrhoea, trichomoniasis and syphilis: Global prevalence and incidence estimates, 2016. Bull. World Health Organ. 2019, 97, 548–562. [Google Scholar] [CrossRef] [PubMed]
- Lenz, J.D.; Dillard, J.P. Pathogenesis of Neisseria gonorrhoeae and the Host Defense in Ascending Infections of Human Fallopian Tube. Front. Immunol. 2018, 9, 2710. [Google Scholar] [CrossRef] [PubMed]
- Rowlinson, E.; Soge, O.O.; Hughes, J.P.; Berzkalns, A.; Thibault, C.; Kerani, R.P.; Khosropour, C.M.; Manhart, L.E.; Golden, M.R.; Barbee, L.A. Prior exposure to azithromycin and azithromycin resistance among persons diagnosed with Neisseria gonorrhoeae infection at a Sexual Health Clinic 2012–2019. Clin. Infect. Dis. 2022, 76, e1270–e1276. [Google Scholar] [CrossRef]
- Sánchez, N.O.; Pérez, N.F.; Martínez, S.B. Evaluation of the viasure Neisseria gonorrhoeae ciprofloxacin resistant assay for the simultaneous identification and direct detection of ciprofloxacin susceptibility. Diagn. Microbiol. Infect. Dis. 2022, 104, 115798. [Google Scholar] [CrossRef]
- Mlynarczyk-Bonikowska, B.; Kowalewski, C.; Krolak-Ulinska, A.; Marusza, W. Molecular Mechanisms of Drug Resistance and Epidemiology of Multidrug-Resistant Variants of Neisseria gonorrhoeae. Int. J. Mol. Sci. 2022, 23, 10499. [Google Scholar] [CrossRef]
- Vaezzadeh, K.; Sepidarkish, M.; Mollalo, A.; As’Adi, N.; Rouholamin, S.; Rezaeinejad, M.; Mojtahedi, M.F.; Hosseini, S.M.M.; Taheri, M.; Mahjour, S.; et al. Global prevalence of Neisseria gonorrhoeae infection in pregnant women: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2022, 29, 22–31. [Google Scholar] [CrossRef]
- Mathew, B.J.; Vyas, A.K.; Khare, P.; Gupta, S.; Nema, R.K.; Nema, S.; Gupta, S.; Chaurasiya, S.K.; Biswas, D.; Singh, A.K. Laboratory diagnosis of COVID-19: Current status and challenges. Iran. J. Microbiol. 2021, 13, 1–7. [Google Scholar] [CrossRef]
- Ojeda, V.D.; Strathdee, S.A.; Lozada, R.; Rusch, M.L.A.; Fraga, M.; Orozovich, P.; Magis-Rodriguez, C.; De La Torre, A.; Amaro, H.; Cornelius, W.; et al. Associations between migrant status and sexually transmitted infections among female sex workers in Tijuana, Mexico. Sex. Transm. Infect. 2009, 85, 420–426. [Google Scholar] [CrossRef] [Green Version]
- Santos, F.S. Reemergencia de Sífilis y Gonorrea en México. Enfermedades Infecc. Y Microbiol. 2018, 38, 103. [Google Scholar]
- Ye, X.; Li, F.R.; Pan, Q.; Li, Z.; Yu, G.Q.; Liu, H.; Liu, J.; Huai, P.-C.; Zhang, F.-R. Prevalence and associated factors of sexually transmitted infections among methamphetamine users in Eastern China: A cross-sectional study. BMC Infect. Dis. 2022, 22, 7. [Google Scholar] [CrossRef]
- NOM-039-SSA2-2014; Para la Prevención y Control de las Infecciones de Transmisión Sexual. Diario Oficial de la Federación: Ciudad de México, México, 2013.
- NOM-017-SSA2-2012; Para la Vigilancia Epidemiológica. Diario Oficial de la Federación: Ciudad de México, México, 2013.
- Red Nacional de Vigilancia Epidemiológica. RENAVE. Protocolo de Vigilancia de Infección Gonocócica. Available online: https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Documents/archivos%20A-Z/INFECCION%20GONOCOCICA/Protocolo%20de%20Vigilancia%20de%20Infección%20gonocócica.pdf (accessed on 31 May 2023).
- Sentís, A.; Prats-Uribe, A.; López-Corbeto, E.; Montoro-Fernandez, M.; Nomah, D.K.; de Olalla, P.G.; Mercuriali, L.; Borrell, N.; Guadalupe-Fernández, V.; Reyes-Urueña, J.; et al. The impact of the COVID-19 pandemic on Sexually Transmitted Infections surveillance data: Incidence drop or artefact? BMC Public Health 2021, 21, 1637. [Google Scholar] [CrossRef] [PubMed]
- Lőrincz, K.; Meznerics, F.A.; Jobbágy, A.; Kiss, N.; Madarász, M.; Belvon, L.; Tóth, B.; Tamási, B.; Wikonkál, N.M.; Marschalkó, M.; et al. STIs during the COVID-19 Pandemic in Hungary: Gonorrhea as a Potential Indicator of Sexual Behavior. Int. J. Environ. Res. Public Health 2022, 19, 9627. [Google Scholar] [CrossRef]
- Nazir, A.; Masood, W.; Ahmad, S.; Nair, A.M.; Aborode, A.T.; Khan, H.D.; Farid, S.; Raza, M.A.; Audah, K.A. Rise of syphilis surge amidst COVID-19 pandemic in the USA: A neglected concern. Ann. Med. Surg. 2022, 80, 104239. [Google Scholar] [CrossRef]
- Pagaoa, M.; Grey, J.; Torrone, E.; Kreisel, K.; Stenger, M.; Weinstock, H. Trends in Nationally Notifiable Sexually Transmitted Disease Case Reports During the US COVID-19 Pandemic, January to December 2020. Sex. Transm. Dis. 2021, 48, 798. [Google Scholar] [CrossRef] [PubMed]
- Moitra, E.; Tao, J.; Olsen, J.; Shearer, R.D.; Wood, B.R.; Busch, A.M.; LaPlante, A.; Baker, J.V.; Chan, P.A. Impact of the COVID-19 pandemic on HIV testing rates across four geographically diverse urban centres in the United States: An observational study. Lancet Reg. Health Am. 2022, 7, 100159. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.R.; Reid, D.; Howarth, A.R.; Mohammed, H.; Saunders, J.; Pulford, C.V.; Hughes, G.; Mercer, C.H. Changes in STI and HIV testing and testing need among men who have sex with men during the UK’s COVID-19 pandemic response. Sex. Transm. Infect. 2022, 99, 226–238. [Google Scholar] [CrossRef]
- Aniskevich, A.; Shimanskaya, I.; Boiko, I.; Golubovskaya, T.; Golparian, D.; Stanislavova, I.; Jacobsson, S.; Adaskevich, A.; Unemo, M. Antimicrobial resistance in Neisseria gonorrhoeae isolates and gonorrhoea treatment in the Republic of Belarus, Eastern Europe, 2009–2019. BMC Infect. Dis. 2021, 21, 520. [Google Scholar] [CrossRef]
- Vigilancia Epidemiológica Convencional de Casos Nuevos de Enfermedad. Definiciones Operacionales de Enfermedades Sujetas a Vigilancia Convencional. Secretaria de Salud/Subsecretaría de Prevención y Promoción de la Salud/Dirección Adjunta de Epidemiología. Available online: https://epidemiologia.salud.gob.mx/gobmx/salud/documentos/manuales/DefinicionesOperacionales_Padecimientos_Sujetos_a_VE.pdf (accessed on 31 May 2023).
- Sharifi, A.; Khavarian-Garmsir, A.R. The COVID-19 pandemic: Impacts on cities and major lessons for urban planning, design, and management. Sci. Total Environ. 2020, 749, 142391. [Google Scholar] [CrossRef]
- Kularatne, R.S.; Niit, R.; Rowley, J.; Kufa-Chakezha, T.; Peters, R.P.H.; Taylor, M.M.; Johnson, L.F.; Korenromp, E.L. Adult gonorrhea, chlamydia and syphilis prevalence, incidence, treatment and syndromic case reporting in South Africa: Estimates using the Spectrum-STI model, 1990–2017. PLoS ONE 2018, 13, e0205863. [Google Scholar] [CrossRef]
- Walker, C.K.; Sweet, R.L. Gonorrhea infection in women: Prevalence, effects, screening, and management. Int. J. Womens Health 2011, 3, 197–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maduna, L.D.; Kock, M.M.; van der Veer, B.M.J.W.; Radebe, O.; McIntyre, J.; van Alphen, L.B.; Peters, R.P.H. Antimicrobial Resistance of Neisseria gonorrhoeae Isolates from High-Risk Men in Johannesburg, South Africa. Antimicrob. Agents Chemother. 2020, 64, e00906–e00920. [Google Scholar] [CrossRef] [PubMed]
- Cámara, J.; Serra, J.; Ayats, J.; Bastida, T.; Carnicer-Pont, D.; Andreu, A.; Ardanuy, C. Molecular characterization of two high-level ceftriaxone-resistant Neisseria gonorrhoeae isolates detected in Catalonia, Spain. J. Antimicrob. Chemother. 2012, 67, 1858–1860. [Google Scholar] [CrossRef] [PubMed]
- Kandinov, I.; Dementieva, E.; Filippova, M.; Vinokurova, A.; Gorshkova, S.; Kubanov, A.; Solomka, V.; Shagabieva, J.; Deryabin, D.; Shaskolskiy, B.; et al. Emergence of Azithromycin-Resistant Neisseria gonorrhoeae Isolates Belonging to the NG-MAST Genogroup 12302 in Russia. Microorganisms 2023, 11, 1226. [Google Scholar] [CrossRef] [PubMed]
- Escobedo-Guerra, M.R.; Katoku-Herrera, M.; Lopez-Hurtado, M.; Gutierrez-Trujillo, R.; Guerra-Infante, F.M. Use of the mtrR Gene for Rapid Molecular Diagnosis of Neisseria gonorrhoeae and Identification of the Reduction of Susceptibility to Antibiotics in Endocervical Swabs. Mol. Diagn Ther. 2018, 22, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Hale, T.; Angrist, N.; Goldszmidt, R.; Kira, B.; Petherick, A.; Phillips, T.; Webster, S.; Cameron-Blake, E.; Hallas, L.; Majumdar, S.; et al. A global panel database of pandemic policies (Oxford COVID-19 Government Response Tracker). Nat. Hum. Behav. 2021, 5, 529–538. [Google Scholar] [CrossRef]
- Ivarsson, L.; Campa, M.d.A.S.d.l.; Elfving, K.; Yin, H.; Gullsby, K.; Stark, L.; Andersen, B.; Hoffmann, S.; Gylfe, A.; Unemo, M.; et al. Changes in testing and incidence of Chlamydia trachomatis and Neisseria gonorrhoeae–the possible impact of the COVID-19 pandemic in the three Scandinavian countries. Infect. Dis. 2022, 54, 623–631. [Google Scholar] [CrossRef]
- Hammerschlag, M.R. Chlamydial and gonococcal infections in infants and children. Clin. Infect. Dis. 2011, 53, S99–S102. [Google Scholar] [CrossRef]
- Kellogg, N.D.; Melville, J.D.; Lukefahr, J.L.; Nienow, S.M.; Russell, E.L. Genital and Extragenital Gonorrhea and Chlamydia in Children and Adolescents Evaluated for Sexual Abuse. Pediatr. Emerg. Care 2018, 34, 761–766. [Google Scholar] [CrossRef]
- Whaitiri, S.; Kelly, P. Genital gonorrhoea in children: Determining the source and mode of infection. Arch. Dis. Child. 2011, 96, 247–251. [Google Scholar] [CrossRef]
- Yue, X.L.; Gong, X.D.; Li, J.; Wang, Y.J.; Gu, H. Gonorrea en China, 2018. Gonorrhea in China, 2018. Int. J. Dermatol. Venereol. 2019, 2, 65–69. [Google Scholar] [CrossRef]
- Huneeus, A.; Schilling, A.; Fernandez, M.I. Prevalence of Chlamydia Trachomatis, Neisseria gonorrhoeae, and Trichomonas Vaginalis Infection in Chilean Adolescents and Young Adults. J. Pediatr. Adolesc. Gynecol. 2018, 31, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Nacher, M.; Koendjbiharie, A.; Michaud, C.; Rabier, S.; Leborgne, C.; Rousseau, C. Sexually transmitted infections on the border between Suriname and French Guiana: A scoping review. Front. Med. 2022, 5, 994964. [Google Scholar] [CrossRef] [PubMed]
- Lannoy, L.H.; Santos, P.C.; Coelho, R.; Dias-Santos, A.S.; Valentim, R.; Pereira, G.M.; Miranda, A.E. Gestational and congenital syphilis across the international border in Brazil. PLoS ONE 2022, 25, e0275253. [Google Scholar] [CrossRef] [PubMed]
- Patterson, T.L.; Strathdee, S.A.; Semple, S.J.; Chavarin, C.V.; Abramovitz, D.; Gaines, T.L.; Mendoza, D.; Staines, H.; Aarons, G.A.; Magis-Rodriguez, C. Prevalence of HIV/STIs and correlates with municipal characteristics among female sex workers in 13 Mexican cities. Salud. Publ. Méx. 2019, 61, 116–124. [Google Scholar] [CrossRef]
- Rosales, C.B.; Carvajal, S.C.; devZapien, J.E. Emergent Public Health Issues in the US-Mexico Border Region. Front. Public Health 2016, 17, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraser, H.; Borquez, A.; Stone, J.; Abramovitz, D.; Brouwer, K.C.; Goodman-Meza, D.; Hickman, M.; Patterson, T.L.; Silverman, J.; Smith, L.; et al. Overlapping Key Populations and HIV Transmission in Tijuana, Mexico: A Modelling Analysis of Epidemic Drivers. AIDS Behav. 2021, 25, 3814–3827. [Google Scholar] [CrossRef]
- Tan, N.X.; Tan, G.X.; Yang, L.-G.; Yang, B.; Powers, K.A.; Emch, M.E.; Tucker, J.D. Temporal trends in syphilis and gonorrhea incidences in Guangdong province, China. J. Infect. Dis. 2014, 209, 426–430. [Google Scholar] [CrossRef] [Green Version]
- Ibáñez-Cervantes, G.; León-García, G.; Vargas-De-León, C.; Castro-Escarpulli, G.; Bandala, C.; Sosa-Hernández, O.; Mancilla-Ramírez, J.; Rojas-Bernabé, A.; Cureño-Díaz, M.; Durán-Manuel, E.; et al. Epidemiological behavior and current forecast of syphilis in Mexico: Increase in male population. Public Health 2020, 185, 386–393. [Google Scholar] [CrossRef]
- Cornelisse, V.J.; Chow, E.P.; Chen, M.Y.; Bradshaw, C.S.; Fairley, C.K. Summer heat: A cross-sectional analysis of seasonal differences in sexual behaviour and sexually transmissible diseases in Melbourne, Australia. Sex. Transm. Infect. 2016, 92, 286–291. [Google Scholar] [CrossRef]
- Fortenberry, J.D.; Orr, D.P.; Zimet, G.D.; Blythe, M.J. Weekly and seasonal variation in sexual behaviors among adolescent women with sexually transmitted diseases. J. Adolesc. Health 1997, 20, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.A.; Judson, F.N. Relative and seasonal incidences of the sexually transmitted diseases. A two-year statistical review. Br. J. Vener. Dis. 1978, 54, 433–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loyola-Cruz, M.Á.; Fernández-Sánchez, V.; Durán-Manuel, E.M.; Calzada-Mendoza, C.C.; Castro-Escarpulli, G.; Quijano-Soriano, M.F.; Nicolás-Sayago, L.; Razo-Blanco Hernández, D.M.; Villegas-Castañeda, M.; Cárdenas-Cantero, A.; et al. Epidemiological Overview of Urogenital Gonorrhea in Mexico (2003–2020). Healthcare 2023, 11, 2118. https://doi.org/10.3390/healthcare11152118
Loyola-Cruz MÁ, Fernández-Sánchez V, Durán-Manuel EM, Calzada-Mendoza CC, Castro-Escarpulli G, Quijano-Soriano MF, Nicolás-Sayago L, Razo-Blanco Hernández DM, Villegas-Castañeda M, Cárdenas-Cantero A, et al. Epidemiological Overview of Urogenital Gonorrhea in Mexico (2003–2020). Healthcare. 2023; 11(15):2118. https://doi.org/10.3390/healthcare11152118
Chicago/Turabian StyleLoyola-Cruz, Miguel Ángel, Verónica Fernández-Sánchez, Emilio Mariano Durán-Manuel, Claudia Camelia Calzada-Mendoza, Graciela Castro-Escarpulli, María Fernanda Quijano-Soriano, Liliana Nicolás-Sayago, Dulce Milagros Razo-Blanco Hernández, Marcela Villegas-Castañeda, Alejandro Cárdenas-Cantero, and et al. 2023. "Epidemiological Overview of Urogenital Gonorrhea in Mexico (2003–2020)" Healthcare 11, no. 15: 2118. https://doi.org/10.3390/healthcare11152118







